Abstract
Approximately 70% of male rats receiving severe T8 spinal contusions develop allodynia in T5-7 dermatomes (at-level) beginning two weeks post-injury. In contrast, rats having either complete transections or dorsal hemisections do not develop allodynia at-level following chronic spinal cord injury (SCI). In the present study, incomplete laceration and contusion injuries were made to test for neuroanatomical correlates between areas of white matter damage/sparing at the lesion epicenter and the presence/absence of allodynia. Following incomplete laceration lesions and six weeks of behavioral testing, histological reconstruction and analysis of the lesion epicenters revealed a significant difference (p<.001) in the amount of ventrolateral funiculus (VLF) asymmetry between rats showing pain-like responses evoked by touch (74.5% ± 8.4% side-to-side difference in VLF damage) versus those not responding to touch (11.3% ± 4.4% side-to-side difference in VLF damage). A five week mean allodynia score for each rat that incorporates a full range of forces that are all innocuous in intact controls revealed that the degree of hypersensitivity at level is related to the extent of VLF asymmetry post-SCI. No other damaged spinal white matter or gray matter area was correlated with sensitivity to touch. Similar findings were obtained for rats receiving T8 contusions, a more clinically relevant injury. These data suggest that different extents of damage/sparing between the two sides of VLF are likely a requisite for the development of allodynia after SCI.
Introduction
Neuropathic pain is associated with diseases such as multiple sclerosis, diabetic neuropathy, shingles, post herpetic neuralgia, and stroke as well as mechanical injuries such as amputation, spinal cord injury (SCI) and brain trauma 4, 41. Confounding variables include gender, age, the subjective nature of pain, and differences depending on the neural structure(s) affected 31, 32, 37, 38. Neuropathic pain can spontaneously occur or can be evoked by normally non-noxious (allodynia) or noxious (hyperalgesia) stimuli 10, 39. While some of the causes and symptoms of neuropathic pain are understood, more investigation is required to understand the neural mechanisms underlying neuropathic pain so that more effective treatment strategies can be developed 12. For example, SCI patients often experience pain despite being treated with various therapeutic strategies such as surgery to stabilize the spine and decompress impinged nerve roots 53, antidepressant therapy 11 or other types of pharmacological treatment, or electrical stimulation of the spinal cord 46 or brain 19.
Many types of SCI pain occur above, at, and below the level of injury. These types include muscle spasm pain, visceral pain, central dysesthesia syndrome, and transitional zone or segmental pain 50. Central sensitization caused by damage to nervous tissue post-SCI leads to allodynia and hyperalgesia 4 due in part to pre and postsynaptic cellular changes 34.
We have shown previously that many (70%) but not all rats with severe contusions at the T8 spinal level develop ‘at level’ allodynia in the T5-7 dermatomes beginning around two weeks post injury 21, 24. In contrast, rats with complete transections and dorsal hemisections do not develop allodynia ‘at level’ following chronic SCI 21, 25. There are, however, conflicting reports about the type of injury necessary to evoke pain after spinal cord damage. Some studies have reported allodynia after lateral hemisection lesions 7 and complete spinal transections 62. Although we previously found a positive correlation between behavioral and electrophysiological (thalamic recordings 30 days post-injury) evidence of allodynia and the sparing of some myelinated axons within the core of the contused lesion epicenter 24, the source of these axons is not known.
The purpose of the present study was to determine in laceration at T8 of a specific amount or region of white matter results in the development and maintenance of ‘at level’ allodynia. Since these lesions are accompanied by some degree of gray matter damage, the degree of both white and gray matter damage was examined. The lesion epicenters were then analyzed for T8 contused rats tested for allodynia to see if the laceration injury findings are consistent with this more clinically-relevant SCI model. Evidence for a strong association between the degree of allodynia and extent of asymmetrical ventrolateral funiculus (VLF) tissue damage/sparing is presented for both injury models.
Materials and Methods
Male Wistar rats (Harlan Sprague Dawley, Inc.) approximately 60–70 days old weighing 220–250 grams were individually housed in an animal room with a 12 hour light and dark cycle. Before undergoing SCI surgery at the T8 spinal level, each rat was handled a few times daily and baseline behavioral measurements were obtained for comparison with measurements obtained during the six week post-SCI recovery period. All animal procedures were reviewed and approved by the Institutional Animal Use and Care Committee at the University of Louisville, School of Medicine.
Spinal Cord Injury Protocol
Two groups of rats were used in the present study. The first group (n=18) received select lesions at the T8 spinal level to determine if there was a correlation between specific regions of white matter damage/sparing and the presence/absence of at level allodynia. The second group (n=18) received a contusion lesion at T8 to confirm the select lesion data in a more clinically relevant animal model.
Select Laceration Lesions
Lesions within the dorsal three-fifths of the spinal cord were performed under aseptic conditions. Eighteen animals were anesthetized with a mixture of ketamine (80mg/kg of body weight) and xylazine (10 mg/kg of body weight) injected intraperitoneally 26. Additional 0.2cc doses of the mixture were given as needed. After induction of anesthesia, animals were given 0.5cc subcutaneous injection of Ambi-Pen (Penicillin G and Penicillin G Procrain, Butler). The spinal cord was exposed by laminectomy of the T7 vertebra, which overlies the T8 portion of the spinal cord 21. In order to make a select lesion, a custom-made 0.12 mm thick and 1.6 mm wide blade attached to the Vibraknife™ of a modified VIBRATOME® Series 1000 63 was used, a device which was made at the University of Louisville.
The target vertebra of the rat was stabilized on a platform and this platform was elevated until the oscillating blade contacted the dorsal surface of the spinal cord. The spinal cord was cut by gradually elevating the platform. The spinal cord and the Vibraknife blade were immersed in Hanks balanced salt solution (Gibco, Grand Island, NY) and a dorsal to ventral transverse lesion of varying depths and medial-lateral placements were made 63. Some treatment groups received either partial or complete dorsal hemisection lesions, all of which included the dorsal columns, while other groups had lesions that included portions of the VLF. The two separated pieces of spinal cord that had been created by the laceration were apposed against each other as if no laceration had been made, and the area was covered between the dura/arachnoid mater and the dorsal surface of the spinal cord 2 mm caudal and 2 mm rostral to the laceration with a thin coat of Fibrin adhesive sealant (Tisseel; Baster Healthcare Corp., Glendale, CA) 63. After the Tisseel placement, the dura was closed using two No. 10-0 sutures (Ethicon, Somerville, NJ)63. All musculature and subcutaneous tissue was sutured closed (Ethicon 4-0 nonabsorbable surgical suture) and Michel clips were used to close the skin 22.
Contusion
Spinal cord contusions were also performed using the same protocols as described above for laceration surgeries. To make a spinal cord contusion, the Infinite Horizon impactor device (Precision Systems and Instrumentation, Lexington, KY) was used 48. The impactor was attached to a computer with a software program that used predetermined forces, which have been shown to produce consistent injuries 48. The actual force of compression of the spinal cord was measured by the tip of the impounder which has a force sensor attached to it. The rostral and caudal sections of vertebrae (T6 and T8) were stabilized during impaction by stabilizing forceps. For all animals given contusions during this study, the force applied ranged from 200 to 250 kilodynes. After completion of the injury, the contused area of spinal cord was covered with Durafilm®. All musculature and subcutaneous tissue was sutured closed (Ethicon 4-0 nonabsorbable surgical suture) and Michel clips were used to close the skin 22.
