Synopsis
At the beginning of the 21st century, we are facing the convergence of several epidemics. These include tobacco smoking, tuberculosis, HIV infection, influenza, and chronic obstructive pulmonary disease (COPD). These epidemics interact by way of increasing disease susceptibility and worsening outcomes. To control these interacting epidemics, we need to better understand each infection and how it influences the others. Multi-faceted approaches will be necessary to reduce the impact on those in developing nations most likely to be affected by the convergence of all epidemics.
Keywords: Tuberculosis, smoking, HIV, Influenza, COPD, pneumonia, epidemics
Introduction
History appears to be repeating itself. This time however, it might have a sting in its tail. At the end of the 19th century a vaguely familiar picture was developing, with consequences for global respiratory health. Tobacco smoking was widespread and increasing after the invention of the cigarette rolling machine in 1881(1). The discovery of Mycobacterium tuberculosis by Robert Koch one year later in 1882 occurred during a time that tuberculosis (TB) was rampant across many parts of the world.
By the turn of the 20th century, tobacco consumption had increased to approximately 50 billion cigarettes per year(1) at a time that TB mortality was declining in Europe most likely due to improved nutrition and social circumstances. A potential association between smoking and TB was suggested by Webb in 1918. During the same year the “Mother of all pandemics”(2), the 1918 Spanish flu killed nearly 50 million people worldwide. (2, 3)
Nearly 100 years later, smoking rates are at an all time high with over 6,319 billion cigarettes consumed per year(1). Faced with declining markets in the North, the tobacco industry is moving south, where smoking is on the increase, especially in countries such as China and India. Tuberculosis remains uncontrolled in the developing world with increasing rates of multi-, extensively- and pan-drug resistant tuberculosis. HIV has added a new face to respiratory disease with increased rates of TB and pneumonia and in addition we are facing the looming possibility of a highly virulent H5N1 “avian” influenza pandemic, with the novel strain of H1N1 “swine” flu recently added to the mix.
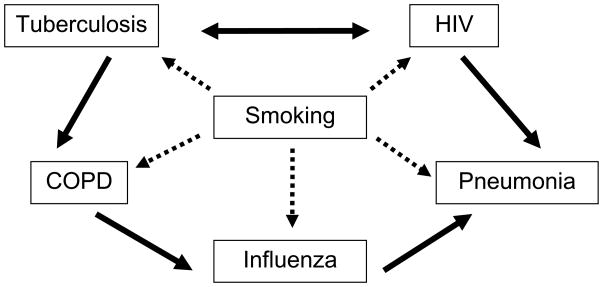
In this overview, we build on our previous reviews (4, 5) and consider the predisposing effects of tobacco smoking, and the potential interactions of tuberculosis, HIV, COPD, influenza, and other respiratory infection epidemics (figure 1).
Figure 1.
The central role of smoking in pulmonary infections, HIV and COPD, and the interactions of several of the individual diseases. The dashed lines indicate increased risk of disease associated with tobacco smoking. The solid line indicates increased risk associated with other diseases.
Methodology and Search strategy
We searched PubMed for peer-reviewed literature over the last 3 decades with a focus on studies that reported data on the associations among tuberculosis, smoking, HIV, influenza, pneumonia and COPD. No language restrictions were imposed, although only English language studies were eventually included. In addition, we identified three systematic reviews (6–8) on the association between tobacco and TB, one systematic review on the association between tobacco and HIV(9), and several narrative reviews(4, 5, 10–16) on the association between tobacco and all the conditions of interest. The reference lists of these reviews were also used to supplement the search. In addition, we identified a comprehensive report entitled “A WHO / The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics”(17) this was used as an additional resource to supplement our searches. Key words included Tuberculosis, HIV, COPD, pneumonia, influenza and tobacco smoking.
