Abstract
The rabbit large clot embolic stroke model has been used for over 23 years to study methods to manipulate hemorrhage and to test drugs and devices for safety, because the rabbit model is particularly sensitive to embolism-induced hemorrhage. This study refined the original embolization procedure using an automated, pump-assisted injection method to introduce large blood clots or macroscopic emboli into the middle cerebral artery (MCA) via an indwelling carotid artery catheter. The study shows that rapid injection of blood clots (3ml/30 seconds) produced a model where there is a high hemorrhage incidence rate (79%) and a high stroke success rate (63%), compared to a low stroke success rate (19%) with no hemorrhages when clots were injected at a slow rate (3ml/90 seconds). The rapid injection method, which produces a high hemorrhage rate is particularly useful to study neuroprotective agents to attenuate embolism-induced hemorrhage. In addition, we show that manual injection of blood clots, which produces a lower baseline hemorrhage rate (41%) with a similar stroke success rate (65%), may allow investigators to study pharmacological agents to either up or down-regulate hemorrhage incidence. Lastly, we show that in the rabbit embolic stroke model, hemorrhages are adjacent to areas of 2,3,5-triphenyltetrazolium (TTC)-negative tissue, normally associated with infarcted or ischemic tissue. Thus, there is clear separation of ischemia and hemorrhage in the model, suggesting that therapeutics that are neuroprotective may also be useful to limit the evolution of ischemic damage associated with a hemorrhage, if not attenuate hemorrhage itself.
Keywords: Mechanism, Hemorrhage, Survival, Translational Science, Infarct Zones, Histochemistry
1. Introduction
Recently, there has been some progress in the translational development of hemorrhage therapy that successfully reduces the escalating morbidity and mortality rate associated with brain bleeding (Lapchak, 2002; Lapchak and Araujo, 2007). Perseverance and applied translational drug development using a variety of animal models representative of the human condition will be required for additional success (Lapchak, 2010). Rosenberg and colleagues have made substantial advancements in the understanding of cellular mechanisms involved in intracerebral hemorrhage (ICH) damage and blood brain barrier (BBB) breakdown using a rodent model that relies on altering the cellular matrix by introducing collagenase intracerebrally (Mun-Bryce and Rosenberg, 1998; Rosenberg, 1995; Rosenberg and Navratil, 1997). Their studies, like that of Lapchak and colleagues have shown that matrix metalloproteinases(MMP) are involved in ICH and tissue plasminogen activator (tPA)-induced ICH(Lapchak et al., 2000; Rosenberg, 1995; Rosenberg and Navratil, 1997; Rosenberg et al., 2001) and that MMP inhibitors can attenuate hemorrhage damage(Lapchak et al., 2000; Rosenberg and Navratil, 1997). Other investigator have also shown that MMP's are involved in ICH and a variety of MMP inhibitors may be useful to reduce ICH(Asahi et al., 2000; Romanic et al., 1998; Sumii and Lo, 2002; Wang and Tsirka, 2005; Zhao et al., 2007). Moreover, both Rosenberg's and Lapchak's research(Lapchak, 2007; Rosenberg et al., 1995) have found that tumor necrosis factor-α is involved in BBB breakdown and ICH, an affect that may involve MMP's. Additional research teams have shown that oxidative stress may be involved in ICH using the collagenase model(Lekic et al., 2010) and embolic stroke model(Hu et al., 1999; Lapchak and Araujo, 2001; Lapchak et al., 2001; Lapchak et al., 2002a). Thus, there are some common mechanisms involved in hemorrhage damage pathways induced by enzyme injection or embolus introduction.
The rabbit large clot embolic stroke model(RLCEM), which is the model being refined in the current study, was originally developed by Lyden and colleagues(Lyden et al., 1987) to study the effects of anticoagulants on ICH. Since 1987, the model has remained relatively intact with only minor technical modifications. However, a survey of the literature related to the model shows that the baseline hemorrhage rate (i.e incidence) achieved in each independently controlled study varies dramatically, making pharmacological studies aimed at reducing ICH or tPA-induced ICH challenging. For instance, in the original Lyden paper(Lyden et al., 1987), the baseline hemorrhage rate was 25% in the study control group. However, in subsequent studies, the baseline hemorrhage rate varied from 22-52%(Chapman et al., 2001; Lapchak et al., 2000; Lapchak, 2007; Lapchak et al., 2008) and tissue plasminogen activator(tPA) -induced hemorrhage rate varied from 60% to 87%(Chapman et al., 2001; Lapchak et al., 2000; Lapchak, 2007; Lapchak et al., 2008). The reason for the variability in both baseline and tPA-induced hemorrhage rate is unknown, but may be related to the manner and or rate in which the emboli is manually injected through the indwelling carotid catheter by each investigative team.