Post-op Care
In the days following surgery, all animals were monitored 3 times per day to express bladders, monitor wounds, to note changes in an animal’s general condition, and to clean the animals, if necessary. During the two days immediately following surgery, animals were given subcutaneous injections of an analgesic, Ketoprofen (2.5 mg/kg of body weight), twice daily. In addition, animals were given Gentamicin (5 mg/kg of body weight) for four days following surgery for prevention of possible bladder infections. Approximately seven days after surgery, Michel clips were removed from the skin.
Behavioral Measurements
Behavioral testing of SCI rats for sensitivity to normally innocuous stimuli (touch and gentle squeeze/pressure) was done in a dedicated facility in close proximity to where the animals are housed. The facility is a well-lit room where levels of noise and other distractions are kept to a minimum. All test sessions were started at approximately the same time of day (1 pm). Two baseline measures at least three days apart were done pre-injury for all rats.
Touch-evoked Agitation
The top of each cage was removed and the animal was allowed to acclimate to the environment for 2 minutes. Each animal was stroked (while still in its home cage) with a #5 paint brush (1.5 × 0.5 cm bristles – average pressure of 17 grams), as previously described 25. A total of 10 stimuli, with an inter-stimulus interval of at least one minute, were applied to the dorsolateral trunk just above (T6-7) the T8 spinal injury level alternating between the right and left sides (5 each) and to test for touch-evoked agitation 59 ‘at level’. After each stroking stimulus, the presence/absence of any one of three evoked responses that are indicative of pain was noted. The three typical evoked pain-like responses, as observed previously with both noxious stimulation in uninjured rats and touch stimuli in SCI rats (based on previous studies 21, 24, 25, 45), were (1) a freezing response (stopping of normal activity and staying still in response to the stimulus), (2) escape (any movement of the animal away from the stimulus probe), and (3) grabbing at or pushing away the stimulus probe with their forepaws. Woolf 57 demonstrated that these evoked responses are lost in decerebrate rats, indicative of higher order processing and thus perception of pain. As with our previous studies, head orientation, which is reflexive in nature (present in decerebrate rats 57), was not counted as an evoked pain-like behavioral response. In order for an animal to be considered responsive to the stimulus, the animal must show an evoked pain-like behavioral response at least 60% of the time in a given session 24.
Each animal was then further assessed for degree of hypersensitivity to normally innocuous stimulation as the information obtained from the commonly used touch evoked method is limited to a single application force. Responses to brush, if present, were assessed further for threshold values using a set of Semmes-Weinstein monofilaments (20 filament set, 15 of which are in the range of 0.008 g to 15 g; obtained from Stoelting Co., Wood Dale, IL). Depending on the level of responsiveness a given animal exhibited during the brush test, the experimenter chose an appropriate Semmes-Weinstein stimulus probe (usually in mid-range). The monofilaments are placed on different areas of the sensitive parts of the dermatomes and pressed until it is bent. If the animal responded to the initial Semmes-Weinstein stimulus, a lower stimulus (in grams) was used to test the animal’s sensitivity. In between probing, the animal is left alone for the same one minute inter-stimulus interval. This process is repeated until the animal no longer responds to a specific monofilament force. At that point, the last stimulus the animal was found responsive to was the pressure recorded for that animal during that testing session. If the animal was not responsive to the initial Semmes-Weinstein stimulus, the next greatest stimulus (in grams) was used to assess the sensitivity of the animal. This process was repeated until the threshold of the animal’s sensitivity was reached and recorded for that testing session. The Semmes-Weinstein monofilaments were chosen over our electrovonfrey anesthesiometer (IITC Model 2390), which was not very sensitive in the lower range of forces.
Gentle Mechanical Pressure
For those animals not responsive to brush stroke (i.e., evoked pain-like behavioral response to less than 60% of the stimuli), a gentle squeeze/pressure test was conducted in order to ascertain if the animal had increased sensitivity to a stronger mechanical stimulus over a wider surface area (which normally does not provoke avoidance behaviors and is thus considered innocuous). The gentle squeeze test is conducted in much the same way that the brush stroke test is done. The animal’s skin is gently squeezed with a pair of modified Adson tissue forceps (2.0 mm wide tips) with a load cell mounted to the end for force measurements. The mean force used averages 66.2 ±4.3 g/cm2 (recorded for 10 trials in each of 4 intact control rats), which is equivalent to the 60g Semmes-Weinstein monofilament. Note that mean avoidance threshold for intact rats, as assessed using a hand-held ElectroVonfrey anesthesiometer (IITC Model 2390; www.iitcinc.com) with the rigid tip adapter (for deep pressure) is 111.9 ± 3.3 g/cm2 45. Gentle pressure/squeeze testing was done 10 times just above the T8 level (5 on the right and 5 on the left). Between squeeze stimuli the animals were left alone to resume normal behavior for one minute before the next stimulus was conducted. The animal is observed as with touch-evoked agitation. Any evoked pain-like behavioral responses are recorded for that testing session.
Scoring Sensitivity of the Behavioral Response
To score the touch evoked and gentle squeeze/pressure test data, scores were calculated based on degree of sensitivity for each animal at each testing period. Five scores, obtained from 2 to 6 weeks post injury, were averaged together in order to get a mean sensitivity score for each rat, reflecting the extent of at level allodynia. This period was chosen because our lab and others have shown that allodynia develops around two weeks following SCI (the sensitivity on the dorsolateral trunk ‘at level’ is unrelated to some initial sensitivity associated with the midline trunk incision during the first week post injury). Note that in previous studies 24, we report only the presence or absence of evoked pain-like responses to touch that meet the 60% response criteria. The criterion for a rat to be considered allodynic is a score greater than 0 on three of five weeks, two of which are on consecutive weeks. If this criterion is not met, the rat receives a 5 week mean score of 0, thereby insuring that the response is consistent and not a onetime phenomenon.
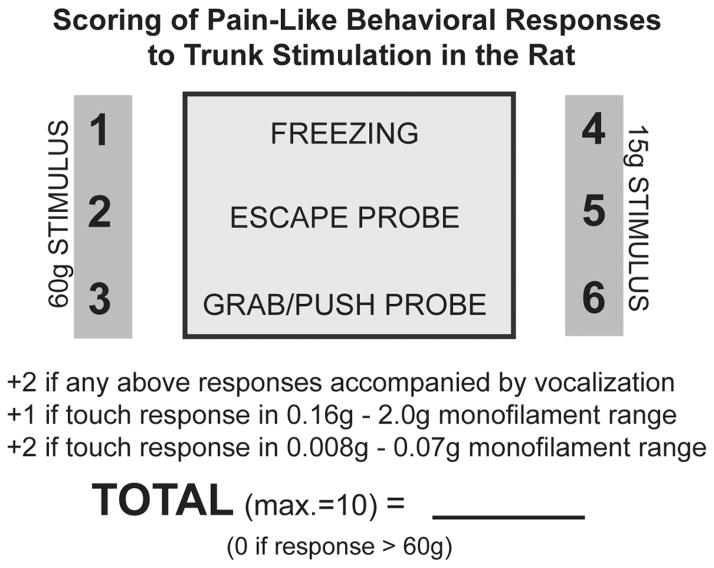
The scoring of pain-like behavioral responses to trunk stimulation in the rat, shown in Figure 1, accounts for the type of evoked pain-like response, the response threshold, and the presence or absence of vocalization. The rats found to be responsive to the 17g brush stroke test versus those only responsive to the 66g gentle squeeze/pressure test are considered more sensitive and receive higher scores, depending on their evoked responses to the brush stroke stimulus (freezing= 4, escaping stimulus= 5, and grab/pushing stimulus= 6). The freeze/escape/grab differential is based upon observations of the nature of the pain-like response; from being more passive to more aggressive (the greater action to avoid is taken to mean “it hurts more”). Animals that responded to the gentle squeeze stimulus were given lower scores (freezing= 1, escaping stimulus= 2, and grab/pushing stimulus= 3). If any of the responses were accompanied by vocalization (audible range), 2 points were added to the score. While vocalization is not considered a higher order process (can be evoked, for example, in decerebrate rats – e.g., 57), it still may be indicative of greater sensitivity. Also, Semmes-Weinstein monofilaments were used when responses to stroke occurred, so points were added to the score depending upon the threshold determined. The size ranges were considered based on the color coding of the monofilaments, which were grouped into ranges based upon light touch sensation (per manufacturer specifications). Evoked pain-like responses to the group of coded monofilaments (green) having the smallest diameters was given a score of two. Evoked responses to the next sized group of monofilaments (blue coded) were given a score of one. Thus, only animals that were very sensitive to touch (≤a force of 2g) received additional points. The maximum score that an animal can receive is 10 points.