Smoking and pulmonary immunity and disease
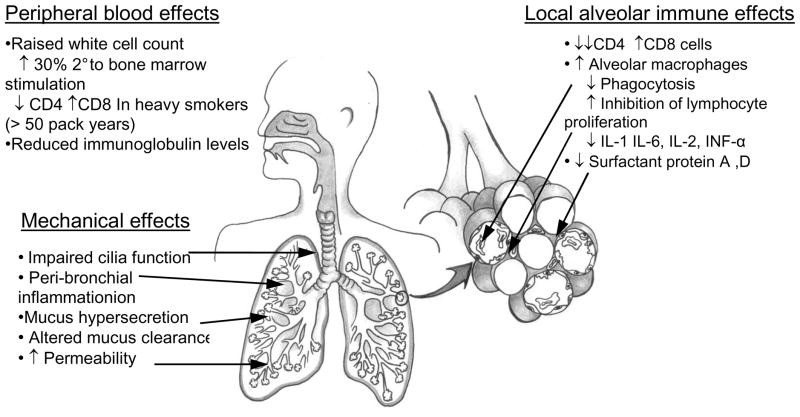
Current estimates of tobacco smoking rates are 49% for men and 8% for women in low- and middle-income countries, and 37% for men and 21% for women in high-income countries(6). Tobacco is the single most preventable cause of death in the world today. It kills more than five million people per year, with more than 80% of those deaths occurring in the developing world (18). There are a multitude of effects of tobacco smoke on the immune system, predisposing individuals to respiratory infections (14–16). These effects have been summarised in figure 2. Briefly, smoking affects circulating immune cells, mucosal surface defences (mucus, cilia) as well as local innate immune cell functions (macrophages, neutrophils). The associations with tobacco smoking have been well described for several of the diseases depicted in figure 1.
Figure 2.
Overview of the systemic and local effects of tobacco smoke on the immune system and pulmonary defence mechanisms against infection.
Smoking and TB
The association between tobacco smoke and tuberculosis was suggested many years ago(19). Evidence of the impact of tobacco smoking on TB infection has been confounded by its almost universal association with poverty, overcrowding and alcohol usage. Similar pathological mechanisms induced by malnutrition, alcohol abuse and smoking may indeed all predispose an individual to TB. There are now three comprehensive independent systematic reviews and meta-analyses that have synthesized the evidence for the association between TB and tobacco smoking (6–8).
The association of smoking and TB has been analysed for three outcomes: TB infection (defined by a positive tuberculin skin test), active TB disease, and death due to TB. Table 1 provides the outcome-specific pooled relative risk estimates from the 3 independent meta-analyses.
Table 1.
The association between smoking and the relative risk of latent tuberculosis infection, progression to active disease and mortality from active TB.
| Meta-analyses: pooled relative risks (95%C.I) | ||||
|---|---|---|---|---|
| Slama et al. 2007(6) | Lin et al. 2007(7) | Bates et al. 2007(8) | ||
| TB outcome | TB infection | ~1.8 (1.5–2.1) | 1.7 – 2.2 (1.5–2.8) | ~1.7 (1.5–2.0) |
| TB disease | ~2.3 (1.8–3.0) | ~2.0 (1.6–2.6) | ~2.3 (2.0–2.8) | |
| TB mortality | ~2.2 (1.3–3.7) | ~2.0 (1.1–3.5) | ~2.1 (1.4–3.4) | |
Adapted from van Zyl-Smit RN, Pai M, Yew WW, Leung CC, Zumla A, Bateman ED, et al. Global Lung Health: The Colliding Epidemics of Tuberculosis, Tobacco Smoking, HIV and COPD. Eur Respir J. 2010; 35: 27–33
It is evident from these meta-analyses that smoking approximately doubles the risk of each outcome, namely TB infection (RR ~1.5), active TB disease (RR ~2.0) and TB mortality (RR ~2.0). The evidence is strong for TB disease, but relatively weaker for TB infection. Due to the widespread nature of tobacco smoking the population attributable risk (PAR %) is likely to be high. For example, if the relative risk for TB disease is estimated at 1.5, and population exposure to tobacco smoke 30%, the PAR% will be approximately 15%. In other words, 15% of the TB cases in the world each year may be attributable to tobacco exposure(4).
Since the publication of these meta-analyses, newer studies have been published, and these studies confirm the association between smoking and TB. In a large case-control study from India, Jha et al.(20) reported excess TB deaths among smokers, as compared with non-smokers, among both women (RR, 3.0; 99% CI, 2.4 to 3.9) and men (RR, 2.3; 99% CI, 2.1 to 2.6). A subsequent large case-control study from India (21) reported that those who both smoked cigarettes and drank alcohol had considerably higher active TB incidence rates than those who did neither (TB incidence RR 3.5).