To refine the model and further characterize the hemorrhage model, we have modified the way in which emboli were injected into brain. For this study, we directly compared manual embolization to pump-assisted embolization to determine the best method to produce reproducible hemorrhages in rabbits so that the model can further be used to develop novel treatment to reduce the deleterious sequelae of brain hemorrhage. In addition, we have used TTC staining of a cohort of embolized rabbits to show areas of ischemia surrounding hemorrhage, which are independent of other infarct or ischemic zones. The implication of the finding will be discussed in the context of developing new neuroprotective drugs to attenuate ischemia and hemorrhage.
2. Results
2.1 Stroke Success Rate
The behavioral manifestations of embolization included nystagmus, head lean, hemiparesis, vocalization, seizures and /or uncoordinated jerking movements. There was a positive correlation between the appearance of abnormal behaviors after embolization and stroke success rate. As shown in Table 1, we measured the behavioral reaction to embolization in all groups studies and determined if there was a correlation between the behavioral response and stroke success rate defined by the presence of greater than 10% in brain of the total 57Co amount administered during embolization. Even though we monitored the behavioral reaction to the embolus and this is shown in the Table, the strict quantitative criteria for inclusion were based on the presence of 57Co-label in brain.
Table 1.
Effect of Embolization on Behavioral Response Rate
| Measure |
Manual 3 ml/ 45 seconds |
Auto 1 3ml/ 90 seconds |
Auto 2 3 ml/ 45 seconds |
Auto 3 3 ml/ 30 seconds |
|---|---|---|---|---|
| Behavioral Response Rate | 23/26 (89%) | 14/21 (66%) P=0.119 |
19/22 (86%) P=0.900 |
14/22 (77%) P=0.468 |
| Stroke Success Rate | 17/26 (65%) | 4/21 (19%)* P=0.004 |
7/22 (31%)* P=0.039 |
14/22 (63%) P=0.874 |
| Ratio Stroke Success Rate/ Behavioral Response Rate | 73.1% | 28.78% | 36.0% | 81.2% |
| Co57 Recovery | 53.88 ± 8.39% | 35.76 ± 12.49% p>0.05 |
54.06 ± 13.79% p>0.05 |
45.25 ± 6.68% p>0.05 |
| Hemorrhage Rate | 7/17 (41%) | 0/4 (0%)* P<0.0001 |
3/7 (43%) P=0.71 |
11/14 (79%) P=0.077 |
| Ratio Hemorrhage Rate/ Stroke Success Rate | 63% | 0% | 139% | 125% |
Significance compared to Manual injection method
For manual injection, the behavioral rate was 89% whereas the stroke success rate was 65%, and 53.88 ± 8.39% of radiolabel was recovered in brain. There was a good correlation between the behavioral response rate and stroke success rate for manual embolization. Moreover, for manual injection the ratio of Stroke Success Rate /Behavioral Response Rate was 73.1%
However, for pump-assisted embolization, the behavioral response rate varied between 66 and 86% with 35.76 ± 12.49% to 54.06 ± 13.79% of label recovered in the brain of those rabbits included in the study (Table 1). Thus, there is a positive correlation between radiolabel measured in brain and “stroke success rate”, but the initial behavioral response to the introduction of saline and blood clots through the carotid catheter cannot be used as a reliable measure of successful embolization, since clots injected using the 3 ml/45-90 seconds method overestimated actual stroke success defined by other criteria.
Table 2 documents the 24 and 48 hour survival rate in the 4 different embolization groups. There are no statistically significant effects of embolization method on survival rate.
Table 2.
Effect of Embolization on Survival Rate