Figure 1.
Grading scale to calculate allodynia scores for each animal at each testing session. Two different types of normally innocuous stimuli were used. Animals responsive to the brush stroke test were considered more sensitive and given higher scores than animals responsive to the gentle squeeze test. Vocalization and responses to the finest monofilaments added points to the allodynia score, as they reflect greater sensitivity to touch, as does the aggressiveness of the behavioral response (freezing vs. escape vs. grab/push).
Histology
Tissue Processing
After completion of a terminal electrophysiology experiment, which was part of another study in the laboratory, each animal was euthanized with an anesthetic overdose of urethane. Perfusions were performed transcardially with 0.9% saline followed by 4% paraformaldehyde 26. The animal’s spinal cord was removed and placed in a 30% sucrose/phosphate buffer solution with 1% sodium azide for at least 24 hours and until the tissue was cut on the cryostat (Leica CM1850). A small piece of spinal cord tissue, which contained the lesion site, was mounted onto a metal chuck and frozen using TBS tissue freezing media (Triangle biomedical sciences, Raleigh-Durham, NC). The tissue was cut into 18μm thick dorsal to ventral transverse sections and mounted onto charged glass microscope slides (Fisher Scientific premium frosted microscope slides). Following the cutting and mounting of tissue, the slides were placed onto a slide warmer to dry.
The Klüver-Barrera method (as adapted from the protocol presented by the Armed Forces Institute of Pathology) was used to stain the tissue 36. This protocol combines the luxol fast blue stain, which is used to indicate the presence of myelin, with the cresyl echt violet stain, which is used to stain Nissl substance in nervous tissue 5.
Slide Staining
The staining technique that was used in this study is as follows. In order to dissolve freezing media and hydrate the tissue, slides were briefly rinsed with distilled water. Slides were placed in a coplin jar with luxol fast blue and left in the oven overnight at a temperature of 55 degrees Celsius. The following day, luxol fast blue stain was drained and slides were rinsed briefly with 95% ethanol. Next, slides were rinsed again with distilled water and placed in a 0.05% lithium carbonate solution for 10 minutes. Then, slides were rinsed with 70% ethanol for two minutes and afterward rinsed briefly with distilled water. Slides were again immersed in lithium carbonate for 10 minutes followed by a two minute 70% ethanol rinse. After rinsing with distilled water, slides were placed into a cresyl violet solution for two minutes and 15 seconds. The cresyl violet stain was drained, and slides were rinsed again with distilled water before being placed in two rinses of 95% ethanol for two minutes followed by two rinses of 100% ethanol for one minute. To finalize the procedure, slides were soaked in xylene and coverslipped with a 50:50 Gurr mounting adhesive: xylene mixture.
After drying, the slides were viewed under a light microscope at 40× magnification to locate the epicenter of the lesion. Using the Spot Insight color camera, which was mounted on a Nikon E400 microscope, pictures were taken of a section from a more intact portion of spinal cord (located 1 mm or more away from the epicenter) and the epicenter at 40× magnification. A comparison and quantification was then done between the intact spinal cord pictures and their respective epicenters for each animal.
Quantification of Lesion Size
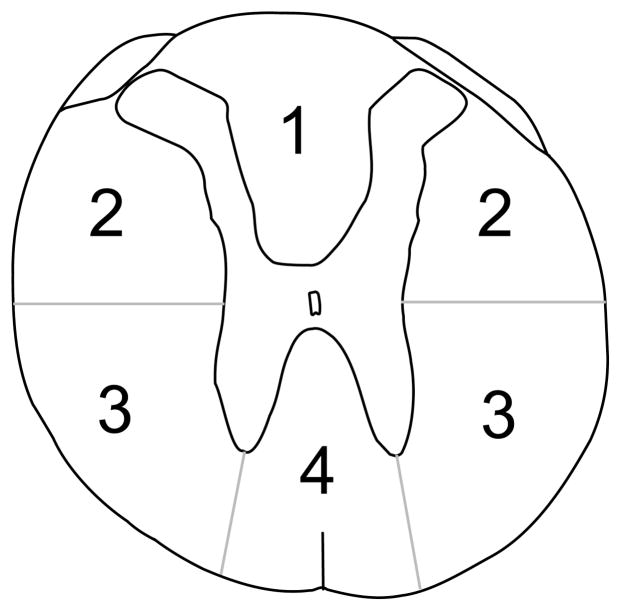
In order to determine the area of damage to the spinal cord at the lesion epicenter, measurements were taken using the Spot Advanced software interfaced with a camera that was connected to the Nikon E400 microscope. The white matter was divided into four areas. These regions were the dorsal columns, dorsolateral funiculus, ventrolateral funiculus, and the ventromedial funiculus (each of these regions were subdivided into right and left sides for analysis since asymmetrical lesions were being made). An example of the divisions used to separate the spinal cord into different sections can be found in Figure 2. The landmarks for the divisions are the central canal, the medial edges of the dorsal horn, and the tips of the ventral horn. The gray matter, however, was divided into two regions (area above and below the central canal; also separate measurements for left and right sides).
Figure 2.
The spinal cord was divided into four areas in order to quantify the damage to different regions of white matter after a select spinal cord injury. These areas were the dorsal columns (1), dorsolateral funiculus (2), ventrolateral funiculus (3), and the ventromedial funiculus (4).
If the central canal was not visible at the lesion epicenter, two lines were digitally imposed on the section image, one running horizontally through the greatest mediolateral width of the cord section and the other running vertically down the center 30. The intersection of these two lines was used to estimate the location of the central canal 30. If the upper medial edge of the dorsal horns and/or lower tip of the ventral horns were not visible at the epicenter, surface notches where the dorsal roots enter and the ventral roots exit the cords were used as landmarks. The percent of white matter sparing was determined by dividing the white matter remaining at the epicenter by the average area of white matter present in more intact sections. The intact area of white matter for a given region (Figure 2) was estimated by averaging together measurements from two sections within 2 mm rostral and caudal to the epicenter.