Similar results were also obtained in Taiwan and China. In a prospective cohort study from Taiwan, Lin et al.(22) reported that current smoking was associated with an increased risk of active TB (adjusted OR, 1.94; 95% confidence interval, 1.01–3.73). In a case-control study from two rural areas of China, Wang et al. (23)reported an adjusted OR of 1.93 (95% CI: 1.51–2.48) for smoking and TB disease. Thus, these studies from India, Taiwan and China added further support to the already strong evidence from the meta-analyses.
A cohort study in Brazil looked at the association between smoking and a fourth outcome, TB relapse.. They reported that smoking was independently associated with relapse of TB, as defined by the requirement for re-treatment within 3–5 years after succesful completion of TB treatment. After adjusting for socioeconomic variables and alcohol, the OR for relapse was 2.53 (95% CI, 1.23, 5.21)(24).
Lastly, Lin et al (25) did a mathematical modelling study, where they modelled future COPD and lung cancer mortality and TB incidence, taking into account the accumulation of hazardous effects of risk factors on COPD and lung cancer over time, and dependency of the risk of TB infection on the prevalence of disease. Their model suggested that complete cessation of smoking and solid-fuel use by 2033 could avoid 26 million deaths from COPD and 6.3 million deaths from lung cancer. Smoking cessation would also reduce the projected annual TB incidence in 2033 by 14–52% if 80% directly observed treatment short-course (DOTS) coverage is sustained. Based on these results, they concluded that reducing smoking and solid-fuel use can substantially lower predictions of COPD and lung cancer burden and would contribute to effective TB control in China.
Smoking and Pneumonia
Pneumonia occurs more frequently in individuals with impaired immunity. This is evident in the elderly, alcoholics, malnourished, HIV-infected and those with underlying COPD(26). In addition, tobacco smoking appears to be an independent risk factor for pneumonia (27, 28). It is evident from several studies that smoking is a particularly important risk factor for streptococcal pneumonia (OR 1.88 to 4.1) (29, 30). Passive smokers and importantly children also have an increased risk of infection, OR 2.5 (95% CI 1.2–5.1) and 1.88 (95% CI 1.04–3.39) respectively (29, 31). Severe pneumococcal disease is more frequently seen in smokers (29, 32), which may be partially explained by enhanced pneumococcal epithelial adherence caused by tobacco smoking (33).
Although the frequency of pneumonia caused by other organisms is low, there is also strong evidence for increased risks of Legionella (OR 3.48; 95%CI 2.09 – 5.79) (34), Mycoplasma, (OR 5.6; 95%CI, 1.5–20.4) (35) and Haemophilus influenzae (36) infection. In HIV-infected individuals the already increased risk of community acquired pneumonia is compounded by the increased risk associated with smoking (37–42) with an apparent dose response association. (39) In addition, the risks of other infection such as PCP are also increased by smoking (40, 43) (discussed below)
Smoking and Influenza
With the prospect of an avian influenza epidemic ever increasing(3), multiple measures will be required to control the epidemics. Although not recognised as a risk factor in the 1918 pandemic (data not collected)(2, 3) there is substantial evidence in both mouse and human models for a negative effect of smoking on influenza (44–48). The risk of infection is increased in smokers (OR 1.4–2.4)(46) (47) as well as likelihood for severe disease and complications (OR 4.3 95%CI 1.1- 16.1)(49). In addition to predisposing individuals to infection, the efficacy of influenza vaccines is reduced in smokers (50–52).
The predisposing risk factors for localized influenza outbreaks have been studied. An influenza A/USSR/90/77(H1N1) outbreak in Israeli military recruits in 1979 found a relative risk of 1.44 (95% CI; 1.03–2.01)(48). In a 1986 influenza A/Taiwan/1/86(H1N1) outbreak at a Florida naval air base, the seasonal trivalent vaccination offered no protection and smokers had a non significant trend to increased infection OR 1.4 95%CI 0.9–2.2; p=0.27)(53). There are little data on the current H1N1 epidemics and their potential association with tobacco smoking. Several recent observational studies have not reported on the smoking status of patients (54–57). It is likely however, given the extent of the global pandemic, that the association with tobacco smoking will become evident as the number of reported cases increases.
