Abstract
The vaccinia virus (VACV) entry-fusion complex (EFC) is comprised of at least nine membrane proteins. Immunization of mice with individual EFC genes induced corresponding protein-binding antibody but failed to protect against VACV intranasal challenge and only DNA encoding A28 elicited low neutralizing antibody. Because the A28 and H2 proteins interact, we determined the effect of immunizing with both genes simultaneously. This procedure greatly enhanced the amount of antibody that bound intact virions, neutralized infectivity and provided partial protection against respiratory challenge. Neither injection of A28 and H2 plasmids at different sites or mixing A28 and H2 sera enhanced neutralizing antibody. The neutralizing antibody could be completely removed by binding to the A28 protein alone and the epitope was located in the C-terminal segment. These data suggest that the interaction of H2 with A28 stabilizes the immunogenic form of A28, mimicking an exposed region of the entry-fusion complex on infectious virions.
Introduction
Poxviruses are large, complex, enveloped DNA viruses that replicate in the cytoplasm of infected cells (Moss, 2007). The best-characterized members belong to the orthopoxvirus genus of the chordopoxvirus subfamily, which includes variola virus and vaccinia virus (VACV) – the causative agent of smallpox and the vaccine virus used to prevent smallpox, respectively (Damon, 2007). Two major infectious forms of VACV have been characterized. The mature virion (MV) contains more than 80 proteins (Chung et al., 2006; Resch et al., 2007; Yoder et al., 2006) and consists of a nucleoprotein core surrounded by a lipoprotein membrane (Condit et al., 2006). The MV can be released by cell lysis or wrapped by modified trans-Golgi or endosomal cisternae, which facilitate virion movement to the cell periphery and exocytosis as the enveloped virion (EV) (Smith and Law, 2004). Thus, the EV is essentially a MV with an additional lipoprotein membrane. The EV membrane does not fuse with the cell membrane but must be disrupted to expose the MV (Law et al., 2006).
More than 20 viral proteins are associated with the MV membrane (Moss, 2007). There is evidence that four MV membrane proteins (A26, A27, D8, H3) are involved in attachment to the cell by binding to glycosaminoglycans (Chung et al., 1998; Hsiao et al., 1999; Lin et al., 2000) or laminin (Chiu et al., 2007), while others are dedicated to membrane fusion (Moss, 2006). Nine of the fusion proteins, namely A16 (Ojeda et al., 2006b), A21 (Townsley et al., 2005b), A28 (Senkevich et al., 2004), G3 (Izmailyan et al., 2006), G9 (Ojeda et al., 2006a), H2 (Senkevich and Moss, 2005), J5 (Senkevich et al., 2005), L5 (Townsley et al., 2005a) and the recently discovered O3 (Satheshkumar and Moss, 2009) form a stable entry-fusion complex known as the EFC. Of the three additional entry proteins, L1 (Bisht et al., 2008) and F9 (Brown et al., 2006) have a weak association with the complex; the association of the I2 entry protein (Nichols et al., 2008) has not been analyzed. The overall organization of the EFC is unknown, but there is evidence for direct interactions between the A28 and H2 (Nelson et al., 2008b) and between the A16 and G9 (Wagenaar et al., 2008) components.
Of the six viral proteins associated with the EV membrane, four (A33, A34, B5 and F13) are involved in MV wrapping, intracellular movement, and the formation of “actin tails” on the cell surface (Smith et al., 2002). Two additional proteins, A56 and K2, are present in both the EV membrane and the plasma membrane; they interact with the A16 and G9 components of the EFC (Wagenaar and Moss, 2007; Wagenaar et al., 2008) and function to prevent fusion of progeny virions with infected cells (Turner and Moyer, 2008; Wagenaar and Moss, 2009) and fusion of infected cells with each other (Ichihashi and Dales, 1971; Law and Smith, 1992; Turner and Moyer, 1992; Zhou et al., 1992).
The use of cowpox or VACV to prevent smallpox was a pivotal event in the history of vaccinology (Fenner et al., 1988). Nevertheless, because of the implementation and early success of the vaccine prior to modern immunology, we know relatively little regarding the mechanism of protection against smallpox (Kennedy et al., 2009). Specific antibody and memory B and T cells persist for decades in humans after smallpox vaccination (Crotty et al., 2003; Hammarlund et al., 2003; Putz et al., 2005; Taub et al., 2008; Viner and Isaacs, 2005). Studies with animal models suggest that interferons, natural killer cells, CD4 and CD8 T cells, and antibody are all involved in clearing a primary orthopoxvirus infection, but that antibodies are central for prevention of a secondary infection or a primary infection following vaccination (Panchanathan et al., 2008). MVs can be neutralized with antibodies to A27 (Rodriguez and Esteban, 1987), D8 (Hsiao et al., 1999), H3 (Lin et al., 2000), L1 (Wolffe et al., 1995) and A28 (Nelson et al., 2008a). EVs can be neutralized directly or in a comet assay with antibody to B5 (Galmiche et al., 1999) and A33 (Galmiche et al., 1999). Immunization with individual proteins or DNA encoding them can partially protect mice against VACV infection (Davies et al., 2005b; Fogg et al., 2004; Galmiche et al., 1999; Hooper et al., 2000; Lai et al., 1991). Combinations of at least one MV and one EV protein, however, achieve far greater protection than individual proteins (Fogg et al., 2004; Hooper et al., 2000; Hooper et al., 2003). A similar benefit was obtained by prophylactic administration of polyclonal and monoclonal antibodies to both MV and EV proteins (Lustig et al., 2005).
We are interested in determining whether the components of the EFC can induce neutralizing antibodies. A previous report showed that rabbits immunized multiple times with a secreted recombinant form of the A28 protein with Freund’s adjuvant produced low levels of neutralizing antibody (Nelson et al., 2008a). In the present study, we used a DNA vaccine approach to compare the immunogenicity of eight EFC proteins alone and in combinations. Only DNA encoding A28 induced a barely measurable level of neutralizing antibody, which was markedly increased when administered with DNA encoding H2. No other individual or combination of EFC gene immunizations lacking H2 enhanced the neutralizing antibody response. In addition, only the combination of A28 and H2 provided partial protection to a VACV intranasal challenge. The neutralizing antibody from mice immunized with A28 and H2 genes was depleted by adsorption with immobilized A28 protein alone and the epitope was located in the C-terminal segment of A28. H2 and A28 have been shown to physically interact (Nelson et al., 2008b), suggesting that this interaction enhances immunogenicity by stabilizing the neutralizing epitope on the latter.
