Abstract
Infection by Leptospira interrogans has been causally associated with human and equine uveitis. Studies in our laboratories have demonstrated that leptospiral lipoprotein LruA and LruB are expressed in the eyes of uveitic horses, and that antibodies directed against LruA and LruB react with equine lenticular and retinal extracts, respectively. These reactivities were investigated further by performing immunofluorescent assays on lenticular and retinal tissue sections. Incubation of lens tissue sections with LruA-antiserum and retinal sections with LruB-antiserum resulted in positive fluorescence. By employing two-dimensional gel analyses followed by immunoblotting and mass spectrometry, lens proteins cross-reacting with LruA antiserum were identified to be α-crystallin B and vimentin. Similarly, mass spectrometric analyses identified β-crystallin B2 as the retinal protein cross-reacting with LruB-antiserum. Purified recombinant human α-crystallin B and vimentin were recognized by LruA-directed antiserum, but not by control pre-immune serum. Recombinant β-crystallin B2 was likewise recognized by LruB-directed antiserum, but not by pre-immune serum. Moreover, uveitic eye fluids contained significantly higher levels of antiibodies that recognized α-crystallin B, β-crystallin B2 and vimentin than did normal eye fluids. Our results indicate that LruA and LruB share immuno-relevant epitopes with eye proteins, suggesting that cross-reactive antibody interactions with eye antigens may contribute to immunopathogenesis of Leptospira-associated recurrent uveitis.
Author Summary
Leptospira is the most common infectious cause of uveitis, a potentially debilitating inflammation of the eye. In our earlier work, we discovered that eye fluids of uveitic horses contain high levels of antibodies directed against novel leptospiral proteins, which we named LruA and LruB (Leptospiral recurrent uveitis associated proteins A and B). Significantly, antibodies raised against LruA and LruB also recognize lens and retinal tissue. We have now identified the cross-reactive eye proteins as alpha-crystallin B, vimentin and beta-crystallin B2. We also demonstrated that ocular fluids from uveitic horses contain high levels of antibodies recognizing alpha-crystallin B, vimentin and beta-crystallin B2. These data suggest that antibodies directed against leptospiral LruA and LruB during infection can also react with eye proteins, alpha-crystallin B, vimentin and beta-crystallin B2, potentially contributing to the severity of this eye disease.
Introduction
Infectious disease caused by spirochetes of the genus Leptospira is a veterinary and public health problem of global proportions [1], [2]. Humans and other mammals are exposed to the organism when they contact groundwater contaminated with urine from carrier animals. The disease in humans varies from a mild flu-like form to a more severe syndrome involving multiorgan failure and death [3]. Uveitis is a common complication of systemic infection in humans affecting one or both eyes [4]. In equines, infection is mainly associated with spontaneous abortion in mares and recurrent uveitis [3]. After an initial infection, some horses develop a recurrent inflammation of the uveal tract of eye (iris, ciliary body and choroid), known as equine recurrent uveitis (ERU) or ‘moon blindness’. First described in 1819 by James Wardrop as a “specific inflammation” of uveal origin, it is the most common cause of blindness in horses worldwide [5], [6] with a prevalence of approximately 8–10% in the United States [7]. Onset of the disease is usually acute with variable degrees of severity and duration. The acute phase is followed by a quiescent phase of no or low inflammation [8]. Subsequent recurrence of inflammation results in pronounced lesions with guarded prognosis for preservation of visual acuity [8], [9], [10], [11]. The Appaloosa breed and horses with MHC class I haplotype ELA-A9 have been observed to be at increased risk of developing uveitis [12], [13].
Leptospira interrogans serovar Pomona is the most common and well-documented infectious cause of ERU in the United States [14]. Its association with pathogenic leptospires has been well established by presence of high titers of leptospiral agglutinins in the blood and aqueous humor [15], [16], by isolation of Leptospira from ocular fluids [17], [18] and the detection of leptospiral DNA by polymerase chain reaction in vitreous humor of uveitic horses [17]. Initial evidence of the association was provided by Morter et al. [19] when they induced uveitis in ponies by subcutaneous injection of guinea pig blood containing live L. interrogans serovar Pomona. The resulting ocular pathology in experimental ponies was found to be similar to that of spontaneous cases of Leptospira-associated ERU.
By using ERU uveitic fluids to screen a lambda phage library of L. interrogans, we identified leptospiral lipoproteins, LruA and LruB, associated with recurrent uveitis in horses [20]. Uveitic equine eye fluids contained significantly higher levels of immunoglobulin A (IgA) and IgG specific for LruA and LruB than did companion sera, indicating strong local antibody responses. Moreover, monospecific antiserum to LruA and LruB reacted with extracts of equine ocular tissue. In the present study we have examined the reactivity of LruA- and LruB-antiserum with sections of lens and retinal tissue and identified the ocular proteins involved in the interaction. In addition, the significance of the identified autoantigens was assessed by measuring their immuno-reactivities in eye fluids of uveitic and healthy animals.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with relevant national and international guidelines, and all animal work was approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC#2009-0477).