Since all lesion epicenters for the contusion injuries made in the current study do not have any gray matter sparing (i.e., those resulting from forces in the 200 to 250 kilodyne range), analyses were made for gray matter 1 mm rostral to the lesion epicenter, which is far enough away from the epicenter that there is partial damage but not too far that all of the gray matter is intact (±1.5 to 2.0 mm from the epicenter).
For all tests, ANOVAs were used to determine statistical significance between groups of animals at each time point. Percent change from pre-injury baseline data was also calculated. In an effort to determine if a correlation exists between the allodynia scores and damage to the spinal cord, the final allodynia scores were compared versus the average (left side and right side of spinal cord) percentage of white matter damage in all four divisions of the spinal cord with a Spearman Rank Order Correlation. Final allodynia scores were also compared using the Spearman Rank Order Correlation to the difference in the percentage of white matter damage (left versus right side) for each of the four divisions of the spinal cord, calculated by subtracting the percent damage from the side of the spinal cord having less damage from the side with more damage. Finally, allodynia scores were compared versus total white matter damage in each division of the spinal cord and overall white matter damage at the lesion epicenter with a Spearman Rank Order Correlation.
Results
Part 1: Mechanical Sensitivity At Level Post Incomplete Laceration Injuries
Incomplete laceration injuries in 18 rats involving varying amounts of white and gray matter damage/sparing were used to test for a correlation between specific areas of damage and the presence/absence of allodynia. Fifteen of eighteen rats met our minimum sensitivity criteria (allodynia score greater than 0 on three of five weeks, at least two of which were on consecutive weeks) for stimuli applied to ’at level’ territories (T6-8). The average allodynia score for these 15 rats was 3.24 ± 0.52, with a range of 1.2 to 7.8. Five (33.3%) rats were consistently very sensitive to touch (≤ a force of 2g) and received additional points on the 10 point scale. The range of monofilament sizes inducing avoidance responses for these 5 rats was 0.008 – 0.6 g, with a median/mode of 0.008g, the smallest monofilament available. None of the rats had any pain-like behavioral responses to touch during baseline testing pre-injury.
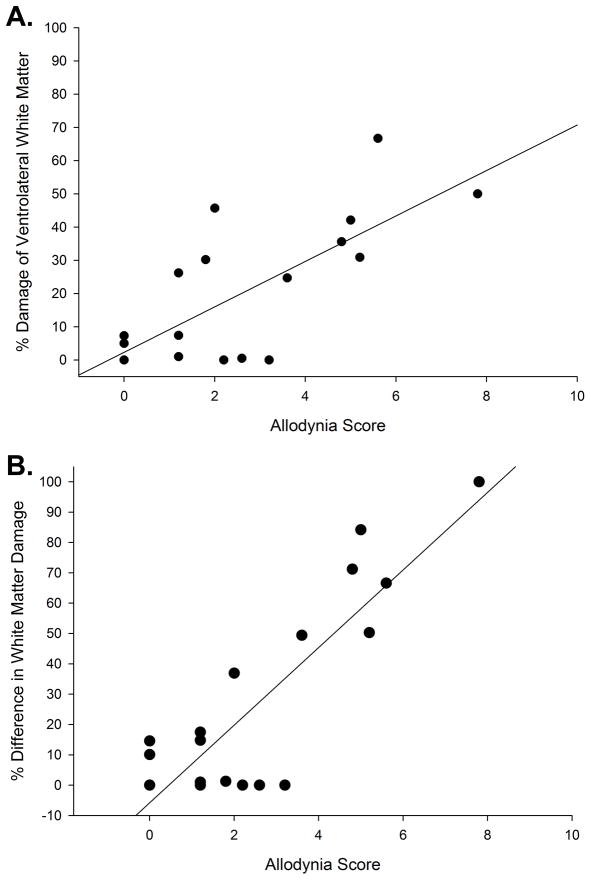
A comparison was first done for the allodynia scores versus the percentage of epicenter white matter damage in each of four divisions of the spinal cord (dorsal funiculus, dorsolateral funiculus, VLF, and the ventromedial funiculus – see Figure 2). A significant correlation was only found between the allodynia score and the percent white matter damage (average of the left and right side) for the VLF (rS= .582, p=.011). The positive correlation indicates that the greater the amount of VLF damage, the higher the allodynia score. A plot showing the data from each individual rat is provided in Figure 3A. The rS values (none of which are significant) for the comparison of allodynia scores vs. the percent white matter damage (average of the left and right side) of the dorsal columns, dorsolateral funiculus, and ventromedial funiculus are .0642, – .373, and .365 respectively (none are significant, p>.05).
Figure 3.
A - Graph showing a positive correlation (rS= .582, p=.011) between the allodynia score of the incomplete laceration animals and the average (left and right side) damage within the ventrolateral funiculus. B - Graph showing a positive correlation (rS = .619, p<.01) between the allodynia score of the incomplete laceration animals and the percent difference of white matter damage from side of greater damage to side of lesser damage in the ventrolateral funiculus.
Upon examination of the VLF at the lesion epicenter in the allodynic vs non-allodynic rats, it was apparent that there were side-to-side differences in damage/sparing in the allodynic rats. To address the potential significance of having an asymmetrical lesion, the rats were divided for initial analyses into two groups based on whether they met our response criteria for only the touch evoked agitation (assessment method taking into account only the responses to the 17g force brush stimulus). Five of 18 rats met the brush criteria (note in Figure 3A the five data points falling above the mean allodynia score of 4). A highly significant difference in VLF asymmetry was found between the SCI rats responding to brush (74.5% ± 8.4% side-to-side difference in VLF damage) versus those not responding to brush (11.3% ± 4.4% difference in VLF damage) (t test, p<.001).
In order to see if there was a relationship between extent of asymmetry and the level of sensitivity to stimulation, allodynia scores that account for the extent of hypersensitivity over a wide range of forces were compared to the percent difference of white matter damage (side of greater damage to side of lesser damage) for all four regions (per Figure 2). A graphic representation of the data for the % difference in VLF, the only white matter region where a significant correlation was found (rS = .619, p<.01), is presented in Figure 3B. The positive correlation indicates that the more asymmetry in the amount of VLF damage, the higher the allodynia score. Representative examples of the lesion epicenters are provided in Figure 4.
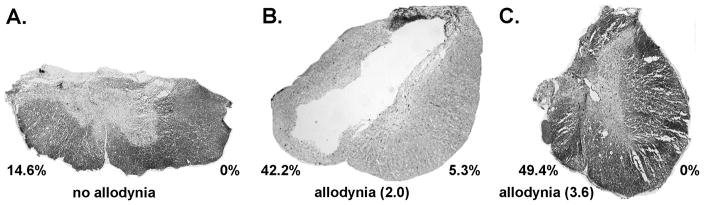
Figure 4.
Photomicrographs at the epicenter of spinal cord incomplete laceration injuries from non-allodynic animals and allodynic animals. Note the differences in damage/sparing within the ventrolateral funiculus in example A versus examples in B and C. The percentage values indicate the amount of area 3 damage (re Figure 1) on each side of the spinal cord. Magnification factor of 40X.
There were no significant correlations found for the allodynia scores vs. the percent difference of white matter damage from side of greater damage to side of lesser damage in the dorsal columns, dorsolateral funiculus, and ventromedial funiculus (.159, −.199, and .246 respectively). Studies of peripheral nerve injury, however, have implicated the dorsal columns as the projection that conveys afferent signals mediating tactile allodynia 52. Only 4 of the 15 allodynic rats in the present study had sparing (26.2% ± 5.8%) within the dorsal funiculus (area 1 in Figure 2). The mean allodynia score did not differ significantly (ANOVA, p>.05) between those rats with some dorsal column sparing and those without (3.2 ± 0.7 vs. 3.3 ± 0.7, respectively).