Smoking and HIV
Although both tobacco smoking and HIV infection may be associated through their common associations with poverty and high-risk behaviour, tobacco smoking appears to be an independent and important risk factor for contracting HIV (58–61). Other studies have demonstrated higher viral loads (62) and rate of progression of HIV infection to AIDS in smokers(63, 64), but this association has not been observed in all studies(65–68).
Smoking further raises the extremely high risk of contracting TB in HIV-positive persons (43) in addition to increased susceptibility of community-acquired bacterial pneumonia (37–40). HIV infected smokers also have higher respiratory symptoms and risk of mortality (hazard ratio 1.99; 95%CI 1.03 to 3.86) when compared to non-smokers (41).
Several studies have examined the risk of other opportunistic infection such as Pneumocystis jiroveci pneumonia (PCP) with conflicting results. Some have shown significantly increased risk but this has not been confirmed by others (38–40, 66).
Tobacco smoking and HIV are associated with an accelerated form of obstructive pulmonary disease (69–73). If this form of COPD is phenotypically similar to that seen with HIV uninfected smokers, the risk of influenza and pneumonia may be further magnified.
Smoking and COPD
Although the focus of this review is primarily on the convergence of respiratory infections, HIV and tobacco smoking, the importance of COPD in the interactions needs to be stressed.
The causal link between smoking and COPD is well described and the multitude of consequences well known (74). There is now a growing recognition of the importance of non-smoking causes for COPD (75). There are several studies examining respiratory infections (particularly TB) as a cause of COPD (76–80). The long-term effect on pulmonary function and response to treatment is not well documented (75) although the increased susceptibility to other respiratory infections, particularly influenza and pneumonia are likely to be similar.
Convergence of TB, HIV, COPD, pneumonia and influenza
Tobacco smoking is undoubtedly at the centre of convergence of the epidemics we are currently facing (not discounting the role of alcohol, malnutrition, poverty, indoor biomass fuel exposure and outdoor air pollution).
As smoking increases the risk of TB and COPD, the long-term damage to pulmonary structures and respiratory function further increases the risk of bacterial pneumonia and influenza. Similarly the increased susceptibility to influenza seen in smokers will magnify the risk of superadded bacterial pneumonia following severe influenza. The multitude of infections associated with HIV infection, particularly TB and pneumonia, are likely to be exacerbated by smoking especially if the accelerated form of COPD further impairs pulmonary immunity to infection.
Even without the effect of tobacco smoke, the interactions of HIV, Influenza, TB and pneumonia compound the health on the individuals suffering from them as well as society in general. The interactions summarized in figure 1 are likely to be far more complex and wide ranging. This is no more evident than what is being seen in many parts of the developing world where in addition to high smoking rates, malnutrition, overcrowding and indoor biomass fuel usage, HIV is driving the TB epidemic. The net outcome, in several parts of the world (mainly low income countries), is that these epidemics are simultaneously converging. These high-burden low income countries are least likely to be able to put measures in place to effectively combat the spread of a world-wide influenza epidemic. The already overburdened TB control programs are struggling to cope with increasing HIV-positive subjects as well as complicated and expensive treatment regimes for MDR-and XDR- TB. Whether local and national government structures are able to effectively improve service delivery, drug supplies and infection control measures remains to be seen. The H1N1 epidemic clearly showed the inability of weak healthcare systems to launch any concerted, evidence-based programs to manage clinical cases and prevent transmission.
It is clear that smoking cessation and respiratory infection control are likely to be the cornerstone to halting the convergent epidemics. In addition, effective treatment strategies will be required to treat those who contract community-acquired pneumonia, tuberculosis, influenza or HIV. The management of multiple infections with potentially interacting drug therapies, the need for quarantine and rational allocation of critical care services will further challenge the most advanced health care services let alone those in under-resourced areas.