Eye fluids and eye tissue extracts
Eye fluids and companion sera from horses of varied age, breed, and origin were obtained from a commercial horse slaughter plant in North America. Eyes with gross evidence of uveitis were enucleated after slaughter, and aqueous humor was removed with a 10-ml syringe and stored at −20°C. The eyes were placed in 10% formaldehyde for subsequent embedding, sectioning, and staining with hematoxylin and eosin for histological examination. Eye fluids and sera were assayed for antibodies to serovars Pomona, Canicola, Icterohemorrhagiae, Hardjo, Bratislava, and Grippotyphosa in the microscopic agglutination test (MAT) [20]. Eye fluids and sera from each horse were also tested by ELISA using recombinant antigens LigA, Lk73.5 and Qlp42). Extracts were prepared from the ciliary body, cornea, lens, and retina of a normal eye from a young horse serologically negative for Leptospira, as described by Parma et al., 1985 [21].
Recombinant protein and antiserum
Identification, cloning, and expression of recombinant LruA and LruB has been described previously [20]. Briefly, following PCR amplification of chromosomal DNA of L. interrogans serovar Pomona type kennewicki (JEN4) with gene-specific primers, amplicons were inserted into pET-15b (Novagen, Madison, WI). Recombinant plasmids were transformed into Escherichia coli BL21 (DE3) (Novagen, Madison, WI), and recombinant His-tagged proteins were isolated and their purity tested as previously described [20]. Three New Zealand white rabbits were immunized to obtain polyclonal antiserum directed against recombinant LruA.
Immuno-fluorescence assay (IFA)
Lenses were dissected from the eyes of three healthy horses immediately after euthanasia, frozen in liquid nitrogen, stored at −70°C, and later embedded in tissue freezing medium for mounting in a Tissue-Tek (Miles, Elkhart, IN) cryostat. Sections (8–10 µ) were placed on glass slides treated with 2% solution of 3-aminopropyltriethoxysilane in acetone. The sections were fixed in acetone at 20°C for 20 min followed by two washes with phosphate buffer saline (PBS, pH 7.4) for 5 min each. Blocking was performed using 2% bovine serum albumin (BSA; Sigma, St. Louis, MO) in PBS for 30 min. Sections were again washed thrice with PBS and incubated with 1∶100 polyclonal rabbit antiserum or pre-immune serum overnight at 4°C in a humidifying chamber. Sections were washed three times for 5 min each and subsequently incubated with 1∶250 dilution of FITC conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) for 1 h at room temperature in a humidifying chamber. Slides were mounted in a mounting medium containing anti-fading reagent Mowiol (EMD Chemicals, Gibbstown, NJ) and screened by epifluorescence microscopy (Axioscope-20; Zeiss, Thornwood, NY, USA) and image analysis was carried out using the QUIPS-XL and QUIPS-AKS system (Vysis, Downer's Grove, IL, USA). The same IFA protocol as above was used for testing lens tissues obtained from two healthy sheep.
Two dimensional polyacrylamide gel electrophoresis
Lenticular and retinal aqueous extracts were separated by two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) using MultiPhor-II system (GE Healthcare, Piscataway, NJ). Briefly, the aqueous extracts were subjected to isoelectric focusing using precast IPG strips (Bio-Rad, Hercules, CA) for 3000 V· h (500 V, 6 h, 10°C). Strips were then equilibrated and subjected to conventional sodium dodecylsulfate-12.5% polyacrylamide gel electrophoresis (SDS-PAGE). Gels were either stained with SYPRO Ruby (Invitrogen) or transferred to nitrocellulose membranes for immunoblot analysis with LruA- or LruB-directed antiserum. Immunoblot positive protein spots were extracted from gels and analyzed by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry (University of Louisville Mass Spectrometry Core Laboratory, Louisville, KY). Spectrometry outputs were compared with known sequences using Mascot (Matrix Science, Boston, MA).
Eye protein antibody assays
Recombinant human α-crystallin B (Abcam, Cambridge, MA), purified vimentin from bovine lens (Sigma) or human recombinant β-crystallin B2 (Abnova) were separated by SDS-PAGE, transferred to a nitrocellulose membrane and blocked with 5% nonfat dry milk in Tris-buffered saline (20 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 7.5). Membranes were incubated with LruA or LruB-antiserum (1∶400) followed by incubation with protein G conjugated to horseradish peroxidase (Zymed, San Francisco, CA). Membranes were developed with the SuperSignal West Pico enhanced chemiluminescence substrate (Pierce), and bands were visualized with BioMax Light film (Kodak).
ELISA measured alpha-crystallin B, vimentin and β-crystallin B2 antibody levels in leptospiral uveitic and normal eye fluids as described previously [20], [22]. Briefly, ELISAs were performed in Maxisorp 96-well plate wells (Nalge-Nunc, Rochester, NY) coated with 200 ng human recombinant alpha-crystallin B (Abcam), purified vimentin from bovine lens (Sigma) or human recombinant β-crystallin B2 (Abnova) followed by blocking with 5% nonfat dry milk. Uveitic and normal eye fluids (1∶100) were added and incubated for 1 h at 37°C. Bound antibodies were detected using HRP conjugated Protein G (1∶4000; Zymed, San Francisco, CA). Plates were developed using ready-to-use 3,3′,5,5′-tetramethyl benzidine substrate solution (1-Step Turbo TMB-ELISA, Thermo Scientific, Rockford, IL). Reactions were stopped by addition of 2N H2SO4, 50 µl/well. Absorbance was read at 450 nm in a Spectramax plate reader using SoftMax Pro (Molecular Devices, Sunnyvale, CA). Statistical analyses were performed using Student's t-test assuming unequal variances.