There were no significant differences between trunk allodynia scores obtained from the side of greater VLF damage versus the side having less VLF damage at the lesion epicenter. In fact, for the rats having the greatest % difference (>50%; n=5), the scores for the two sides of the trunk at level were identical (average allodynia score was 4.1). The similarities for allodynia scores for the left and right sides of the trunk despite the anatomical differences in the VLF implicate the involvement of supraspinal influences. Note that there were no animals with unilateral at level allodynia over the six week post-injury test period.
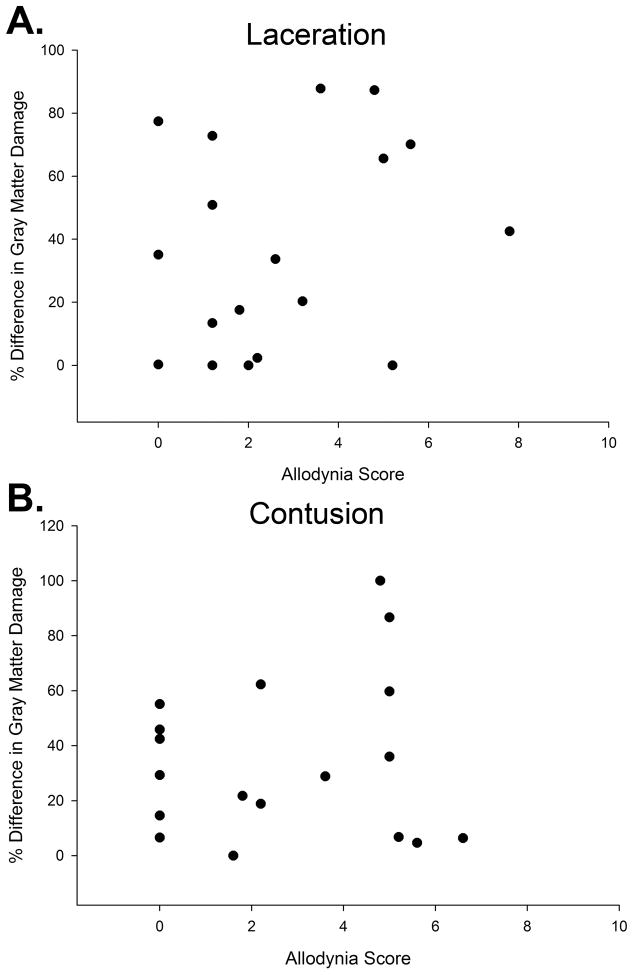
With regards to gray matter damage, no significant correlations were found between allodynia scores and either total gray matter damage/sparing (rS = .171, p>.05), percent difference in total gray matter damage between the left and right sides (rS = −.092, p> .05), and percent difference in ventral gray matter damage (looked at separately since tissue is adjacent to VLF) between the left and right sides (rS = .152, p>.05). The individual data for the total gray matter damage between the left and right sides is provided in Figure 5A.
Figure 5.
Graph showing no correlation between the allodynia score and the percent difference of gray matter damage from side of greater damage to side of lesser damage for the (A) laceration (rS = −.092, p> .05) and (B) contused (rS = −0.138, p>.05) groups of animals.
Part 2: Mechanical Sensitivity At Level Post Contusion
The relevance of asymmetry in the amount of VLF damage on our behavioral outcome measure of allodynia at level was further evaluated by assessing tissues obtained from 18 male rats that had received contusions as part of a different study (using all male rats tested during the same period of time as those examined in Part 1). Allodynia scores were obtained from experimental data records of 18 rats. Twelve of the 18 (67%) met the response criteria for avoidance responses at level. This proportion is consistent with previous results in our lab which show approximately 69% of contused rats develop allodynia in the weeks following SCI 21, 24. Of the 12 animals showing consistent avoidance responses to touch and/or gentle squeeze/pressure, the range of allodynia scores was 1.6 to 6.6 (out of 10), with a mean score of 4.1 ± 0.4. Seven (58.3%) rats were consistently very sensitive to touch (≤ a force of 2g) and received additional points on the 10 point allodynia scale. The range of monofilament sizes inducing avoidance responses for these 7rats was 0.008 – 2.0 g, with a median/mode of 0.04g. None of the rats had any pain-like behavioral responses to touch during baseline testing pre-injury.
At the lesion epicenters of rats receiving contusion injuries, there was very little, if any, gray matter sparing. Only three of the 18 rats had any gray matter sparing (some superficial dorsal horn is spared bilaterally). Analyses of gray matter side to side differences at 1 mm rostral to the epicenter (where there is partial sparing) revealed no significant correlations with allodynia scores. The data for percent difference in gray matter damage is provided in Figure 5B (rS = −0.138, p>.05).
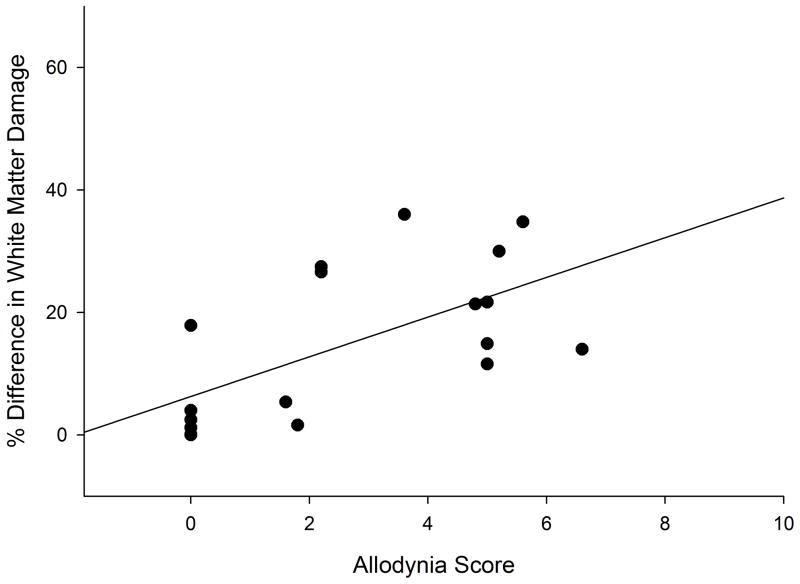
As with the select laceration injuries, a significant positive correlation (rS = 0.660, p<.01) was found between the histological and behavioral measures (Figure 6), which again indicates that the more asymmetry in the amount of VLF damage, the higher the allodynia score. Note that regardless of the allodynia score, there was a significant difference in the percent difference in VLF damage between those rats that had at level allodynia (27.4 ± 5.3%; n=27) and those rats that didn’t (5.6 ± 2.3%, n=9) (p=.021; values for laceration and contused rats combined).
Figure 6.
Graph showing a positive correlation (rS = 0.660, p<.01) between the allodynia score of the contused animals and the percent difference of white matter damage from side of greater damage to side of lesser damage in the ventrolateral funiculus.