Conclusions
At the beginning of the 21st century, the globe is facing an economic crisis in addition to the convergence of several potentially devastating infection epidemics. The emergence of H1N1 is adding to the burden on healthcare delivery systems, and is already diverting precious human and laboratory resources. This was clearly evident in countries such as Mexico and India. The final number of people who will succumb to TB, HIV and influenza will depend on the combined efforts of governments, health agencies and non-governmental bodies. Interventional strategies will need to target preventative strategies as well as locally sustainable treatment options.
Karl Marx said: “History repeats itself – first as a tragedy, second as a farce”. It remains to be seen if we have learnt sufficiently over the past hundred years to effectively combat the looming epidemics or whether we are doomed to see history repeat itself.
Acknowledgments
RVZS is supported by a Discovery Foundation fellowship and by the Fogarty International Clinical Research Scholars/Fellows Support Centre NIH grant R24TW007988. MP is supported by grants from the Canadian Institutes of Health Research (CIHR) and European Commission (TBSusgent; EU FP-7).
References
- 1.Mackay JEM. The Tobacco Atlas. 2. Hong Kong: World Health Organisation; 2006. [Google Scholar]
- 2.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006 Jan;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007 Apr 1;195(7):1018–28. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 4.Pai M, Mohan A, Dheda K, Leung CC, Yew WW, Christopher DJ, et al. Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther. 2007 Jun;5(3):385–91. doi: 10.1586/14787210.5.3.385. [DOI] [PubMed] [Google Scholar]
- 5.van Zyl-Smit RN, Pai M, Yew WW, Leung CC, Zumla A, Bateman ED, et al. Global Lung Health: The Colliding Epidemics of Tuberculosis, Tobacco Smoking, HIV and COPD. Eur Respir J. 2010;35:27–33. doi: 10.1183/09031936.00072909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slama K, Chiang CY, Enarson DA, Hassmiller K, Fanning A, Gupta P, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007 Oct;11(10):1049–61. [PubMed] [Google Scholar]
- 7.Lin HH, Ezzati M, Murray M. Tobacco Smoke, Indoor Air Pollution and Tuberculosis: A Systematic Review and Meta-Analysis. PLoSMed. 2007;4(1):e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. ArchInternMed. 2007;167(4):335–42. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 9.Furber AS, Maheswaran R, Newell JN, Carroll C. Is smoking tobacco an independent risk factor for HIV infection and progression to AIDS?. A systemic review. Sexually Transmitted Infections. 2007;83(1):41–6. doi: 10.1136/sti.2005.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baris E, Ezzati M. Should interventions to reduce respirable pollutants be linked to tuberculosis control programmes? BMJ. 2004 Nov 6;329(7474):1090–3. doi: 10.1136/bmj.329.7474.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang CY, Slama K, Enarson DA. Associations between tobacco and tuberculosis. IntJ TubercLung Dis. 2007;11(3):258–62. [PubMed] [Google Scholar]
- 12.Davies PD, Yew WW, Ganguly D, Davidow AL, Reichman LB, Dheda K, et al. Smoking and tuberculosis: the epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg. 2006 Apr;100(4):291–8. doi: 10.1016/j.trstmh.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Maurya V, Vijayan VK, Shah A. Smoking and tuberculosis: an association overlooked. IntJTubercLung Dis. 2002;6(11):942–51. [PubMed] [Google Scholar]
- 14.Arcavi L, Benowitz NL. Cigarette smoking and infection. ArchInternMed. 2004;164(20):2206–16. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 15.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002 May;2(5):372–7. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 16.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009 May;9(5):377–84. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation. A WHO /The Union monograph on TB and tobacco control: joining forces to control two related global epidemics [WHO/HTM/TB/2007.390] Geneva: World Health Organization; 2007. [Google Scholar]
- 18.WHO. The MPOWER package. Geneva: World Health Organisation; 2008. WHO Report on the Global Tobacco Epidemic, 2008. [Google Scholar]