Results
Cross-reactivity of LruA and LruB with equine lens and retina, respectively
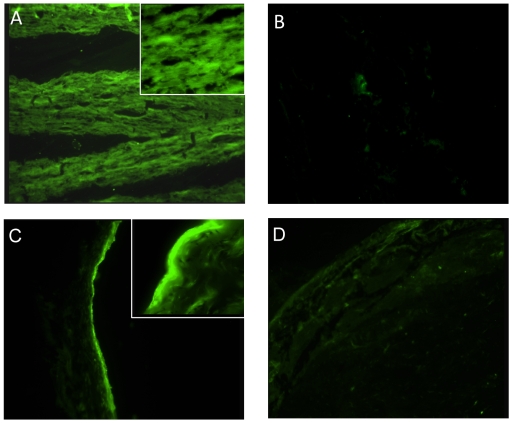
In a previous study [20] LruA-antiserum was shown to recognize a ∼22 kDa protein in lens extract and a ∼65 kDa protein in ciliary body extract. In the same work, LruB-antiserum reacted with a ∼30 kDa band in retinal extract. To further examine the observed cross-reactivity between equine ocular tissue and LruA and LruB specific antisera, immunofluorescent assays were performed. Frozen lenticular and retinal tissue sections (8–10 µ) were fixed, blocked and incubated with antiserum or preserum to LruA and LruB (diluted 1∶100). The lens fibers showed uniform homogenous pattern of fluorescence when incubated with LruA-specific antiserum but not with normal rabbit serum ( Figure 1A and B ).
Figure 1. LruA and LruB-antiserum reacts with lenticular and retinal tissues.
(A) Photomicrographs showing uniform homogeneous fluorescence in a section of equine lens incubated with LruA-antiserum (1∶100) but not with preserum (B) (×100). (C) Photomicrographs showing a positive fluorescence in a frozen section of equine retina incubated with LruB-antiserum (1∶100) but not with pre-immunization serum (D) (×100). Inset (×400).
Similarly, fluorescence was observed when equine retinal tissue sections were incubated with LruB-specific antiserum but not with normal rabbit serum ( Figure 1C and D ). The positive fluorescence seen in retinal tissue incubated with LruB-antiserum was restricted to one or more deeper retinal layers, which include the inner limiting membrane, layer of nerve fiber and may be the ganglion cell layer, in contrast to a diffused positive fluorescence seen in lens tissue sections incubated with LruA-antiserum. In addition, sclera and choroid were devoid of any fluorescence in the same tissue section ( Figure 1 ).
Similar results were obtained with lenticular tissues from a healthy sheep. Sections of sheep lens incubated with LruA-antiserum showed positive fluorescence but not when these sections were incubated with pre-immune serum (not shown). The reactivities of equine and sheep lenticular tissue sections with LruA-antiserum indicated that the observed interaction is not unique to equines lens.
Identification of lenticular and retinal proteins cross-reactive with LruA and LruB
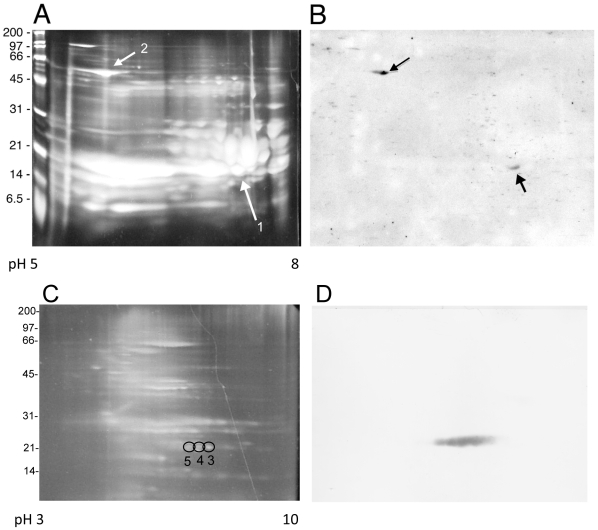
To identify the eye protein(s) recognized by LruA-directed antibodies, proteins in equine lenticular extract were separated on two-dimensional polyacrylamide gels, transferred to nitrocellulose membranes and probed with LruA-specific antiserum ( Figure 2 A and B ). LruA-antiserum recognized protein spots with apparent molecular masses of approximately 20 and 60 kDa. The immunoblot was aligned with the stained gel, to locate the corresponding protein spots which were then subjected to mass spectrometric analysis. The 20 and 60 kDa protein spots were identified as α-crystallin B and vimentin, respectively ( Table 1 ) with 66% and 44% coverage (not shown). Alpha-crystallin B and vimentin have molecular masses of 20188 Daltons and 53727 Daltons, respectively. Attempts to identify ciliary body protein(s) reactive to LruA-specific antiserum by this method have not yet been successful.
Figure 2. Two-dimensional electrophoretic analysis of proteins in equine lenticular and retinal tissue extracts.