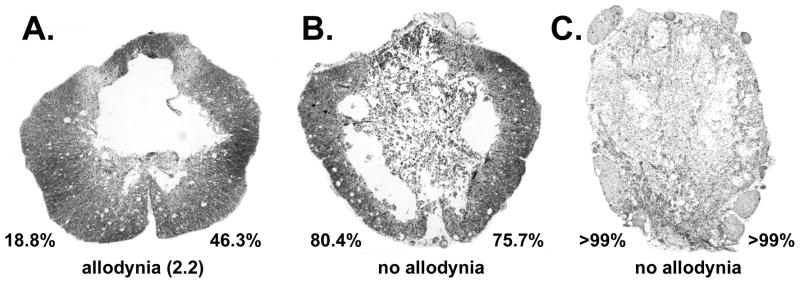
For the rats receiving contusion injuries, there was an average impact force of 225 ± 4.7 kilodynes with a mean spinal cord displacement of 1.23 ± 0.06 mm for the 12 spinal cord contusion rats showing behavioral signs of allodynia. The six non-allodynic animals had an average injury force of 227 ± 5.1 kilodynes with a spinal cord displacement of 1.21 ± 0.06 mm. No significant differences were found (t-test; p > .05) between the allodynic and non-allodynic animals’ values for either the impactor force or displacement measurements. Likewise, no significant differences were found for overall percent white matter sparing (14.2% ± 3.6%; n=18) at the injury epicenter. Representative photomicrographs of contused spinal cords taken at the lesion epicenter from animals with and without allodynia are presented in Figure 7.
Figure 7.
Photomicrographs taken at the epicenter of spinal cord contusion injuries from allodynic and non-allodynic animals. The percentage values indicate the amount of area 3 damage (re Figure 1) on each side of the spinal cord. Magnification factor of 40X.
Discussion
The results of the present study suggest that different extents of damage/sparing between the two sides of the VLF may be a requisite for the development of allodynia after SCI. Knowledge of the location and extent of tissue damage that underlies the development and perpetuation of at level allodynia will lead to a more consistent and reliable model for assessing both the mechanisms underlying central neuropathic pain as well as the effectiveness of therapeutic interventions.
VLF Damage
Histological analyses of the lesions in combination with the behavioral outcome measures indicate that asymmetrical damage to the VLF white matter but not the gray matter is likely an important determinant for the development of at level allodynia; i.e., some degree of sparing on one side of the VLF is necessary. Support comes from (1) the absence of allodynia following very severe (but anatomically discomplete) injury bilaterally post contusion (as shown in Figure 7C), (2) the absence of allodynia following anatomically complete spinal transection (see 25), (3) the absence of allodynia in rats with partial but symmetrical VLF sparing (as shown in Figure 7B), (4) a lack of a correlation between the presence of allodynia and the degree of gray matter sparing, and (5) the rat with the greatest extent of asymmetry had the highest allodynia score, a finding consistent with studies that use unilateral lesions to model central pain syndrome55.
In SCI patients, the presence of residual spinothalamic pathways in the VLF, assessed by topical application to distal skin areas below level of chemicals known to activate small diameter afferents, distinguished individuals with versus without pain 56. In addition, in a diffusion tensor imaging and tractography study of patients with syringomyelia (a SCI in which allodynia is one of the defining symptoms), individuals with very severe spinothalamic tract lesions (bilateral) did not develop central neuropathic pain 18. Furthermore, quantitative sensory testing of SCI patients indicate that individuals with below level pain have at level pain more frequently 14, suggesting that complete damage of the spinothalamic pathway is not a requisite for segmental pain.
Spinothalamic but not medial lemniscal pathways have been associated with the development of central pain, based on studies of patients with central post-stroke pain and syringomyelia 3, 18. Spinothalamic tract axons terminate in one of three thalamic nuclei: the ventral posterolateral thalamic nucleus, the centrolateral thalamic nucleus 8, or the posterior thalamic nuclear group 8, 44. The thalamus of SCI patients experiencing segmental pain has been explored during stereotaxic procedures for treatment of pain. All patients exhibit abnormal bursting activity in thalamic regions where stimulation evokes pain 35. These findings in addition to alterations in the somatotopic organization 35 indicate that major reorganization of inputs has occurred supraspinally, making the thalamus an important target for studies on SCI pain. More recently, MR spectroscopy has been used in SCI individuals to demonstrate alterations of metabolites in thalamic nuclei in male SCI patients with versus without chronic pain 43.
In previous electrophysiological recordings, the occurrence of hypersensitivity of at level inputs to somatovisceral convergent neurons with large (often whole body) receptive fields in the ventroposterolateral and posterior nuclei was demonstrated in allodynic but not non-allodynic SCI rats 24. Specifically, these convergent neurons that normally responded to only noxious levels of stimulation23, including the trunk, respond to touch in at level dermatomes in only the allodynic rats. This shift in the responses of a specific sub-group of thalamic neurons (those likely contributing to affective/motivational aspects of pain perception) is different from the classical contralateral small receptive field type of ventroposterolateral nucleus type neuron (sensory-discriminitive type of input via the dorsal column-medial lemniscal pathway). An electrophysiological study using brush as the search stimulus 16 reported that although the evoked firing of ventroposterolateral neurons differed between allodynic rats (defined by vocalization only) and uninjured controls, the firing rate did not differ from SCI non-allodynic rats. However, their recordings were done at seven days post-SCI, and it is well established by several groups, including our own, that at level neuropathic pain is not evident for several weeks post-SCI 28.
Note that although the spinothalamic tract is a major pathway within the VLF for this at level input to reach the brain for higher order processing, it is not the only pathway. Other neurons located in lamina I of the spinal gray matter send signals from mechanical and thermal inputs to spinal, bulbar, and telencephalic regions associated with autonomic, emotional, and somatosensory processing 54. Also, deep dorsal horn neurons from laminae V–VII send signals from the dorsal horn to the cervical spinal cord, medullary reticular formation, and to parts of the parabrachial area in the mesencephalon. Neurons running from the dorsal horn to these areas of the spinal cord and brain comprise part of the spinocervicothalamic, spinoreticular, and spinomesencephalic tracts respectively 54. With a myriad of possibilities leading from stimulus to perception, it is understandable that the nervous system is dynamic and ever-changing rather than static and inflexible 2.
Potential Central Effects of Asymmetrical VLF Damage
The results of the present study indicate that the asymmetry of the lesion is a likely requisite for the cellular changes in the thalamus in response to SCI and the increased neuronal excitability and behavioral signs of allodynia that ensue 16, 24, 64, but the mechanisms through which this occurs are not known. The injury-induced changes involving a variety of thalamic subnuclei is consistent with models for mononeuropathy in which neuropathic-like syndromes persist with very select thalamic lesions in the lateral thalamus 47.
One possible mechanism for the manifestation of nociceptive behaviors in rats having asymmetrical VLF lesions is a disruption in the balance of the bilateral inputs to somatovisceral convergent thalamic neurons that normally receive widespread (often whole body) inputs involved in nociceptive processing. Disrupting the laterality and not just a partial loss of inputs may be an important trigger necessary to precipitate a local cascade of cellular events leading to bursting and ultimately to altered responsiveness of the spared inputs (i.e. dorsal trunk) and subsequent to that the eventual behavioral output (pain-like behaviors to touch). For example, in a study using a unilateral lesion centered on the spinothalamic tract at the T8/9 vertebral level55, a selective T-type calcium channel blocker ethosuximide abolished bursting in thalamic brain slices (VPL) obtained from the lesion rats (no effect on lamina III–V spinal cord slices), and when given to lesion rats i.p., alleviated hyperexcitability of the hind and forepaw (withdrawal reflex).
