Abstract
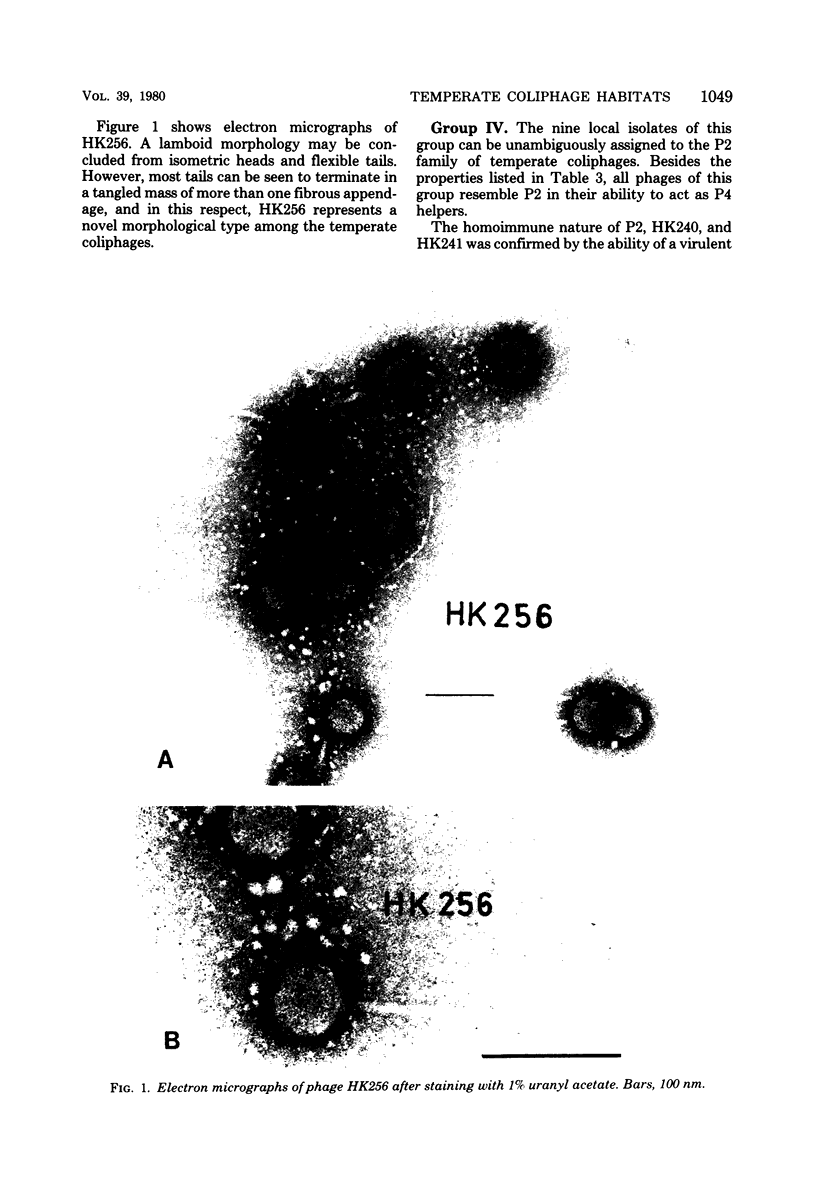
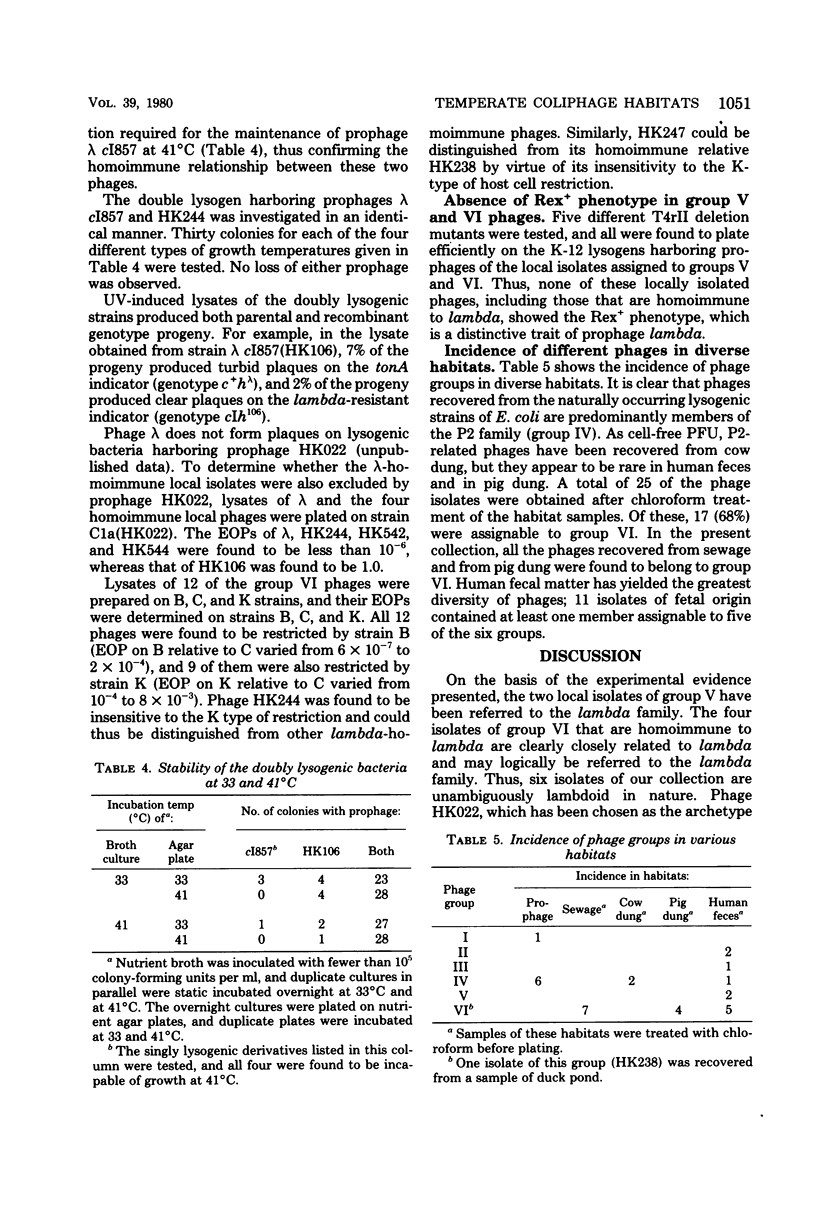
Temperate coliphages were recovered from sewage, mammalian feces, and lysogenic strains of Escherichia coli. A total of 32 phages of independent origin were divided into six groups by applying the criteria of host range, antigenic homology, and the ultraviolet inducibility of the prophage. The demonstration of genetic interactions in some cases has confirmed the classification scheme. Nine phages were assigned to the P2 family and 19 to the lambda family. The remaining four isolates may represent some novel phylogenetic types. Phages recovered from the lysogenic strains of E. coli were all found to be P2 related, whereas a majority of the phages recovered as cell-free plaque-forming units were assignable to the lambda family. It is proposed that the biological attributes of the phages belonging to the two principal families are reflected in the distribution patterns observed. The virions of phage HK256 show multiple tail fibers and may thus represent a "new" virion form among the temperate coliphages.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S., Matsushiro A. Studies on temperature sensitive growth of phage phi 80. I. Prophage excision. Virology. 1975 Sep;67(1):168–178. doi: 10.1016/0042-6822(75)90414-6. [DOI] [PubMed] [Google Scholar]
- Bertani L. E. Abortive induction of bacteriophage P2. Virology. 1968 Sep;36(1):87–103. doi: 10.1016/0042-6822(68)90119-0. [DOI] [PubMed] [Google Scholar]
- Bertani L. E., Bertani G. Genetics of P2 and related phages. Adv Genet. 1971;16:199–237. doi: 10.1016/s0065-2660(08)60359-4. [DOI] [PubMed] [Google Scholar]
- Dhillon E. K., Dhillon T. S. HK239: a P2 related temperate phage which excludes rII mutants of T4. Virology. 1973 Sep;55(1):136–142. doi: 10.1016/s0042-6822(73)81015-3. [DOI] [PubMed] [Google Scholar]