(A) Lens extract separated on a polyacrylamide gel stained with the fluorescent dye SYPRO-Ruby. (B) Lens proteins transferred from a second gel to nitrocellulose membrane and blotted with LruA-antiserum. The arrowheads indicate the protein spots excised from the stained gel for analysis by mass spectrometry. (C) Retinal extract separated on a polyacrylamide gel stained with SYPRO-Ruby. (D) Retinal proteins transferred from a second gel to nitrocellulose membrane and blotted with LruB-antiserum. Three protein spots (numbered 3, 4 and 5) were excised from the stained gel for analysis by mass spectrometry. Results of mass spectrometric analyses are tabulated in Table 1.
Table 1. Identification of ocular proteins cross-reactive with LruA and LruB antiserum (Figure 2) by mass spectrometry.
| Protein Spot | Identified Protein | Accession Number | Predicted Mass* |
| Spot 1 | Alpha-crystallin chain B | CYBOAB | 20024 |
| Spot 2 | Vimentin | VIM_BOVINE | 53752 |
| Spot 3 | Beta-crystallin chain B2 | Q2 LEC2_CANFA | 23318 |
| Spot 4 | Beta-crystallin chain B2 | Q2 LEC2_CANFA | 23318 |
| Spot 5 | Beta-crystallin chain B2 | Q2 LEC2_CANFA | 23318 |
*Peptide masses were analyzed using the MASCOT Database search engine v1.9 (www.matrixscience.com) (Matrix Science Ltd.).
Similarly, LruB-antiserum recognized three spots of retinal proteins ( Figure 2C and D ), which were identified by mass spectrometry to be β-crystallin B2 ( Table 1 ).
LruA-directed antiserum recognizes human eye lens α-crystallin B and vimentin
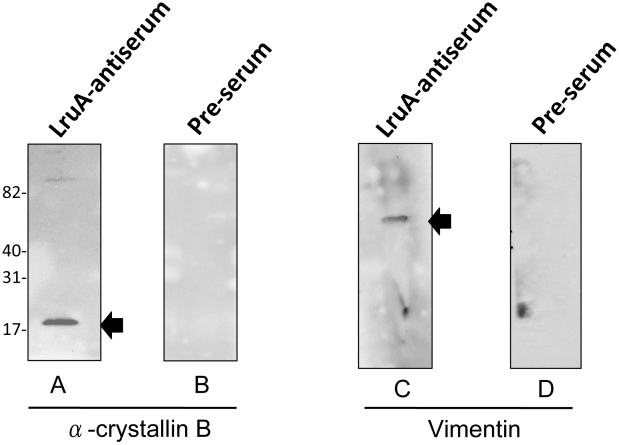
Mammalian α-crystallin B protein sequences are highly conserved across species ( Figure 3 ). Therefore, purified recombinant human α-crystallin B was used in immunoblot analyses to confirm α-crystallin B as a cross-reacting antigen. LruA-directed antiserum, but not the pre-immune serum, reacted with recombinant α-crystallin B, indicating that this lenticular protein is indeed the cross-reacting antigen ( Figure 4A and B ).
Figure 3. Multisequence alignment of alpha-crystallin B of horse (Equus caballus), man (Homo sapiens), cow (Bos taurus), sheep (Ovis aries) and mouse (Mus musculus) using T-COFFEE Version 5.05 [http://www.tcoffee.org].
Figure 4. LruA antiserum reacts with recombinant human alpha-crystallin B and purified vimentin.
(A) Immunoblot showing reactivity of LruA-specific antiserum (1∶400) with recombinant human alpha-crystallin B (1µg). (B) Pre-immune serum (1∶400) did not react with this protein. (C) Immunoblots showing reactivity of LruA-antiserum (1∶400), but not the pre-immunization serum (D), with purified vimentin (1µg). Molecular mass markers are indicated in kilodaltons.
The amino acid sequence identity between equine and bovine vimentin is 91% (not shown). So, purified vimentin from bovine lens was used in immunoblot to examine its reactivity to LruA-directed antiserum. LruA-directed antiserum but not the pre-immune serum, reacted with purified vimentin in an immunoblot ( Figure 4C and D ).
LruB-directed antiserum recognizes human β-crystallin B2
Recombinant human β-crystallin B2 was used in immunoblot analyses to confirm β-crystallin B2 as a cross-reacting antigen. LruB-directed antiserum, reacted with recombinant β-crystallin B2, indicating that this lenticular protein is indeed the cross-reacting antigen ( Figure 5A ). Pre-immunization serum did not react with β-crystallin B2 ( Figure 5B ).
Figure 5. LruB antiserum reacts with human β-crystallin B2.