An alternate explanation regarding the relationship between asymmetrical damage, neuronal hyper-excitability and allodynia is a disruption in the balance of primary afferent inputs to spinothalamic dorsal horn neurons in the segments immediately rostral to the lesion epicenter. Primary axons conveying nociceptive inputs will ascend and descend up to several segments upon entry to the CNS. Thus, an asymmetrical central lesion may also produce an imbalance of inputs to the dorsal horn (as with the direct projections to the thalamus), which likewise would precipitate a cascade of cellular events (changes in sodium channel expression - 17; persistent activation of intracellular signaling kinases - 28 ) leading to altered responsiveness to spared inputs. This explanation is less likely given that damage to the dorsal cord for the contused group (1.2 mm impactor displacement) is similar in allodynic and non-allodynic rats.
In terms of damage to the gray matter, there were no correlations found between the presence/absence of allodynia at level and overall extent of gray matter damage/sparing. Alternatively, it is possible that there is an association between the presence/absence of at level allodynia and damage to specific laminae, as shown for excessive grooming behavior (self-directed biting and scratching) following select chemical lesions with quisqualic acid, an AMPA-metabotropic recptor agonist which produces an excitotoxic SCI (damage restricted to only the spinal gray matter) 1, 60, 61. However, unlike the select intraspinal injection technique, specific laminar damage/sparing assessments is not possible given the contusion and laceration types of lesions. Note, however, that no association was found between specific regions of the dorsal horn and mechanical or thermal hypersensitivity of the hind paw 60. It is possible though that nervous tissue damage can, for example, induce apoptosis of a select group of inhibitory GABAergic interneurons 40. Without these inhibitory interneurons, weak nociceptive signals that would normally be blocked in the spinal cord can be transmitted to higher centers in the brain. Note that the finding of no side-to-side differences in extent of gray matter damage does not preclude an alternate possibility that a combination of asymmetrical VLF and gray matter damage may be necessary. The results of an MRI study of SCI patients by Finnerup and colleagues suggest that some gray matter damage in addition to spinothalamic tract damage may be necessary for the development of central pain13.
Likewise, sensitization of dorsal horn neurons due to a loss of descending modulatory pathways from the rostral medulla 44 could not be accounted for by the histological findings as there were no side-to-side differences in white matter sparing within the dorsolateral funiculus (allodynic versus non-allodynic rats). It is important to note that damage to the VLF requires a contusion in the “severe” range, but this type of lesion also affects the dorsal and dorsolateral funiculus. Thus, whether damage to VLF alone would lead to at level allodynia is not known. Current studies are underway using well controlled chemical lesions to establish the location and extent of white matter asymmetrical damage that is necessary for allodynia to develop. These studies will also help distinguish the contribution of asymmetrical VLF versus primary axonal injury resulting from damage to the incoming dorsal roots.
Scoring of Pain-Like Behavioral Responses
In the current study, graded responses were used to evaluate the degree of at level mechanical allodynia. Some studies use Semmes-Weinstein monofilaments to measure at level allodynia, but only a single monofilament is used. In one study, a filament calibrated to generate a force of 2 g/cm² was used to stimulate dermatomes corresponding to the at level segments 42, while in another, a 5g filament size was used 7. In the present study, brush (equivalent to 17g stimulus) is first used to determine the presence or absence of allodynia. If an avoidance response is present, then the series of Semmes-Weinstein monofilaments is used to determine the threshold. Our allodynia scoring also reflects only higher order processing. Although many studies include responses indicating higher order processing to test for allodynia 6, 7, 49, 57, the response criteria in the present study was restricted to only evoked pain-like behaviors. Woolf 58 demonstrated that these evoked responses were lost in decerebrate rats, whereas responses that were reflexive in nature (involving regions below the level of the tectum) were still present. A previous animal study 33 that used von Frey hair testing on the hind paw after spinal cord contusion injury, for example, concluded that the chances of developing below level mechanical allodynia was significantly increased if less than 10% of spinal cord white matter remained at the lesion epicenter after spinal cord contusion injury. As the outcome measure was a reflex withdrawal, their results may reflect below level plasticity of the local circuitries that is induced following only a very severe level of injury.
The present study is also the first to demonstrate freezing in response to trunk stimulation following SCI. This observation is likely related to how we test the rat in the open cage. In other studies 9, 29, a girdle is used to hold the animals in place while testing. Under those experimental circumstances, the freezing response would not be noticed. During the freezing response, our animals displayed tightening of the skeletal musculature and stopping of all movement including movement of the whiskers and facial structures. One study examining the freezing or arresting response 57 found that decerebrate rats did not show a freezing response (characterized as a state of immobility for a short period in the midst of normal activities) where intact normal animals did show this response. Freezing is a behavior that has been implicated in studies centering on fear and stress 20, 51. Several studies 15, 20, 27, 51 have shown that various higher order structures related to the limbic system, such as the hippocampus, lateral septum, nucleus accumbens, and amygdala, are CNS regions involved in the processing of fear and stress. Thus, higher order processing is necessary to evoke the feelings of fear and stress which is considered part of the freezing response.
Perspective: A side-to-side lesion asymmetry following chronic spinal cord injury in a rodent model was found to be highly correlated with the presence and degree of allodynia. Greater insight of key factors contributing to the development and maintenance of chronic neuropathic pain is important for improving quality of life.