Results
Construction of plasmids expressing proteins of the poxvirus EFC
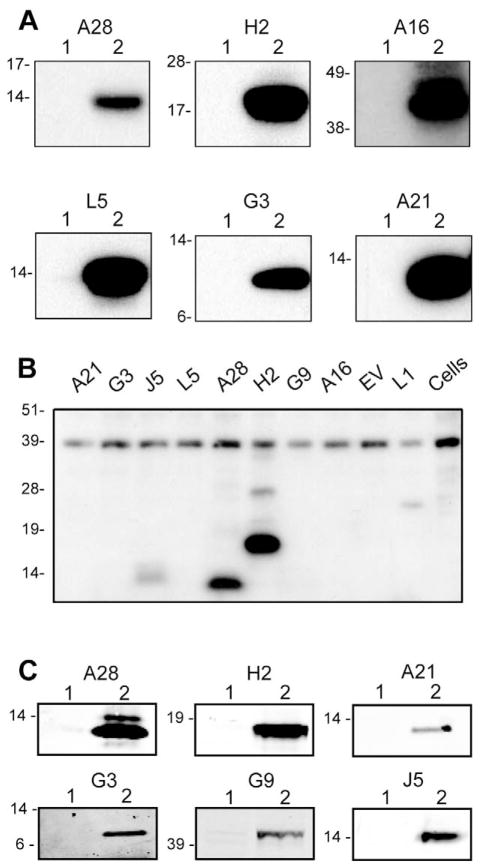
The recently described EFC on the VACV MV is comprised of at least nine viral membrane proteins (A16, A21, A28, G3, G9, H2, J5, L5 and O3) that are conserved in all poxviruses. We constructed plasmids expressing each of these genes, except for O3, which had not been discovered when these experiments were initiated, for subsequent mouse immunization. Since these proteins are not glycosylated during a VACV infection, potential glycosylation sites were mutated and the sequences were codon optimized prior to cloning into a eukaryotic expression vector, VRC8400, with a cytomegalovirus promoter adapted for expression in mice (Barouch et al., 2005). After transfection of BS-C-1 cells with the individual plasmids, expression of A28, H2, A16, L5, G3, and A21 was documented by Western blotting using specific anti-peptide rabbit polyclonal antibodies (Fig. 1A). Protein expression was greater after plasmid transfections than after VACV infection (data not shown). Expression of J5 and G9 could not be assessed in this experiment because of the absence of specific rabbit antibody but was confirmed by other means below. Antisera produced by infecting rabbits with VACV reacted with A28, H2 and J5 in addition to L1 (Fig. 1B); however the other EFC proteins were not detected suggesting that they are not highly immunogenic during a virus infection or that the antibodies do not react with the denatured proteins on Western blots.
Fig. 1.
Western blots showing expression and immunogenicity of EFC proteins. (A) Expression of VACV EFC genes from plasmids. BS-C-1 cells were transfected with the empty vector plasmid or a plasmid expressing A28, H2, A16, L5, G3 or A21, harvested after 24 h and analyzed by SDS-PAGE under reducing conditions. Following membrane transfer, strips were incubated with the corresponding polyclonal antibody and bound proteins were detected by chemiluminescence. Numbers: 1, empty plasmid vector; 2, plasmid encoding indicated EFC gene. Positions and masses in kDa of protein markers are at the left of each panel. (B) Reactivity of serum from a rabbit immunized with VACV. Western blotting was performed as in panel A except that the membrane was incubated with antiserum from a rabbit that had been immunized with live VACV (Wyatt et al., 2008). The blot was developed with chemiluminescence. The proteins encoded by the plasmids are indicated above each lane. EV refers to empty vector. (C) Antibody responses of mice following DNA immunizations. Pooled mouse sera (n=10) obtained two weeks following the 3rd immunization of A28, H2, A21, G3, G9 or J5 genes were used to probe Western blots containing the corresponding protein from transfected BS-C-1 cell lysates using infrared fluorescence detection (LI-COR Biosciences, Lincoln, NE).
Immunization of mice with individual plasmids expressing EFC proteins
Gold beads were coated with plasmids and then injected with a gene gun into seven-week BALB/c mice. After three immunizations at intervals of three weeks, the mice were bled and the pooled sera tested by probing Western blots prepared by SDS-polyacrylamide gel electrophoresis (PAGE) of lysates of transfected cells. Western blotting revealed antibodies to A21, A28, G3, G9, H2 and J5 (Fig. 1C); the A16 band was faint and L5 was not detected (not shown). This experiment confirmed the expression of G9 and J5 by the plasmid expression vectors. In addition, a low level of antibody to each of the proteins except A21 was detected by enzyme-linked immunosorbent assay (ELISA) using purified VACV as the antigen (data not shown).
Neutralizing activity was determined by a flow cytometry assay that measures the ability of serum to prevent infection of cells by a recombinant VACV encoding enhanced green fluorescent protein (GFP) (Earl et al., 2003). Sera from mice immunized with A28 exhibited low neutralization with the half maximal inhibitory serum concentration (IC50) of 82, whereas the IC50 of the other sera were <50, the cut-off point for this assay. As a positive control, the sera from mice that were immunized in parallel with gold beads coated with a plasmid that expressed L1 had an IC50 of 868.
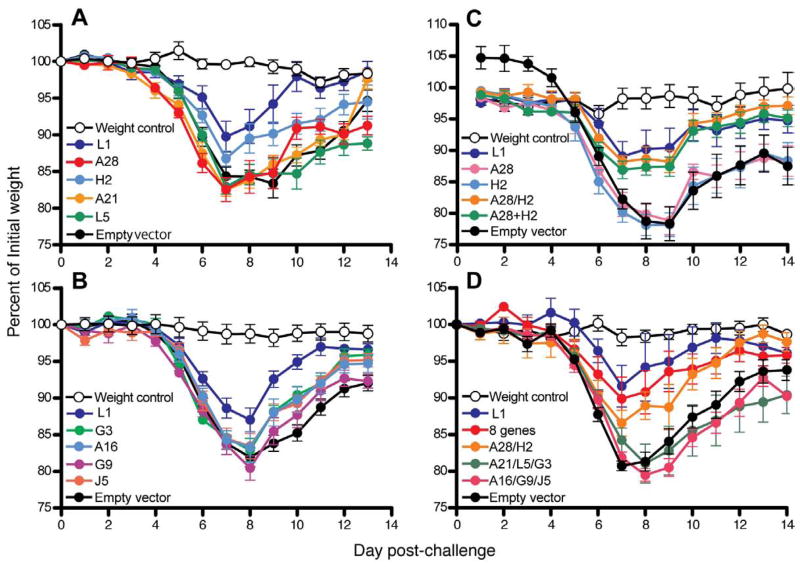
At three weeks after the last gene gun immunization, the mice were challenged with 104 plaque-forming units (PFU) of VACV strain Western Reserve by the intranasal route and weighed daily to assess disease. None of the plasmids expressing individual EFC proteins provided significant protection (Fig. 2A, B). Partial protection was provided by the plasmid expressing L1 as previously described (Shinoda et al., 2009).