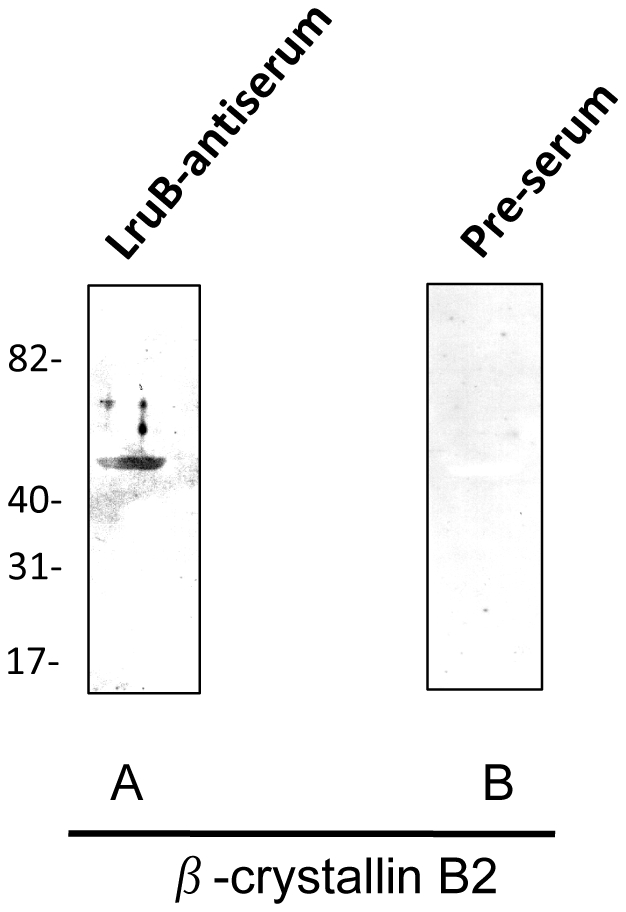
(A) Immunoblots showing reactivity of LruB-antiserum (1∶400) with recombinant human β-crystallin B2(1µg). (B) Pre-immune serum (1∶400) did not react with β-crystallin B2. Molecular mass of recombinant β-crystallin B2 is 50.4 kDa. Molecular mass markers are indicated in kilodaltons.
Antibodies directed to alpha-crystallin B, vimentin and β-crystallin B2 in uveitic fluids
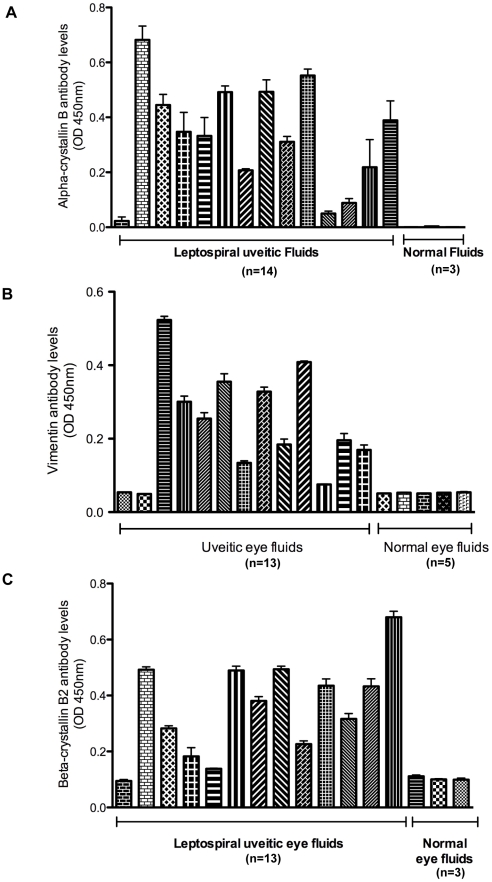
The biological significance of α-crystallin B, vimentin and β-crystallin B2 as a cross-reacting antigen was investigated by examining antibody levels against these lenticular or retinal proteins in eye fluids obtained from clinical cases of leptospiral uveitis and healthy controls. ELISA was performed using recombinant α-crystallin B, purified vimentin or recombinant β-crystallin B2 as coating antigens. Antibody levels to α-crystallin B, vimentin and β-crystallin B2 ( Figure 6 ) were found to be significantly elevated in uveitic compared to normal eye fluids (p<0.001).
Figure 6. Uveitic eye fluids contain antibody to alpha-crystallin B, vimentin and β-crystallin B2.
ELISA results showing significantly higher antibody levels of alpha-crystallin B (A), vimentin (B) and β-crystallin B2-antibodies (C) in uveitic eye fluids. ELISA plate wells were coated with 200 ng of each eye protein. After blocking, wells were sequentially incubated with uveitic or normal eye fluids diluted 1∶100 and HRP-conjugated protein G (1∶5000). Plates were developed using 3,3′,5,5′-tetramethyl benzidine substrate solution and absorbance measured at 450 nm. Presented data is a representative of at least two independent experiments with 3 or more repeats. Error bars indicate standard deviation.
Discussion
The pathogenesis of leptospiral uveitis is currently under investigation and several possible mechanisms have been proposed [6], [9], [12], [17], [20], [21], [23], [24], [25], [26], [27], [28]. How leptospires survive in the eye, causing breach of the ocular immune privilege and initiation of pro-inflammatory changes, is not understood. Although direct Leptospira-mediated injury to eye structures is possible, a growing body of evidence suggests that autoimmune responses to ocular tissue components play a significant role in pathogenesis [6], [9], [12], [17], [20], [21], [22], [23], [24], [25], [26], [27], [28]. Parma et al. [21] demonstrated reactivity of anti-equine cornea antibodies with Leptospira and binding of Leptospira and cornea specific antibodies to equine cornea [21]. Subsequently, an antigenic relationship between equine lens and leptospires was proposed by the same group [27]. Electron microscopic studies revealed that the antigenic protein of L. interrogans that shares epitopes with equine cornea and lens is not exposed on the outer surface of leptospires [28]. However, in those studies, specific leptospiral and/or ocular proteins involved in the antigenic relationship were not identified. In this study, we have shown that the lenticular proteins, α-crystallin B and vimentin, cross-react with LruA and retinal protein, β-crystallin B2, cross-reacts with LruB confirming our previous observations of reactivity of LruA and LruB antibodies with equine lens and retina, respectively.