- 19.Webb GB. The effect of the inhalation of cigarette smoke on the lungs. A clinical study. Am Rev Tuberc. 1918 march;:25–7. [Google Scholar]
- 20.Jha P, Jacob B, Gajalakshmi V, Gupta PC, Dhingra N, Kumar R, et al. A nationally representative case-control study of smoking and death in India. N Engl J Med. 2008 Mar 13;358(11):1137–47. doi: 10.1056/NEJMsa0707719. [DOI] [PubMed] [Google Scholar]
- 21.Gajalakshmi V, Peto R. Smoking, drinking and incident tuberculosis in rural India: population-based case-control study. Int J Epidemiol. 2009 Aug;38(4):1018–25. doi: 10.1093/ije/dyp225. [DOI] [PubMed] [Google Scholar]
- 22.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med. 2009 Sep 1;180(5):475–80. doi: 10.1164/rccm.200904-0549OC. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Shen H. Review of cigarette smoking and tuberculosis in China: intervention is needed for smoking cessation among tuberculosis patients. BMC Public Health. 2009;9:292. doi: 10.1186/1471-2458-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.d’Arc Lyra Batista J, de Fatima Pessoa Militao de Albuquerque M, de Alencar Ximenes RA, Rodrigues LC. Smoking increases the risk of relapse after successful tuberculosis treatment. Int J Epidemiol. 2008 Aug;37(4):841–51. doi: 10.1093/ije/dyn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin HH, Murray M, Cohen T, Colijn C, Ezzati M. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet. 2008 Oct 25;372(9648):1473–83. doi: 10.1016/S0140-6736(08)61345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman C, Anderson R. New insights into pneumococcal disease. Respirology. 2009 Mar;14(2):167–79. doi: 10.1111/j.1440-1843.2008.01422.x. [DOI] [PubMed] [Google Scholar]
- 27.Farr BM, Woodhead MA, Macfarlane JT, Bartlett CL, McCraken JS, Wadsworth J, et al. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir Med. 2000 May;94(5):422–7. doi: 10.1053/rmed.1999.0743. [DOI] [PubMed] [Google Scholar]
- 28.Farr BM, Bartlett CL, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med. 2000 Oct;94(10):954–63. doi: 10.1053/rmed.2000.0865. [DOI] [PubMed] [Google Scholar]
- 29.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000 Mar 9;342(10):681–9. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 30.Almirall J, Gonzalez CA, Balanzo X, Bolibar I. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest. 1999 Aug;116(2):375–9. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 31.O’Dempsey TJ, McArdle TF, Morris J, Lloyd-Evans N, Baldeh I, Laurence BE, et al. A study of risk factors for pneumococcal disease among children in a rural area of west Africa. Int J Epidemiol. 1996 Aug;25(4):885–93. doi: 10.1093/ije/25.4.885. [DOI] [PubMed] [Google Scholar]
- 32.Pastor P, Medley F, Murphy TV. Invasive pneumococcal disease in Dallas County, Texas: results from population-based surveillance in 1995. Clin Infect Dis. 1998 Mar;26(3):590–5. doi: 10.1086/514589. [DOI] [PubMed] [Google Scholar]
- 33.Raman AS, Swinburne AJ, Fedullo AJ. Pneumococcal adherence to the buccal epithelial cells of cigarette smokers. Chest. 1983 Jan;83(1):23–7. doi: 10.1378/chest.83.1.23. [DOI] [PubMed] [Google Scholar]
- 34.Doebbeling BN, Wenzel RP. The epidemiology of Legionella pneumophila infections. Semin Respir Infect. 1987 Dec;2(4):206–21. [PubMed] [Google Scholar]
- 35.Klement E, Talkington DF, Wasserzug O, Kayouf R, Davidovitch N, Dumke R, et al. Identification of risk factors for infection in an outbreak of Mycoplasma pneumoniae respiratory tract disease. Clin Infect Dis. 2006 Nov 15;43(10):1239–45. doi: 10.1086/508458. [DOI] [PubMed] [Google Scholar]
- 36.Kofteridis D, Samonis G, Mantadakis E, Maraki S, Chrysofakis G, Alegakis D, et al. Lower respiratory tract infections caused by Haemophilus influenzae: clinical features and predictors of outcome. Med Sci Monit. 2009 Apr;15(4):CR135–9. [PubMed] [Google Scholar]
- 37.Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006 Jul 1;43(1):90–8. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- 38.Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, et al. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Dec 1;13(4):374–83. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- 39.Conley LJ, Bush TJ, Buchbinder SP, Penley KA, Judson FN, Holmberg SD. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996 Sep;10(10):1121–6. [PubMed] [Google Scholar]