Fig. 2.
Partial protection of immunized mice. (A, B) Groups of 10 mice were immunized 3 times at 3-week intervals by gene gun delivery with gold beads containing 1 μg of the indicated plasmid. At three weeks after the last immunization, mice were challenged intranasally with 104 PFU of VACV and weighed daily. The percent of the initial weights from a representative experiment are plotted and error bars are shown. The weight control mice were not immunized or challenged. (C, D) The protocol was similar to that of the above panels except that the gold beads contained individual or combinations of plasmids (1 μg each except for 0.5 μg in 8 gene combination in 2D). Slashes indicate that plasmids were mixed prior to binding to gold beads; a plus signifies that plasmids were individually bound to gold beads and the beads were mixed prior to injection. The mice (n = 5 to 10) were vaccinated four times at two-week intervals and challenged with 2 × 104 PFU of VACV and weighed daily.
Combination of A28 and H2 provided partial protection against weight loss after VACV challenge
Since the results obtained with the individual genes indicated low or no neutralizing activity, we tried gene combinations. Plasmids expressing A28 and H2 were chosen first because these two proteins are known to interact directly (Nelson et al., 2008b). The plasmids expressing A28 and H2 were either mixed prior to coating the beads (A28/H2) or the beads were coated separately and then mixed (A28+H2). In the experiment depicted in Fig. 2C, separate groups of animals were immunized four times at intervals of two weeks with beads containing A28/H2, A28+H2, A28 alone, H2 alone, L1 as a positive control, or empty plasmid vector. As above, the mice were challenged intranasally with 104 PFU of VACV and weighed daily. Mice immunized with either A28/H2 or A28+H2 exhibited significantly less weight loss at days 8 and 9 (p ≤0.032) than mice immunized with A28 or H2 alone or empty vector (Fig. 2C). The protection achieved with the A28 and H2 plasmid combinations were similar to that resulting from immunization with the L1 plasmid.
Encouraged by results with A28 and H2, we tried additional combinations. The eight EFC proteins can be divided into two subsets: A21, A28, G3, H2 and L5 have N-terminal transmembrane domains and A16, G9 and J5 are sequence related and have C-terminal transmembrane domains (Senkevich et al., 2005). In addition, A16 and G9 were found to interact with each other (Wagenaar et al., 2008). Based on these criteria, we made six plasmid immunization groups: (i) A28/H2, (ii) A21/L5/G3, (iii) A16/G9/J5, (iv) all eight EFC genes, (v) L1, and (vi) empty vector. Mice were immunized four times at three-week intervals and challenged three weeks later. The mice receiving A28/H2, the eight EFC genes or L1 were better protected than those receiving A21/G3/L5, A16/G9/J5 or the empty vector (Fig. 2D). At day 8, the A28/H2 group and eight EFC gene group lost significantly less weight than the mice immunized with the empty vector (p ≤0.02 and p ≤0.032, respectively) or A16/G9/J5 (p ≤0.008 for both). Thus, partial protection correlated with the administration of the genes encoding both A28 and H2.
Analysis of antibodies induced by A28 and H2 immunizations
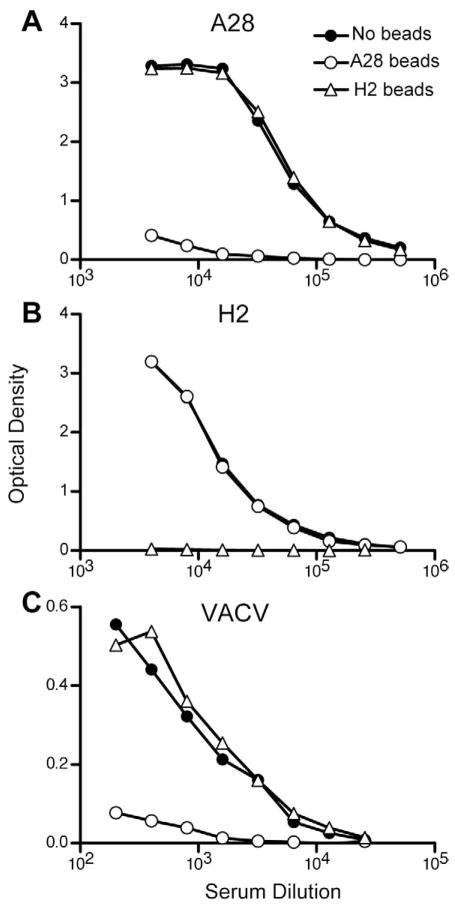
Sera from immunized mice were examined for the presence of binding and neutralizing antibodies. When tested for binding to the A28 protein by enzyme-linked immunosorbent assay (ELISA), sera from the single A28 and combination (A28/H2 and A28+H2) groups had equivalent titers (Fig. 3A). Similarly, when tested against H2 protein, each of the H2 immunization groups had equivalent titers (Fig. 3A). A striking difference was noted, however, when the sera were tested in an ELISA against intact VACV virions. The virus ELISA titers were 6- to 10-fold higher when the mice were immunized with plasmids encoding A28/H2 or A28+H2 compared to either plasmid alone (Fig. 3A). Moreover, the groups immunized with A28/H2 or A28+H2 induced much higher neutralizing antibody than mice immunized with either plasmid alone (Fig. 3B), indicating a direct correlation between the ability of the antibody to bind VACV and neutralize infectivity. In contrast, mixing sera from mice immunized separately with A28 and H2 did not increase the neutralizing activity (Fig. 3B) and mice immunized with A28 and H2 at separate sites raised little neutralizing antibody (Fig. 3C). Furthermore, sera from mice immunized with plasmids expressing A21/L5/G3 and A16/G9/J5 had no measurable neutralizing activity (not shown).
Fig. 3.