- Dhillon T. S., Dhillon E. K., Chau H. C., Li W. K., Tsang A. H. Studies on bacteriophage distribution: virulent and temperate bacteriophage content of mammalian feces. Appl Environ Microbiol. 1976 Jul;32(1):68–74. doi: 10.1128/aem.32.1.68-74.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon T. S., Dhillon E. K. Studies on bacteriophage distribution. II. Isolation and host rage based classification of phages active on three species of Enterobacteriaceae. Jpn J Microbiol. 1972 Jul;16(4):297–306. doi: 10.1111/j.1348-0421.1972.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Dhillon T. S., Dhillon E. K. Temperate coliphage HK022. Clear plaque mutants and preliminary vegetative map. Jpn J Microbiol. 1976 Oct;20(5):385–396. doi: 10.1111/j.1348-0421.1976.tb01004.x. [DOI] [PubMed] [Google Scholar]
- Dove W. The extent of rII deletions in phage T4. Genet Res. 1968 Apr;11(2):215–219. doi: 10.1017/s001667230001140x. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Kondo S. Spontaneous and radiation-induced deletion mutations in Escherichia coli strains with different DNA repair capacities. Mutat Res. 1972 Sep;16(7):13–25. doi: 10.1016/0027-5107(72)90059-0. [DOI] [PubMed] [Google Scholar]
- RUTBERG L., RUTBERG B. ON THE EXPRESSION OF THE RII MUTATION OF T-EVEN BACTERIOPHAGES IN ESCHERICHIA COLI STRAIN B. Virology. 1964 Feb;22:280–283. doi: 10.1016/0042-6822(64)90013-3. [DOI] [PubMed] [Google Scholar]
- Sato K., Nishimune Y., Sato M., Numich R., Matsushiro A. Suppressor-sensitive mutants of coliphage phi-80. Virology. 1968 Apr;34(4):637–649. [PubMed] [Google Scholar]
- Signer E. R. Plasmid formation: a new mode of lysogeny by phase lambda. Nature. 1969 Jul 12;223(5202):158–160. doi: 10.1038/223158a0. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Weil J. Recombination in bacteriophage lambda. I. Mutants deficient in general recombination. J Mol Biol. 1968 Jul 14;34(2):261–271. doi: 10.1016/0022-2836(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Pizer L. I., Pylkas L., Lederberg S. Abortive infection of Shigella dysenteriae P2 by T2 bacteriophage. J Virol. 1969 Aug;4(2):162–168. doi: 10.1128/jvi.4.2.162-168.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]