- 40.Miguez-Burbano MJ, Ashkin D, Rodriguez A, Duncan R, Pitchenik A, Quintero N, et al. Increased risk of Pneumocystis carinii and community-acquired pneumonia with tobacco use in HIV disease. International Journal of Infectious Diseases. 2005;9(4):208–17. doi: 10.1016/j.ijid.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005 Dec;20(12):1142–5. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995 Sep 28;333(13):845–51. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 43.Miguez-Burbano MJ, Burbano X, Ashkin D, Pitchenik A, Allan R, Pineda L, et al. Impact of tobacco use on the development of opportunistic respiratory infections in HIV seropositive patients on antiretroviral therapy. AddictBiol. 2003;8(1):39–43. doi: 10.1080/1355621031000069864. [DOI] [PubMed] [Google Scholar]
- 44.Gualano RC, Hansen MJ, Vlahos R, Jones JE, Park-Jones RA, Deliyannis G, et al. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir Res. 2008;9:53. doi: 10.1186/1465-9921-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins CS, Bauer CM, Vujicic N, Gaschler GJ, Lichty BD, Brown EG, et al. Cigarette smoke impacts immune inflammatory responses to influenza in mice. Am J Respir Crit Care Med. 2006 Dec 15;174(12):1342–51. doi: 10.1164/rccm.200604-561OC. [DOI] [PubMed] [Google Scholar]
- 46.Finklea JF, Sandifer SH, Smith DD. Cigarette smoking and epidemic influenza. Am J Epidemiol. 1969 Nov;90(5):390–9. doi: 10.1093/oxfordjournals.aje.a121084. [DOI] [PubMed] [Google Scholar]
- 47.Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med. 1982 Oct 21;307(17):1042–6. doi: 10.1056/NEJM198210213071702. [DOI] [PubMed] [Google Scholar]
- 48.Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health. 1981 May;71(5):530–2. doi: 10.2105/ajph.71.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanshaoworakul W, Simmerman JM, Narueponjirakul U, Sanasuttipun W, Shinde V, Kaewchana S, et al. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS One. 2009;4(6):e6051. doi: 10.1371/journal.pone.0006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruijff M, Thijs C, Govaert T, Aretz K, Dinant GJ, Knottnerus A. The effect of smoking on influenza, influenza vaccination efficacy and on the antibody response to influenza vaccination. Vaccine. 1999 Feb 5;17(5):426–32. doi: 10.1016/S0264-410X(98)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson WS, Dube SR, Ford ES, Mokdad AH. Influenza and pneumococcal vaccination rates among smokers: data from the 2006 Behavioral Risk Factor Surveillance System. Prev Med. 2009 Feb;48(2):180–3. doi: 10.1016/j.ypmed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Finklea JF, Hasselblad V, Riggan WB, Nelson WC, Hammer DI, Newill VA. Cigarette smoking and hemagglutination inhibition response to influenza after natural disease and immunization. Am Rev Respir Dis. 1971 Sep;104(3):368–76. doi: 10.1164/arrd.1971.104.3.368. [DOI] [PubMed] [Google Scholar]
- 53.Klontz KC, Hynes NA, Gunn RA, Wilder MH, Harmon MW, Kendal AP. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am J Epidemiol. 1989 Feb;129(2):341–8. doi: 10.1093/oxfordjournals.aje.a115137. [DOI] [PubMed] [Google Scholar]
- 54.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009 Jun 18;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 55.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009 Jun 19;324(5934):1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cutler J, Schleihauf E, Hatchette TF, Billard B, Watson-Creed G, Davidson R, et al. Investigation of the first cases of human-to-human infection with the new swine-origin influenza A (H1N1) virus in Canada. CMAJ. 2009 August 4;181(3–4):159–63. doi: 10.1503/cmaj.090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, et al. Pneumonia and Respiratory Failure from Swine-Origin Influenza A (H1N1) in Mexico. N Engl J Med. 2009 August 13;361(7):680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 58.Boulos R, Halsey NA, Holt E, Ruff A, Brutus JR, Quinn TC, et al. HIV-1 in Haitian women 1982–1988. The Cite Soleil/JHU AIDS Project Team. J AcquirImmuneDeficSyndr. 1990;3(7):721–8. [PubMed] [Google Scholar]