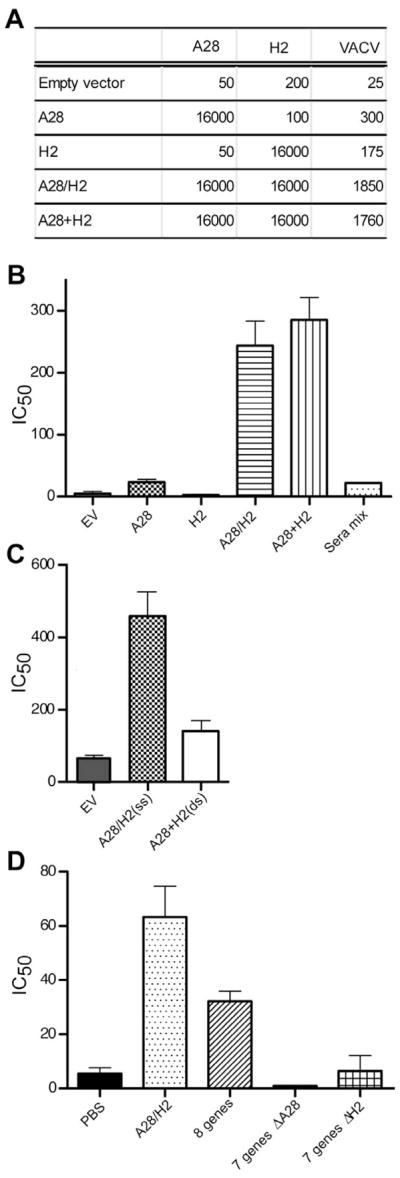
Induction of binding and neutralizing antibodies. (A) ELISA assays. Sera obtained after the fourth DNA immunization (corresponding to Fig. 2C) were pooled and assayed by ELISA. The DNA used for immunization is indicated on the left and the immobilized protein (A28 or H2) or intact virions (VACV) on the top. A28/H2 refers to plasmids attached to the same beads and A28 + H2 refers to mixtures of beads containing A28 and beads containing H2. (B) Neutralization titers after same-site injections. The sera used in panel A were tested by flow cytometry for ability to neutralize recombinant VACV expressing GFP. Sera mix refers to mixing equal volumes of sera from mice immunized with A28 DNA alone and with H2 alone. The data for all samples except the sera mix are the averages from 4 independent assays, each done in duplicate. Error bars are shown. The sera mix was done once in duplicate. (C) Comparison of neutralization titers after same and different site immunizations. Immunizations were as described in Fig. 2C except that some received A28 and H2 in the same site (ss) and others in different abdominal sites (ds). The assay was performed twice each time in duplicate and averages with error bars are shown. (D) Neutralization titers after multi-gene immunizations. Mice were immunized with 0.5 μg of each A28/H2, mixture of all eight plasmids, or seven gene combinations that excluded A28 or H2. Averages of three assays, one which was done in duplicate are shown with error bars.
In order to evaluate whether the combination of A28 and H2 plasmids was uniquely able to induce neutralizing antibody, mice were immunized with A28/H2, all eight plasmids or with seven plasmid mixtures that lacked A28 or H2. The amount of each individual plasmid was 0.5 μg, regardless of the total number of plasmids administered. Only the sera from mice that received both A28 and H2 had much neutralizing activity (Fig. 3D). None of the other EFC proteins was able to substitute for A28 or H2. The neutralization titer obtained with all eight plasmids was less than with A28/H2 alone. Furthermore, immunization with seven plasmids lacking either A28 or H2 did not induce neutralizing antibody. The titer with A28/H2 was lower than in the previous experiments, probably because half the amount of DNA was used for immunizations.
Determination of the target of VACV binding and neutralizing antibodies
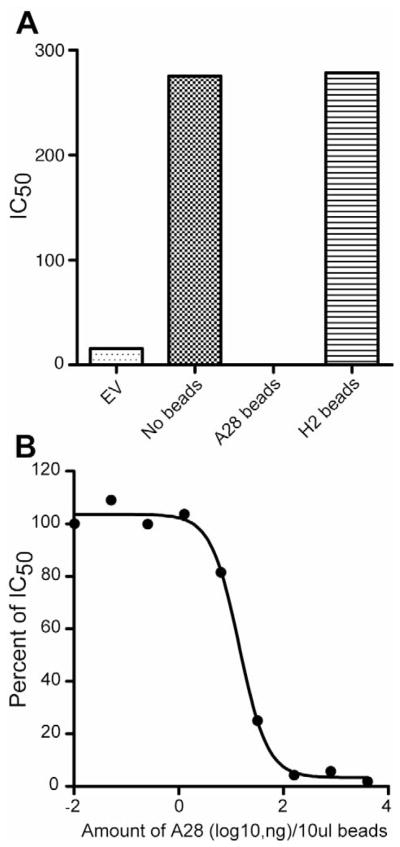
We considered two general explanations for the synergistic effects of A28 and H2 on the induction of virion binding and neutralizing antibodies. One possibility was that the targeted epitope comprises part of the A28-H2 interaction site and is not present in either protein alone. Another is that one of the two proteins stabilizes an immunogenic conformation of the other. Protein adsorption experiments were carried out in an attempt to differentiate between these two models. Pooled serum from mice immunized with A28/H2-coated gold beads was adsorbed with purified A28 or H2 protein coated magnetic beads and the supernatant fractions were analyzed using immobilized A28 and H2 proteins. The A28- and H2-coated beads removed the corresponding antibodies without depleting the non-corresponding ones (Fig. 4A,B). However, the A28-depleted sera failed to bind to immobilized VACV, whereas the H2-depleted sera lost no reactivity (Fig. 4C). In addition, the A28-depleted sera lost all VACV neutralizing activity, which was retained by the H2-depleted sera (Fig. 5A). The adsorption of neutralizing activity was proportionate to the concentration of A28 (Fig. 5B). Non-specific adsorption of VACV binding and neutralizing activity by the A28-coated beads was ruled out by using L1 antiserum generated in a similar manner (data not shown).
Fig 4.
Adsorption of protein and virion-binding antibodies. Samples of pooled sera, following the fourth A28/H2 DNA immunization, were incubated with A28 or H2 protein coated magnetic beads and the depleted sera were analyzed in duplicate by ELISA to immobilized A28 protein (A), H2 protein (B) or intact VACV virions (C).
Fig. 5.
Adsorption of VACV neutralizing antibody. (A) Samples of pooled serum following the fourth A28/H2 DNA immunization were incubated with A28 or H2 coated magnetic beads and the depleted sera tested for ability to neutralize VACV. Averages of duplicate determinations are shown. EV, refers to empty vector. (B) Magnetic beads were coated with five-fold serial dilutions of A28 protein, incubated with pooled A28/H2 sera, and the depleted sera tested for VACV neutralization as in panel A. The percentage of neutralizing activity remaining after adsorption is plotted. Averages of duplicate determinations are shown.