Alpha-crystallin B and vimentin are critical for maintaining lens clarity and thus visual acuity [29]. Alpha-crystallin is the principal constituent of the lens and acts as a molecular chaperone that keeps other lens proteins from precipitating [30]. Disruption of this function may lead to impairment of light refraction and potentially vision. Alpha-crystallin B is a 175-amino acid small heat shock protein and shares high interspecies sequence homology ( Figure 3 ). Its involvement in several disease states including uveitis, Alexander disease, Alzheimer's, Creutzfeldt-Jacob disease and multiple sclerosis are under investigation [31], [32], [33]. In addition to lens and central nervous system (CNS), it is also present in many other tissues including skeletal muscles and kidney epithelial cells.
Vimentin is an important structural determinant in the human lens cell and is mainly expressed in the epithelium of the lens. In a previous study, high expression of vimentin was negatively correlated with the normal differentiation of the lens fibers. In that study, animals developed pronounced cataract and extensive lens degeneration as a result of impairment of lens fiber cell differentiation [34]. A study on expression of vimentin in lens epithelium of age-related cataract suggested that damage to the lens epithelial cells might initiate a decrease in vimentin expression leading to degradation of the lens cytoskeleton [35]. Recently, small interfering RNA (siRNA) mediated downregulation of human pigment epithelium-derived factor (PEDF) expression in primary human lens epithelial cells was shown to result in a decrease in the expression of vimentin and increase of α-crystallin B expression [29]. Interestingly, serum and ocular levels of PEDF have been shown to decrease in uveitic horses, but not the normal horses [36], [37].
Beta-crystallin B2 is present in lens and non-lenticular tissues, including the retina. The appearance and accumulation of beta-crystallin B2 in neural retina coincides with its functional maturation [38]. Recently, antibodies against α-crystallin A, α-crystallin B and β crystallin B1 were found to be significantly elevated in uveitis patients and seroreactivity was found to be significantly associated with cortical cataract [39]. In another study, Çelet and colleagues [31] reported an elevated humoral response to α-crystallin B in neuro-Behçet's disease and Guillain-Barré syndrome. We recently demonstrated that LruA and LruB were recognized by antibodies from Behçet's and Fuchs uveitis patients, without any evidence of those patients having been exposed to Leptospira [22]. Both of these diseases are believed to be autoimmune diseases [22], [40], [41], [42], [43], [44], [45]. In the same study, we also observed an association in humans between high levels of antibodies recognizing LruA and LruB and the presence of cataract [22]. The high levels of antibodies cross-reactive with LruA and LruB in patients with Fuchs or Behçet's uveitis, and the strong association of LruA and LruB antibodies with cataract could be due to increased levels of antibodies to the common autoantigens, α-crystallin B, vimentin and β-crystallin B2, in those diseases. Also, elevated levels of LruA- and LruB-antibodies in sera of human patients with leptospiral uveitis [22] and reactivity of LruA- and LruB-antiserum with human alpha-crystallin B and β-crystallin B2 suggest a similar phenomenon in human leptospiral uveitis. We are presently pursuing those hypotheses to determine the causes of leptospiral and non-leptospiral uveitis.
A linear amino acid similarity or a conformational homology between microbial and host proteins is a potential basis for molecular mimicry. The limited linear amino acid similarity between these leptospiral proteins and their respective cross-reacting ocular proteins (not shown) suggests similarities at the conformational level. Studies to identify the cross-reactive epitopes are underway.
In conclusion, we have identified two lens proteins and a retinal protein that react with antiserum directed against LruA and LruB, leptospiral proteins expressed in uveitic eyes. The presence of antibodies recognizing α-crystallin B, vimentin and β-crystallin B2 in uveitic, but not normal eye fluids, strongly suggests a role for these antibodies in Leptospira-associated recurrent uveitis. In the immune privileged ocular environment, it is likely that the early phase of leptospiral infection involves a non-inflammatory immune responses specific for LruA and LruB. Resulting antibodies may interact with cross-reacting proteins in lens and retinal tissues and may therefore initiate a process of desequestration of these ocular antigens, and possibly other components. How early after an initial infection this interaction results in development of the changes in eye, and what other pro-inflammatory changes, if any, are required remains to be determined.
Acknowledgments
We thank Claire Adams, Sergey Artiushin, Amy Bowman, Catherine Brissette, Logan Burns, Alicia Chenail, Brandon Jutras and Samir Shah for technical assistance and helpful comments.
We dedicate this work to the memory of Dr. George Allen.
Footnotes
The authors have declared that no competing interests exist.