Acknowledgments
We wish to thank Christine Nunn and Dr. Y.P. Zhang for excellent technical assistance and Dr. Christopher B. Shields for use of his labs Vibraknife™ system. This study was supported by grant number RR015576 from the National Center for Research Resources (NCRR), a component of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham KE, McGinty JF, Brewer KL. Spinal and supraspinal changes in opioid mRNA expression are related to the onset of pain behaviors following excitotoxic spinal cord injury. Pain. 2001;90:181–190. doi: 10.1016/s0304-3959(00)00402-4. [DOI] [PubMed] [Google Scholar]
- 2.Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nat Med. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- 3.Boivie J. Hyperalgesia and allodynia in patients with CNS lesions. In: Willis WDJ, editor. Hyperalgesia and Allodynia. Raven Press, Ltd; New York: 1992. pp. 363–373. [Google Scholar]
- 4.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson F. Histotechnology: a self-instructional text. American Society of Clinical Pathologists; Chicago: 1990. [Google Scholar]
- 6.Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- 7.Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- 8.Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Davidoff G, Roth EJ. Clinical characteristics of central (dysesthetic) pain in spinal cord injury patients. In: Casey KL, editor. Pain and Central Nervous System Disease: The Central Pain Syndromes. Raven Press; New York: 1991. pp. 77–83. [Google Scholar]
- 11.Donovan WH, Dimitrijevic MR, Dahm L, Dimitrijevic M. Neurophysiological approaches to chronic pain following spinal cord injury. Paraplegia. 1982;20:135–146. doi: 10.1038/sc.1982.27. [DOI] [PubMed] [Google Scholar]
- 12.Eaton MJ. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J Neurotrauma. 2006;23:549–559. doi: 10.1089/neu.2006.23.549. [DOI] [PubMed] [Google Scholar]
- 13.Finnerup NB, Gyldensted C, Nielsen E, Kristensen AD, Bach FW, Jensen TS. MRI in chronic spinal cord injury patients with and without central pain. Neurology. 2003;61:1569–1575. doi: 10.1212/01.wnl.0000096016.29134.fa. [DOI] [PubMed] [Google Scholar]
- 14.Finnerup NB, Sorensen L, Biering-Sorensen F, Johannesen IL, Jensen TS. Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp Neurol. 2007;207:139–149. doi: 10.1016/j.expneurol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Garcia R, Tocco G, Baudry M, Thompson RF. Exposure to a conditioned aversive environment interferes with long-term potentiation induction in the fimbria-CA3 pathway. Neuroscience. 1998;82:139–145. doi: 10.1016/s0306-4522(97)00285-6. [DOI] [PubMed] [Google Scholar]
- 16.Gerke MB, Duggan AW, Xu L, Siddall PJ. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–722. doi: 10.1016/s0306-4522(02)00961-2. [DOI] [PubMed] [Google Scholar]
- 17.Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatem SM, Attal NM, Gautron M, Parker F, Plaghki L, Bouhassira D. Pathophysiology of central neuropathic pain: psychophysical and electrophysiological correlates in patients with syringomyelia. Society for Neuroscience Online; San Diego: 2007. [Google Scholar]
- 19.Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970–1984) J Neurosurg. 1986;64:543–553. doi: 10.3171/jns.1986.64.4.0543. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubscher CH, Johnson RD. Changes in neuronal receptive field characteristics in caudal brain stem following chronic spinal cord injury. J Neurotrauma. 1999;16:533–541. doi: 10.1089/neu.1999.16.533. [DOI] [PubMed] [Google Scholar]
- 22.Hubscher CH, Johnson RD. Differential effects of chronic spinal hemisection on somatic and visceral inputs to caudal brainstem. Brain Res. 2002;947:234–242. doi: 10.1016/s0006-8993(02)02930-x. [DOI] [PubMed] [Google Scholar]
- 23.Hubscher CH, Johnson RD. Responses of thalamic neurons to input from the male genitalia. J Neurophysiol. 2003;89:2–11. doi: 10.1152/jn.00294.2002. [DOI] [PubMed] [Google Scholar]
- 24.Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol. 2006;197:177–188. doi: 10.1016/j.expneurol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Hubscher CH, Kaddumi EG, Johnson RD. Segmental neuropathic pain does not develop in male rats with complete spinal transections. J Neurotrauma. 2008;25:1241–1245. doi: 10.1089/neu.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubscher CH, Petruska JC, Rau KK, Johnson RD. Co-expression of P2X receptor subunits on rat nodose neurons that bind the isolectin GS-I-B4. Neuroreport. 2001;12:2995–2997. doi: 10.1097/00001756-200109170-00048. [DOI] [PubMed] [Google Scholar]
- 27.Hugues S, Kessal K, Hunt MJ, Garcia R. A conditioned stressful environment causes short-term metaplastic-like changes in the rat nucleus accumbens. J Neurophysiol. 2003;90:3224–3231. doi: 10.1152/jn.00895.2002. [DOI] [PubMed] [Google Scholar]
- 28.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- 30.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keizer D, van Wijhe M, Post WJ, Uges DR, Wierda JM. Assessment of the clinical relevance of quantitative sensory testing with Von Frey monofilaments in patients with allodynia and neuropathic pain. A pilot study. Eur J Anaesthesiol. 2007;24:658–663. doi: 10.1017/S0265021507000221. [DOI] [PubMed] [Google Scholar]
- 32.Keizer D, van Wijhe M, Post WJ, Wierda JM. Quantifying allodynia in patients suffering from unilateral neuropathic pain using von frey monofilaments. Clin J Pain. 2007;23:85–90. doi: 10.1097/01.ajp.0000210950.01503.72. [DOI] [PubMed] [Google Scholar]
- 33.Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol. 1994;72:1570–1587. doi: 10.1152/jn.1994.72.4.1570. [DOI] [PubMed] [Google Scholar]
- 36.Luna L. Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw Hill; New York: 1968. [Google Scholar]
- 37.Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:6S–18S. doi: 10.1016/s0002-9343(96)00133-7. [DOI] [PubMed] [Google Scholar]
- 38.Melzack R, Wall PD, Ty TC. Acute pain in an emergency clinic: latency of onset and descriptor patterns related to different injuries. Pain. 1982;14:33–43. doi: 10.1016/0304-3959(82)90078-1. [DOI] [PubMed] [Google Scholar]
- 39.Merskey H, Bogduk N. Classification of chronic pain. IASP Press; Seattle: 1994. [Google Scholar]
- 40.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niv D, Devor M. Refractory neuropathic pain: the nature and extent of the problem. Pain Pract. 2006;6:3–9. doi: 10.1111/j.1533-2500.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 42.Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110:259–268. doi: 10.1016/j.pain.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 43.Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- 44.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed WR, Chadha HK, Hubscher CH. Effects of 17{beta}-Estradiol on Responses of Viscerosomatic Convergent Thalamic Neurons in the Ovariectomized Female Rat. J Neurophysiol. 2009;102:1062–1074. doi: 10.1152/jn.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson RR, Meyer PR, Cerullo LJ. Neurostimulation in the modulation of intractable paraplegic and traumatic neuroma pains. Pain. 1980;8:75–84. doi: 10.1016/0304-3959(80)90091-3. [DOI] [PubMed] [Google Scholar]
- 47.Saade NE, Al Amin H, Abdel Baki S, Safieh-Garabedian B, Atweh SF, Jabbur SJ. Transient attenuation of neuropathic manifestations in rats following lesion or reversible block of the lateral thalamic somatosensory nuclei. Exp Neurol. 2006;197:157–166. doi: 10.1016/j.expneurol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 49.Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport. 1995;6:1241–1244. doi: 10.1097/00001756-199506090-00003. [DOI] [PubMed] [Google Scholar]
- 50.Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- 51.Spennato G, Zerbib C, Mondadori C, Garcia R. Fluoxetine protects hippocampal plasticity during conditioned fear stress and prevents fear learning potentiation. Psychopharmacology (Berl) 2008;196:583–589. doi: 10.1007/s00213-007-0993-7. [DOI] [PubMed] [Google Scholar]
- 52.Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- 53.Sved P, Siddall PJ, McClelland J, Cousins MJ. Relationship between surgery and pain following spinal cord injury. Spinal Cord. 1997;35:526–530. doi: 10.1038/sj.sc.3100443. [DOI] [PubMed] [Google Scholar]
- 54.Villanueva L, Nathan PW. Multiple pain pathways. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Proceedings of the 9th World Congress on Pain. IASP Press; Seattle: 2000. pp. 371–386. [Google Scholar]
- 55.Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci. 2008;28:11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–2400. doi: 10.1093/brain/awn169. [DOI] [PubMed] [Google Scholar]
- 57.Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- 58.Woolf CJ, Swett JE. The cutaneous contribution to the hamstring flexor reflex in the rat: an electrophysiological and anatomical study. Brain Res. 1984;303:299–312. doi: 10.1016/0006-8993(84)91216-2. [DOI] [PubMed] [Google Scholar]
- 59.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 60.Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 61.Yu CG, Yezierski RP. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Brain Res Mol Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Xie W, Xie Y. Spinal cord injury triggers sensitization of wide dynamic range dorsal horn neurons in segments rostral to the injury. Brain Res. 2005;1055:103–110. doi: 10.1016/j.brainres.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 63.Zhang YP, Iannotti C, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural closure, cord approximation, and clot removal: enhancement of tissue sparing in a novel laceration spinal cord injury model. J Neurosurg. 2004;100:343–352. doi: 10.3171/spi.2004.100.4.0343. [DOI] [PubMed] [Google Scholar]
- 64.Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]