The VACV neutralization epitope resides in the C-terminal region of the A28 protein
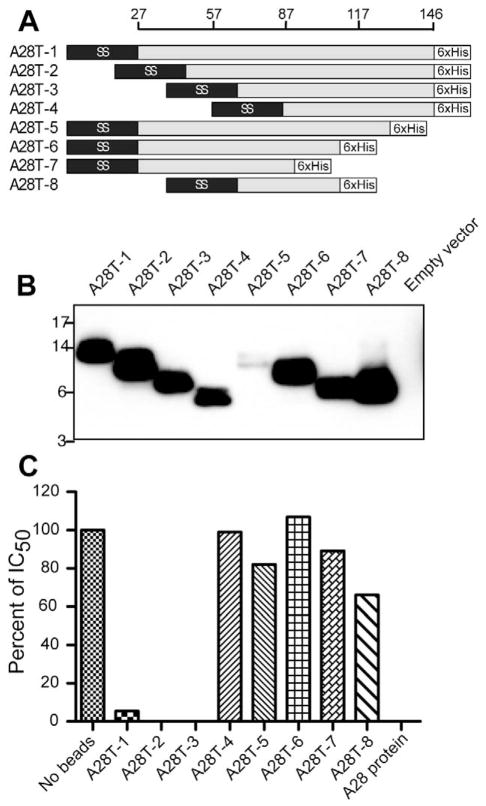
To determine the location of the VACV neutralizing epitope on the A28 protein, plasmids encoding N-terminal and C-terminal truncations were made. In each construct a signal peptide replaced the 26-amino acid transmembrane domain and a six-histidine tag was present at the C-terminus (Fig. 6A). HeLa cells were transfected with individual plasmids. Because the truncated proteins were not efficiently secreted, the cell lysates were analyzed by SDS-PAGE and Western blotting with antibody to the histidine tag (Fig. 6B). The mutant proteins were expressed to similar levels except for the low yields of A28T-4 and A28T-5. The proteins were bound to magnetic affinity beads, which were then incubated with sera from mice that had been immunized with the A28/H2 plasmid. The N-terminal truncated proteins A28T-1, A28T-2, and A28T-3 depleted the serum of neutralizing antibody. In contrast, none of the proteins with C-terminal truncations (A28T-5, A28T-6, A28-T7, or A28T-8) depleted the serum of neutralizing antibody (Fig. 6C). The inability of A28T-5 and possibly A28T-4 to remove neutralizing antibody might have been due to their lower expression and recovery after affinity purification than the other proteins.
Fig. 6.
Localization of VACV neutralizing epitope on the A28 protein. (A) Diagram of A28 truncations. Representation of A28 protein with amino acid numbers is shown at the top. A28T-1 shows the A28 protein with replacement of the 26-amino acid N-terminal transmembrane domain by the Ig μ-chain signal peptide sequence (SS) and addition of a six-histidine (6xHis) tag at the C-terminus. A28T-1 to A28T-4 constructs contain serial N-terminal truncations but retain the signal peptide and six-histidine tag. A28T-5 to A28-T7 constructs have serial C-terminal truncations and A28T-8 has both N- and C-terminal truncations. (B) Western blot. Plasmids encoding truncated proteins were transfected into HeLa cells and the lysates were analyzed by Western blotting with antibody to the histidine tag. (C) The A28 proteins diagrammed in panel A were expressed by transfection and bound to beads via the six-histidine tag. The beads were incubated with A28/H2 sera and the depleted sera were tested for neutralizing activity. A representative of three assays is shown.
Discussion
Identification of the targets of neutralizing antibody is important for understanding the mechanism of immunity to poxviruses and developing safer poxvirus vaccines. In view of the complexity of VACV and the large number of proteins associated with the MV membrane, it is not surprising that multiple targets of neutralizing antibody have been found. The latter include proteins involved in virus attachment to cells e.g. A27 (Rodriguez and Esteban, 1987), H3 (Lin et al., 2000) and D8 (Hsiao et al., 1999) and two proteins involved in entry and membrane fusion, namely L1 (Wolffe et al., 1995) and A28 (Nelson et al., 2008a). Attempts have been made to determine the dominant neutralizing antibody in sera from smallpox vaccine recipients. However, modest or no reduction in overall neutralizing titers were obtained by depletion of antibodies to H3, L1 and A27 (Benhnia et al., 2008; He et al., 2007; Putz et al., 2006). These results can be interpreted in several ways: (i) there are low amounts of neutralizing antibody to many different targets; (ii) the major neutralizing target protein has not yet been identified, or (iii) the dominant neutralizing antibody recognizes a complex on the virion surface.
We were interested in determining whether the proteins involved in virus entry and membrane fusion are immunogenic and targets of neutralizing antibody. Antibodies from humans, macaques and mice that were immunized with VACV and screened against a complete array of VACV proteins made in Escherichia coli detected L1 and occasionally traces of EFC proteins A21, A28, H2 and L5 (Davies et al., 2005a; Davies et al., 2008). For the present study we cloned genes encoding eight of the nine known EFC proteins and L1 into eukaryotic expression plasmids. Using transfected cell lysates for Western blotting, we detected antibodies to A28, H2, J5 and L1 in sera of rabbits that had been infected with VACV. The present and previous studies indicated that antibodies are made to at least some of the EFC proteins.
Gene gun immunizations were used to determine whether individual components of the EFC or combinations of EFC proteins induce neutralizing antibodies in mice. The EFC genes were codon optimized, Asn-X-Ser/Thr sequences altered to prevent unnatural glycosylation and the transmembrane domains retained. Although most individual plasmids induced antibody that bound to the homologous protein, only DNA encoding A28 induced a low level of neutralizing antibody. However, neutralizing antibody was greatly increased when plasmids encoding A28 and H2 were administered together. No other combination of EFC genes, including a mixture of seven genes lacking either A28 or H2, induced neutralizing antibody. It is possible that the majority of EFC proteins may not be targets of neutralizing antibody because of their arrangement in the MV membrane. Alternatively, many of the proteins expressed by DNA immunization may be improperly folded. Therefore, our most significant findings relate to the induction of neutralizing antibody to A28 and particularly its enhancement by H2, rather than the negative findings regarding the other EFC proteins.