This study was supported by National Institutes of Health grant AI-78111 (to B. Stevenson) and the Keeneland Foundation endowment (to J. F. Timoney). A. Verma was funded by a Paul Mellon Fellowship in Equine Studies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thiermann AB. Leptospirosis: Current developments and trends. J Am Vet Med Assoc. 1984;184:722–725. [PubMed] [Google Scholar]
- 2.Vinetz JM. Leptospirosis. Curr Opin Infect Dis. 2001;14:527–538. doi: 10.1097/00001432-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. Melbourne, Australia: MediSci; 1999. [Google Scholar]
- 4.Rathinam SR. Ocular leptospirosis. Curr Opin Ophthalmol. 2002;13:381–386. doi: 10.1097/00055735-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Errington BJ. Ophthalmology in Equidae. J Am Vet Med Assoc. 1941;98:115–123. [Google Scholar]
- 6.Hartskeerl RA, Goris MG, Brem S, Meyer P, Kopp H, et al. Classification of leptospira from the eyes of horses suffering from recurrent uveitis. J Vet Med B. 2004;51:110–115. doi: 10.1111/j.1439-0450.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwink KL. Equine uveitis. Vet Clin North Am Equine Pract. 1992;8:557–574. doi: 10.1016/s0749-0739(17)30441-8. [DOI] [PubMed] [Google Scholar]
- 8.Cook CS, Harling DE. Equine recurrent uveitis. Equine Vet J. 1983;2:2–15. [Google Scholar]
- 9.Gilger BC, Malok E, Cutter KV, Stewart T, Horohov DW, et al. Characterization of T-lymphocytes in the anterior uvea of eyes with chronic equine recurrent uveitis. Vet Immunol Immunopathol. 1999;77:17–28. doi: 10.1016/s0165-2427(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 10.Gilger BC, Michau TM. Equine recurrent uveitis: new methods of management. Vet Clin Equine. 2004;20:417–427. doi: 10.1016/j.cveq.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Rebhun WC. Diagnosis and treatment of equine uveitis. J Am Vet Med Assoc. 1979;175:803–808. [PubMed] [Google Scholar]
- 12.Deeg CA, Marti E, Gaillard C, Kaspers B. Equine recurrent uveitis is strongly associated with the MHC class I haplotype ELA-A9. Equine Vet J. 2004;36:73–75. doi: 10.2746/0425164044864651. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer AE, Gilger BC. Equine recurrent uveitis. In: Gilger BC, editor. Equine ophthalmology. Philadelphia, Pa: W. B. Saunders Co; 2005. [Google Scholar]
- 14.Halliwell RE, Brim TA, Hines MT, Wolf D, White FH. Studies on equine recurrent uveitis. II. The role of infection with Leptospira interrogans serovar Pomona. Curr Eye Res. 1985;4:1033–1040. doi: 10.3109/02713688509003348. [DOI] [PubMed] [Google Scholar]
- 15.Deeg CA, Ehrenhofer M, Thurau SR, Reese S, Wildner G, et al. Immunnopathology of recurrent uveitis in spontaneously diseased horses. Exp Eye Res. 2002;75:127–133. doi: 10.1006/exer.2002.2011. [DOI] [PubMed] [Google Scholar]
- 16.Faber NA, Crawford M, LeFebvre RB, Buyukmihci NC, Madigan JE, et al. Detection of Leptospira spp. in the aqueous humor of horses with naturally acquired recurrent uveitis. J Clin Microbiol. 2000;38:2731–2733. doi: 10.1128/jcm.38.7.2731-2733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandes K, Wollanke B, Niedermaier G, Brem S, Gerhards H. Recurrent uveitis in horses: vitreal examinations with ultrastructural detection of leptospires. J Vet Med A Physiol Pathol Clin Med. 2007;54:270–275. doi: 10.1111/j.1439-0442.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 18.Brem S, Gerhards H, Wollanke B, Meyer P, Kopp H. 35 leptospira isolated from the vitreous body of 32 horses with recurrent uveitis. Berl Munch Tierarztl Wochenschr. 1999;112:390–393. [PubMed] [Google Scholar]
- 19.Morter RL, Williams RD, Bolte H, Freeman MJ. Equine leptospirosis. J Am Vet Med Assoc. 1969;155:436–442. [PubMed] [Google Scholar]
- 20.Verma A, Artiushin S, Matsunaga J, Haake DA, Timoney JF. LruA and LruB, novel lipoproteins of pathogenic Leptospira interrogans associated with equine recurrent uveitis. Infect Immun. 2005;73:7259–7266. doi: 10.1128/IAI.73.11.7259-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parma AE, Santisteban CG, Villalba JS, Bowden RA. Experimental detection of an antigenic relationship between Leptospira and equine cornea. Vet Immunol Immunopathol. 1985;10:215–224. doi: 10.1016/0165-2427(85)90048-0. [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Rathinam SR, Priya CG, Muthukkaruppan VR, Stevenson B, et al. LruA and LruB antibodies in sera of humans with leptospiral uveitis. Clin Vaccine Immunol. 2008;5:1019–1023. doi: 10.1128/CVI.00203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeg CA, Kaspers B, Gerhards H, Thurau SR, Wollanke B, et al. Immune responses to retinal autoantigens and peptides in equine recurrent uveitis. Investig Ophthalmol Vis Sci. 2001;42:393–398. [PubMed] [Google Scholar]