- 59.Halsey NA, Coberly JS, Holt E, Coreil J, Kissinger P, Moulton LH, et al. Sexual behavior, smoking, and HIV-1 infection in Haitian Women. JAMA. 1992;267(15):2062–6. [PubMed] [Google Scholar]
- 60.Chao A, Bulterys M, Musanganire F, Habimana P, Nawrocki P, Taylor E, et al. Risk factors associated with prevalent HIV-1 infection among pregnant women in Rwanda. National University of Rwanda-Johns Hopkins University AIDS Research Team. Int J Epidemiol. 1994;23(2):371–80. doi: 10.1093/ije/23.2.371. [DOI] [PubMed] [Google Scholar]
- 61.Penkower L, Dew MA, Kingsley L, Becker JT, Satz P, Schaerf FW, et al. Behavioral, health and psychosocial factors and risk for HIV infection among sexually active homosexual men: the Multicenter AIDS Cohort Study. Am J Public Health. 1991;81(2):194–6. doi: 10.2105/ajph.81.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, et al. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus seropositive women. Journal of Neurovirology. 2007;13(6):561–8. doi: 10.1080/13550280701620747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM. The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. AIDS. 1993 May;7(5):705–10. doi: 10.1097/00002030-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am J Public Health. 2006 Jun;96(6):1060–5. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eskild A, Petersen G. Cigarette smoking and drinking of alcohol are not associated with rapid progression to acquired immunodeficiency syndrome among homosexual men in Norway. Scand J Soc Med. 1994 Sep;22(3):209–12. doi: 10.1177/140349489402200309. [DOI] [PubMed] [Google Scholar]
- 66.Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Apr 15;14(5):451–8. doi: 10.1097/00042560-199704150-00009. [DOI] [PubMed] [Google Scholar]
- 67.Craib KJ, Schechter MT, Montaner JS, Le TN, Sestak P, Willoughby B, et al. The effect of cigarette smoking on lymphocyte subsets and progression to AIDS in a cohort of homosexual men. Clin Invest Med. 1992 Aug;15(4):301–8. [PubMed] [Google Scholar]
- 68.Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999 Feb 4;13(2):257–62. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 69.Kuhlman JE, Knowles MC, Fishman EK, Siegelman SS. Premature bullous pulmonary damage in AIDS: CT diagnosis. Radiology. 1989 Oct;173(1):23–6. doi: 10.1148/radiology.173.1.2781013. [DOI] [PubMed] [Google Scholar]
- 70.Diaz PT, King ER, Wewers MD, Gadek JE, Neal D, Drake J, et al. HIV Infection Increases Susceptibility to Smoking-Induced Emphysema. Chest. 2000;117(90051):285S. doi: 10.1016/s0012-3692(15)51037-4. [DOI] [PubMed] [Google Scholar]
- 71.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. AnnInternMed. 2000;132(5):369–72. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 72.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC, et al. Increased COPD Among HIV-Positive Compared to HIV-Negative Veterans. Chest. 2006;130(5):1326–33. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 73.Petrache I, Diab K, Knox KS, Twigg HL, III, Stephens RS, Flores S, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008 May 1;63(5):463–9. doi: 10.1136/thx.2007.079111. [DOI] [PubMed] [Google Scholar]
- 74.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007 Sep 15;176(6):532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 75.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009 Aug 29;374(9691):733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 76.Shaheen SO, Barker DJ, Shiell AW, Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):616–9. doi: 10.1164/ajrccm.149.3.8118627. [DOI] [PubMed] [Google Scholar]
- 77.Martin CJ, Hallett WY. The diffuse obstructive pulmonary syndrome in a tuberculosis sanatorium. II. Incidence and symptoms. AnnInternMed. 1961;54:1156–64. doi: 10.7326/0003-4819-54-6-1156. [DOI] [PubMed] [Google Scholar]
- 78.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. RespirMed. 1989;83(3):195–8. doi: 10.1016/s0954-6111(89)80031-9. [DOI] [PubMed] [Google Scholar]
- 79.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005 Oct 1;192(7):1201–9. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 80.Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998 Aug;12(2):351–6. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]