The sera from mice that were immunized with DNA expressing A28 alone or H2 alone contained equivalent amounts of protein-binding antibody as sera from mice that were immunized with DNA expressing both proteins. However, the sera from the combined immunization contained much higher amounts of antibodies that bound to intact virions as determined by an ELISA and neutralized VACV infectivity. The synergistic effect of H2 and A28 on the induction of neutralizing rather than protein binding antibody could have several explanations. The possibility that antibodies to each of the proteins acted synergistically was ruled out by serum mixing experiments. A requirement for a direct association of A28 and H2 proteins was consistent with the enhanced induction of neutralizing antibody when the two DNAs were on the same beads or on separate beads that were injected at the same site and the lack of enhancement when they were delivered to different sites on the mouse. These results can be best explained by the association of A28 and H2, which has been demonstrated to occur when these two proteins are expressed by DNA transfection of uninfected cells (Nelson et al., 2008b). With this explanation in mind, we considered the possibility that the neutralizing epitope is at the A28-H2 interface and hence not present in either A28 or H2 alone or that the epitope is located on H2 or A28 but that the immunogenic conformation is stabilized by the protein-protein interaction. To investigate these possibilities, A28 and H2 were bound separately to beads, which were used to adsorb antibodies from sera of mice that were immunized with both A28 and H2 DNA. The A28 and H2 beads efficiently depleted the antibody that bound to A28 and the H2, respectively. However, only the A28 beads depleted the neutralizing antibody, consistent with the second hypothesis.
Our data indicate that the C-terminal region of A28 either contains the neutralizing epitope elicited by immunization with A28 and H2 or is important for the correct folding of a conformational epitope. In a previous study a synthetic A28 peptide representing amino acids 73–92 was able to bind neutralizing antibody obtained by repeatedly immunizing a rabbit with a soluble form of A28 in adjuvant. We were unable to capture the neutralizing antibody prepared by DNA immunizations with a panel of peptides attached to plastic, including the one above (K.S., unpublished). However, truncated A28 proteins missing the N-terminal 26, 46 or 66 amino acids but not 86 amino acids still depleted the A28/H2 neutralizing antibody, consistent with an epitope including amino acids amino acids 73–92. Further studies are needed to determine whether the targets of neutralizing antibody elicited by soluble A28 protein and by membrane-associated A28/H2 are identical.
The present study was directed to the discovery of targets of neutralizing antibody and no efforts were made to determine whether the modest protection of BALB/c mice to a non-lethal VACV challenge correlated with T cell immunity in addition to neutralizing antibody. Passive transfer experiments were not attempted because of the low neutralizing antibody titers, limitations in quantity of mouse sera and weak protection achieved. In a very recent study, cowpox genetic sequences derived from the whole genome were screened for their ability to protect against a cowpox virus infection of C57Bl/6 mice by DNA immunization (Borovkov et al., 2009). Nine immunogens scored positively and these included non-membrane as well as membrane components. Each of the nine was shown to induce T cell responses and protein binding antibody but virus neutralizing activity was not analyzed. Curiously, no homologs of the VACV protective immunogens, which had been tested in BALB/c mice, were among the nine new cowpox protective immunogens, which included two EFC proteins, namely G9 and J5. Since cowpox virus and VACV are closely related, it is possible that the different results relate to the Th1 and Th2 biases of C57Bl/6 and BALB/c mice respectively (Locksley et al., 1987; Mosmann and Coffman, 1989).
In conclusion, our data confirm a previous report (Nelson et al., 2008b) that A28 is a target of neutralizing antibody and demonstrate by DNA immunizations that co-expression of H2 greatly increases the amount of A28 virion binding and neutralizing antibody. A likely explanation is that the known interaction of H2 with A28 stabilizes an immunogenic conformation of A28. Presumably in solution or attached to beads through an epitope tag, A28 is flexible enough to adopt many different conformations including ones that interact with the antibody, providing an explanation for the difference between immunogenicity and antigenicity.
Materials and Methods
Cell cultures and viruses
BS-C-1 cells (ATCC CCL-26) and monolayer and suspension cultures of HeLa S3 (ATCC CCL-2.2) were grown using standard procedures. VACV strain Western Reserve (ATTC VR1354) and VACV expressing GFP (Earl et al., 2003) were propagated as described (Earl et al., 1998; Earl and Moss, 1998).
Construction of plasmids
The A28, H2, A21, L5, G3, A16, G9, and J5 open reading frame sequences of VACV strain Western Reserve were modified by mammalian codon optimization, removal of potential glycosylation sites, and inserted into pVRC8400 (Barouch et al., 2005) by GENEART (Regenburg, Germany). The plasmid expressing L1, used as a control, was previously described (Shinoda et al., 2009). Plasmids expressing truncations of the A28 gene sequence were constructed as follows: A28T-1, A28T-2, A28T-3 and A28T-4 were truncated 26, 46, 66, or 86 amino acids respectively from the N terminus of A28; A28T-5, A28T-6, and A28T-7 were truncated 20, 40 or 60 amino acids respectively from C terminus of A28; A28T-8 was truncated 66 and 40 amino acid from the N and C termini of A28. All genes encoded a murine Ig κ-chain signal sequence (Coloma et al., 1992) at the N-terminal end and a six-histidine tag at the C-terminus assembled by PCR amplification with terminal PstI and NotI sites, and inserted into the corresponding sites of pVRC8400.
Protein and antibodies
Secreted A28 or H2 protein, in which the honeybee melittin signal peptide was substituted for the transmembrane domain at the N-terminus and a six-histidine tag was at the C terminus, was expressed by recombinant baculovirus in insect cells and purified on Ni-NTA agarose (Qiagen, Valencia, CA) as previously described (Nelson et al., 2008a). Anti-A28 and H2 polyclonal antibodies were obtained by immunizing rabbits with soluble A28 or H2 protein (Nelson et al., 2008a). Antibodies to A21 (Townsley et al., 2005b), L5 (Townsley et al., 2005a), A16 (Ojeda et al., 2006b), and G3 were produced in rabbits with peptides from the corresponding proteins. Tetra-His mouse antibody (Qiagen) was used for Western blotting of proteins expressed with six-histidine tags.
Western blotting
BS-C-1 or HeLa cells were transfected with 1.5 μg of plasmid in 10 μl of Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) per well of a 6-well plate. After 24 h, the cells were suspended with NuPAGE® Lithium Dodecyl Sulfate Sample Buffer (Invitrogen), sonicated, and incubated with NuPAGE® Sample Reducing Agent (Invitrogen). The proteins were resolved by SDS-PAGE, and transferred to a polyvinylidene difluoride or nitrocellulose membrane using iBlot (Invitrogen). The membrane was incubated with antibody to a specific protein or pooled mouse sera (n=10) following single gene gun immunization and then anti-rabbit or mouse IgG conjugated to horseradish peroxidase for chemiluminescence detection (Pierce, Rockford, IL) or infrared-labeled donkey IgG anti-mouse for infrared imaging (Odyssey, LI-COR, Lincoln, NE).