- 24.Deeg CA, Thurau SR, Gerhards H, Ehrenhofer M, Wildner G, et al. Uveitis in horses induced by interphotoreceptor retinoid binding protein is similar to the spontaneous disease. Eur J Immunol. 2002;32:2598–2606. doi: 10.1002/1521-4141(200209)32:9<2598::AID-IMMU2598>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Deeg CA, Pompetzki D, Raith AJ, Hauck SM, Amann B, et al. Identification and functional validation of novel autoantigens in equine uveitis. Mol Cell Proteomics. 2006;5:1462–1470. doi: 10.1074/mcp.M500352-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Deeg CA, Amann B, Raith AJ, Kaspers B. Inter- and intramolecualar epitope spreading in equine recurrent uveitis. Invest Ophthalmol Vis Sci. 2006;47:652–656. doi: 10.1167/iovs.05-0789. [DOI] [PubMed] [Google Scholar]
- 27.Parma AE, Fernandez AS, Santisteban CG, Bowden RA, Cerone SI. Tears and aqueous humor from horses inoculated with Leptospira contains antibodies which bind to cornea. Vet Immunol Immunopathol. 1987;14:181–185. doi: 10.1016/0165-2427(87)90052-3. [DOI] [PubMed] [Google Scholar]
- 28.Parma AE, Sanz ME, Lucchesi PM, Mazzonelli J, Petruccelli MA. Detection of an antigenic protein of Leptospira interrogans which shares epitopes with the equine cornea and lens. Vet J. 1997;153:75–79. doi: 10.1016/s1090-0233(97)80011-1. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Luo L, Liu X, Rosenblatt MI, Qu B, et al. Down regulation of the PEDF gene in human lens epithelium cells changed the expression of proteins vimentin and alphaB-crystallin. Mol Vis. 2010;16:105–112. [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz J. Alpha-crystallin can function as a molecualr chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celet B, Akman-Demir G, Serdaroglu P, Yentur SP, Tasci B, et al. Anti-alpha B-crystallin immunoreactivity in inflammatory nervous system diseases. J Neurol. 2000;247:935–939. doi: 10.1007/s004150070049. [DOI] [PubMed] [Google Scholar]
- 32.Mann E, McDermott MJ, Goldman J, Chiesa R, Spector A. Phosphorylation of alpha crystallin B in Alexander's disease brain. FEBS Lett. 1991;294:133–136. doi: 10.1016/0014-5793(91)81359-g. [DOI] [PubMed] [Google Scholar]
- 33.van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri M, Polman CH, et al. The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature. 1995;375:798–801. doi: 10.1038/375798a0. [DOI] [PubMed] [Google Scholar]
- 34.Capetenaki Y, Smith S, Heath JP. Overexpression of the vimentin gene in transgenic mice inhibits normal lens cell differentiation. J Cell Biol. 1989;109:1653–1664. doi: 10.1083/jcb.109.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Hui Y, Li Y. Expression of vimentin in lens epithelial cells of age-related cataract. Zhonghua Yan Ke Za Zhi. 2001;37:342–345. [PubMed] [Google Scholar]
- 36.Zipplies JK, Hauck SM, Schoeffmann S, Amann B, Stangassinger M, et al. Serum PEDF levels are decreased in a spontaneous animal model for human autoimmune uveitis. J Proteome Res. 2009;8:992–998. doi: 10.1021/pr800694y. [DOI] [PubMed] [Google Scholar]
- 37.Deeg CA, Altmann F, Hauck SM, Schoeffmann S, Amann B, et al. Downregulation of PEDF in uveitic lesions associates with focal VEGF expression and breakdown of the blood retinal barrier. Proteomics. 2007;7:1540–1548. doi: 10.1002/pmic.200600795. [DOI] [PubMed] [Google Scholar]
- 38.Head MW, Sedowofia K, Clayton RM. Beta B2-crystallin in the mammalian retina. Exp Eye Res. 1995;61:423–428. doi: 10.1016/s0014-4835(05)80137-x. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Holland GN, Yu F, Levinson RD, Lampi KJ, et al. Associations of seroreactivity against crystallin proteins with disease activity and cataract in patients with uveitis. Invest Ophthalmol Vis Sci. 2008;49:4476–4481. doi: 10.1167/iovs.08-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Otaibi LM, Porter SR, Poate TWJ. Behcet's disease: a review. J Dent Res. 2005;84:209–222. doi: 10.1177/154405910508400302. [DOI] [PubMed] [Google Scholar]
- 41.Chowers I, Zamir E, Banin E, Merin S. Retinitis pigmentosa associated with Fuch's heterochromic uveitis. Arch Ophthalmol. 2000;118:800–802. doi: 10.1001/archopht.118.6.800. [DOI] [PubMed] [Google Scholar]
- 42.Delunardo F, Conti F, Margutti P, Alessandri C, Priori R, et al. Identification and characterization of the carboxy-terminal region of Sip-1, a novel autoantigen in Behcet's disease. Arthritis Res Ther. 2006;8:R71. doi: 10.1186/ar1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Hey E, Broersma L, van der Gaag R, Baarsma GS, Rothova A, et al. Does autoimmunity to S-antigen play a role in Fuch's heterochromic cyclitis? Br J Ophthalmol. 1993;77:436–439. doi: 10.1136/bjo.77.7.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Y, Ye P, Chen S, Tan EM, Chan EKL. Identification of kinectin as a novel Behcet's disease autoantigen. Arthritis Res Ther. 2005;7:R1133–R1139. doi: 10.1186/ar1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Gaag R, Broersma L, Rothova A, Baarsma GS, Kijlstra A. Immunity to a corneal antigen in Fuch's heterochromic cyclitis patients. Invest Ophthalmol Vis Sci. 1989;30:443–448. [PubMed] [Google Scholar]