Gene gun immunization of mice
Individual cartridges were prepared with approximately 0.5 μg or 1 μg of each plasmid with 0.5 mg of gold particles. Plasmid and 2 micron gold particles (DeGussa, Parsippany, NY) were mixed and dried onto Tefzel tubing (BIO-RAD) according to manufacturer’s directions. Seven weeks old female BALB/c mice were injected with DNA-coated gold particles to three non-overlapping sites on the shaved abdomen using a Helios gene gun (BIO-RAD, Hercules, CA) as previously described (Shinoda et al., 2009). There were a total of 14 gene gun immunization/challenge experiments. The number of immunizations varied from three at 3-week intervals to four at 2-week intervals. The challenges were intranasal with 104, 2 × 104 or 105 PFU from 2 to 6 weeks after the last immunization. Routinely, there were 5 animals per group but in one experiment there were 10. Each combination of immunogens was tested at least twice and most several times. The figures show single representative experiments unless otherwise stated.
Antibody binding assay
Antibody binding to purified A28 or H2 protein and intact VACV particles was carried out by ELISA (Nelson et al., 2008a) with some modifications. For protein ELISA, individual wells of a 96-well plate (Immulon HB plate, Thermolab System, Hertfordshire, UK) were coated with 60 ng of purified A28 or H2 protein in 100 μl of Universal Plate Coating Buffer (Immunochemistry Technologies, Bloomington, MN) and incubated ~24 h at 4 °C. For VACV particle ELISA, individual wells of a 96-well plate were coated with 106 PFU of VACV in 100 μl of Coating Buffer. Plates were fixed with 100 μl/well of 4% paraformaldehyde in phosphate buffered saline at room temperature. Following incubation with diluted serum followed by anti-mouse IgG-peroxidase (Roche, Branchburg, NJ), the plates were incubated with BM Blue substrate (Roche) and read at 370 nm and 492 nm using SpectraMax M5 Microplate Reader and SoftmaxPro Software System (Molecular Devices, Sunnyvale, CA). The endpoint was 0.1 absorbance unit after subtraction of the background absorbance of serum alone.
Neutralization assay
Purified VACV expressing GFP was used for the neutralization assay as described (Earl et al., 2003). The virus was incubated with serum for 1 h, followed by the addition of cytosine arabinoside treated HeLa S3 cells to the plates. The plates were incubated for 16–18 h at 37°C, fixed with 2% paraformaldehyde in phosphate buffered saline and analyzed on a FACSCalibur flow cytometer using CellQuest and FlowJo Software. IC50 values were calculated using PRISM software (GraphPad Software, Inc, La Jolla, CA,)
Adsorption of specific antibodies from serum
For adsorption of A28/H2 sera, 10 μl (0.4 mg) of TALON Dynabeads (Invitrogen) were incubated for 4 h at 4°C with 6 mg of purified histidine-tagged A28 or H2 protein. The washed beads were incubated overnight with 35 μl of 1:10 diluted A28/H2 serum in phosphate buffered saline at 4°C. The beads were removed from the sera with a magnet. The protein-adsorbed sera were used for antibody binding and neutralization assays. For adsorption of A28/H2 sera with different amounts of A28 protein, 0.01 to 4,000 ng of the latter were incubated with magnetic beads. The beads were incubated with A28/H2 sera as above.
Adsorption of sera with truncated A28 proteins
HeLa cells (4 wells of a 6-well plate) were transfected with plasmids expressing truncated proteins and Lipofectamine as described above. After 24 h, the plates were washed and each set of 4 wells were lysed with 600 ml of ice-cold lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1.0 % NP40, and protease inhibitor cocktail, EDTA-free (Roche). Samples were sonicated for 30 sec three times and centrifuged at 14,000 rpm in a Sorvall Legend RT+ centrifuge (Thermo Scientific, Waltham, MA) for 30 min at 4°C. Supernatants were used for Western blotting (10 μl of total lysate). For adsorption studies with A28/H2 mouse sera, 240 μl of total lysate was incubated with 10 ml of TALON Dynabeads, incubated as described above with mouse sera.
Statistical analysis
Weight-loss differences following VACV intranasal challenge of immunized mice were assessed by Mann-Whitney using PRISM software (GraphPad Software, Inc).
Acknowledgments
The excellent assistance of Norman Cooper and Catherine Cotter in preparation of cells and purification of VACV are greatly appreciated. We thank Gary Nabel of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases for providing the VRC8400 plasmid, and Wolfgang Leitner/Dermatology Branch, National Cancer Institute for the gold particles used in the gene gun experiments. The studies were supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, Kishko MG, Arthur JC, Miura A, Mascola JR, Letvin NL, Nabel GJ. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79:8828–8834. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia MR, McCausland MM, Su HP, Singh K, Hoffmann J, Davies DH, Felgner PL, Head S, Sette A, Garboczi DN, Crotty S. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J Virol. 2008;82:3751–3768. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H, Weisberg AS, Moss B. Vaccinia virus L1 protein is required for cell entry and membrane fusion. J Virol. 2008;82:8687–8694. doi: 10.1128/JVI.00852-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovkov A, Magee DM, Loskutov A, Cano JA, Selinsky C, Zsemlye J, Lyons CR, Sykes K. New classes of orthopoxvirus vaccine candidates by functionally screening a synthetic library for protective antigens. Virology. 2009;395:97–113. doi: 10.1016/j.virol.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, Senkevich TG, Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related l1 protein. J Virol. 2006;80:9455–9464. doi: 10.1128/JVI.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WL, Lin CL, Yang MH, Tzou DLM, Chang W. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J Virol. 2007;81:2149–2157. doi: 10.1128/JVI.02302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, Hsiao JC, Chang YS, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparin sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, Chen CH, Ho MY, Huang CY, Liao CL, Chang W. Vaccinia virus proteome: Identification of proteins in vaccinia virus intracellular mature virion particles. J Virol. 2006;80:2127–2140. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloma MJ, Hastings A, Wims LA, Morrison SL. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Meth. 1992;152:89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Damon I. Poxviruses. In: Knipe DM, Howley PM, editors. Field’s Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2947–2976. [Google Scholar]
- Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci USA. 2005a;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu YX, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005b;79:11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, Wyatt LS, Newman FK, Earl PL, Chun S, Hernandez JE, Molina DM, Hirst S, Moss B, Frey SE, Felgner PL. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax. J Virol. 2008;82:652–663. doi: 10.1128/JVI.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J Virol. 2003;77:10684–10688. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 2. John Wiley and Sons; New York: 1998. pp. 16.16.1–16.16.3. [Google Scholar]
- Earl PL, Moss B. Characterization of recombinant vaccinia viruses and their products. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 2. Greene Publishing Associates & Wiley Interscience; New York: 1998. pp. 16.18.1–16.18.11. [Google Scholar]
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. 1. World Health Organization; Geneva: 1988. [Google Scholar]
- Fogg C, Lustig S, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78:10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254:71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nature Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- He Y, Manischewitz J, Meseda CA, Merchlinsky M, Vassell RA, Sirota L, Berkower I, Golding H, Weiss CD. Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine--induced immunity. J Infect Dis. 2007;196:1026–1032. doi: 10.1086/520936. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306:181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J Virol. 1999;73:8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y, Dales S. Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology. 1971;46:533–543. doi: 10.1016/0042-6822(71)90057-2. [DOI] [PubMed] [Google Scholar]
- Izmailyan RA, Huang CY, Mohammad S, Isaacs SN, Chang W. The envelope G3L protein is essential for entry of vaccinia virus into host cells. J Virol. 2006;80:8402–8410. doi: 10.1128/JVI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RB, Ovsvannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009;21:314–320. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CF, Gong SC, Esteban M. The purified 14-kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J Virol. 1991;65:5631–5635. doi: 10.1128/jvi.65.10.5631-5635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KM, Smith GL. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J Gen Virol. 1992;73:549–557. doi: 10.1099/0022-1317-73-3-549. [DOI] [PubMed] [Google Scholar]
- Law M, Carter GC, Roberts KL, Hollinshead M, Smith GL. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc Natl Acad Sci USA. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Chung CS, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol. 2000;74:3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987;138:744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79:13454–13462. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2905–2946. [Google Scholar]
- Nelson GE, Sisler JR, Chandran D, Moss B. Vaccinia virus entry/fusion complex subunit A28 is a target of neutralizing and protective antibodies. Virology. 2008a;380:394–401. doi: 10.1016/j.virol.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GE, Wagenaar TR, Moss B. A conserved sequence within the H2 subunit of the vaccinia virus entry/fusion complex is Important for interaction with the A28 subunit and infectivity. J Virol. 2008b;82:6244–6250. doi: 10.1128/JVI.00434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RJ, Stanitsa E, Unger B, Traktman P. The vaccinia I2L gene encodes a membrane protein with an essential role in virion entry. J Virol. 2008;82:10247–10261. doi: 10.1128/JVI.01035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S, Domi A, Moss B. Vaccinia virus G9 protein is an essential component of the poxvirus entry-fusion complex. J Virol. 2006a;80:9822–9830. doi: 10.1128/JVI.00987-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S, Senkevich TG, Moss B. Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J Virol. 2006b;80:51–61. doi: 10.1128/JVI.80.1.51-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan V, Chaudhri G, Karupiah G. Correlates of protective immunity in poxvirus infection: where does antibody stand? Immunol Cell Biol. 2008;86:80–86. doi: 10.1038/sj.icb.7100118. [DOI] [PubMed] [Google Scholar]
- Putz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL. Prevalence of antibodies to vaccinia virus after smallpox vaccination In Italy. J Gen Virol. 2005;86:2955–2960. doi: 10.1099/vir.0.81265-0. [DOI] [PubMed] [Google Scholar]
- Putz MM, Midgley CM, Law M, Smith GL. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nature Med. 2006;12:1310–1315. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. Protein composition of the vaccinia virus mature virion. Virology. 2007;358:233–247. doi: 10.1016/j.virol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Rodriguez JF, Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987;61:3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satheshkumar PS, Moss B. Characterization of a newly Identified 35 amino acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses. J Virol. 2009;83:12822–12832. doi: 10.1128/JVI.01744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Moss B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J Virol. 2005;79:4744–4754. doi: 10.1128/JVI.79.8.4744-4754.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B. Poxvirus multiprotein entry-fusion complex. Proc Natl Acad Sci USA. 2005;102:18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ward BM, Moss B. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J Virol. 2004;78:2357–2366. doi: 10.1128/JVI.78.5.2357-2366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Wyatt LS, Irvine KR, Moss B. Engineering the vaccinia virus L1 protein for increased neutralizing antibody response after DNA immunization. Virol J. 2009;6:28. doi: 10.1186/1743-422X-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Law M. The exit of vaccinia virus from infected cells. Virus Res. 2004;106:189–197. doi: 10.1016/j.virusres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, Muller D, Moss B, Ferrucci L, Duffey PL, Longo DL. Immunity from Smallpox Vaccine Persists for Decades: A Longitudinal Study. Amer J Med. 2008;121:1058–1064. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley A, Senkevich TG, Moss B. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is requried for cell entry and cell-cell fusion. J Virol. 2005a;79:10988–10998. doi: 10.1128/JVI.79.17.10988-10998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley A, Senkevich TG, Moss B. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J Virol. 2005b;79:9458–9469. doi: 10.1128/JVI.79.15.9458-9469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Moyer RW. An orthopoxvirus serpin-like gene controls the ability of infected cells to fuse. J Virol. 1992;66:2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PC, Moyer RW. The vaccinia virus fusion inhibitor proteins SPI-3 (K2) and HA (A56) expressed by infected cells reduce the entry of superinfecting virus. Virology. 2008;380:226–233. doi: 10.1016/j.virol.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 2005;7:579–583. doi: 10.1016/j.micinf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wagenaar TR, Moss B. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J Virol. 2007;81:6286–6293. doi: 10.1128/JVI.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar TR, Moss B. Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J Virol. 2009;83:1546–1554. doi: 10.1128/JVI.01684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar TR, Ojeda S, Moss B. Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex. J Virol. 2008;82:5153–5160. doi: 10.1128/JVI.00162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe EJ, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211:53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]
- Wyatt LS, Belyakov IM, Earl PL, Berzofsky JA, Moss B. Enhanced cell surface expression, immunogenicity and genetic stability resulting from a spontaneous truncation of HIV Env expressed by a recombinant MVA. Virology. 2008;372:260–272. doi: 10.1016/j.virol.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JD, Chen TS, Gagnier CR, Vemulapalli S, Maier CS, Hruby DE. Pox proteomics: mass spectrometry analysis and identification of Vaccinia virion proteins. Virol J. 2006;3:10. doi: 10.1186/1743-422X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Sun XY, Fernando GJP, Frazer IH. The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell-cell fusion. Virology. 1992;189:678–686. doi: 10.1016/0042-6822(92)90591-c. [DOI] [PubMed] [Google Scholar]