| Survival Time |
Manual 3 ml/ 45 seconds |
Auto 1 3ml/ 90 seconds |
Auto 2 3 ml/ 45 seconds |
Auto 3 3 ml/ 30 seconds |
|---|---|---|---|---|
| 24 hour | 10/17 (58.82%) | 2/4 (50%) p>0.05 |
5/7 (71.43%) p>0.05 |
9/14 (64.29%) p>0.05 |
| 48 hour | 8/17 (47.06%) | 2/4 (50%) p>0.05 |
5/7 (71.43%) p>0.05 |
8/14 (57.14%) p>0.05 |
Significance compared to Manual injection method
2.2 Characterization of Hemorrhage & Ischemic Infarcts
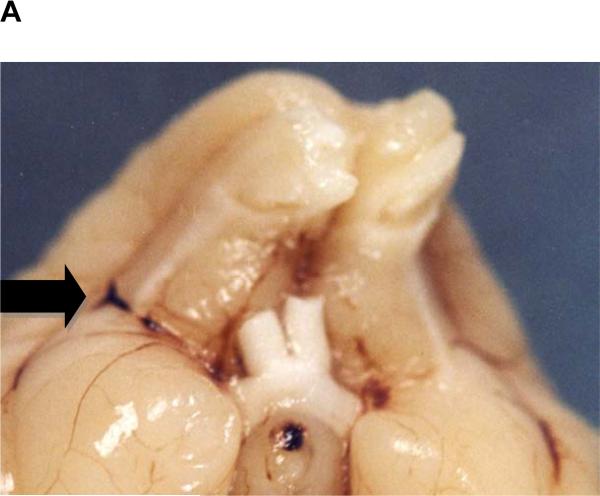
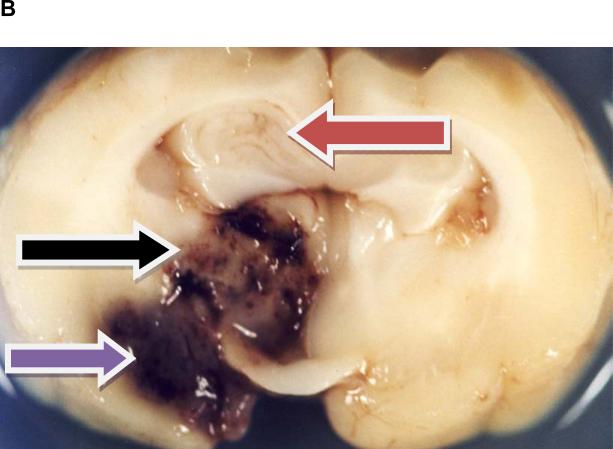
As shown in Figure 1A, large clot embolization via an indwelling catheter results in occlusion of the middle cerebral artery(MCA). In the RLCEM, we have previously shown that the rabbit is “sensitive” to the introduction of an emboli into the MCA resulting in ischemic cores in numerous brain areas (Lapchak et al., 2000; Lapchak et al., 2008). Moreover, in this study, in the manual injection group, 41% of rabbits with successful strokes also had an intracerebral hemorrhage (ICH) classified into one of the following categories: (1) parenchymal hemorrhage, (2) hemorrhagic infarction, (3) punctate hemorrhage and (4) subarachnoid hemorrhage. Figure 1B provides a representative panel to show 3 of the 4 identified types of hemorrhage in rabbit brain. Subarachnoid hemorrhage is not shown since it only occurred in 1 rabbit with a single incidence. The panel also shows ischemia-induced edema in the hippocampus, since there is significant distortion and swelling of the tissue.
Figure 1.
A) An Embolus Located in the Branching of the Middle Cerebral Artery
B) Representative Types of Tissue Damage Following Embolization
A) Panel shows occlusion of middle cerebral artery with blood clot following embolization.; B) Panel shows multiple types of damage following embolizaton. Red arrow points to edema and an ischemic infarct in the hippocampus. Also, note the presence of multiple punctate hemorrhages (HPT). Black arrow points to a Hemorrhagic infarction (HIN) in the midbrain and the Purple arrow points to a Parenchymal hemorrhage (PH) in the ventral cortex.
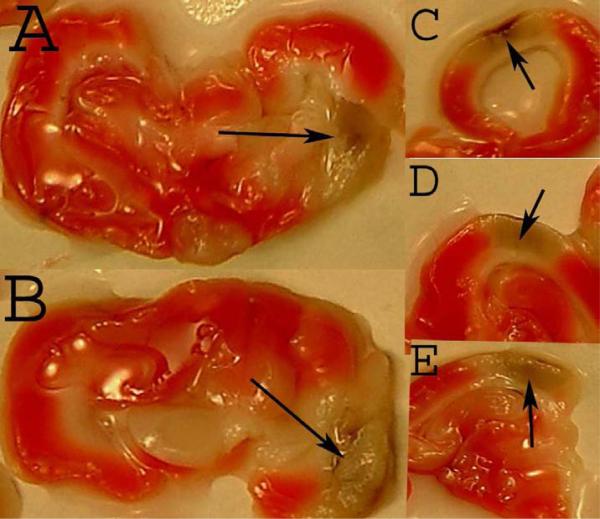
In Figure 1 B, the black arrow is indicating an area with a hemorrhagic infarction, that is a hemorrhage within an ischemic area. However, because of the texture of the tissue and the coloration, it is not readily apparent that the tissue is ischemic, even though the tissue color and texture are not normal. In order to allow for adequate contrast, we used TTC staining of fresh tissues removed from embolized rabbits to determine the staining pattern for living (TTC-positive) and dead (TTC-negative) tissue (due to an infarct) and the presence of hemorrhage in the brain. Figure 2 provides TTC staining evidence for the colocalization of an area of ischemic tissue (TTC-negative) surrounding a parenchymal hemorrhage or hemorrhagic infarction following embolization. This is the first demonstration of TTC visualization of ischemic tissue surrounding a hemorrhage using the rabbit embolic stroke model.
Figure 2. TTC staining of Rabbit Tissue with an ICH.
The panel from 5 different embolized rabbits shows viable living tissue stained by TTC (TTC-positive) and is represented by a pink/red hue. The brown/grey areas within the white/off-white ischemic areas (TTC-negative) in the cortex are hemorrhages, either parenchymal or hemorrhagic infarctions. Note the hemorrhage in the ischemic zone as identified with an arrowhead. Panel C-E show cortical hemorrhages in the midst of an infarct.
2.3 Hemorrhage Rate & Volume Following Embolization
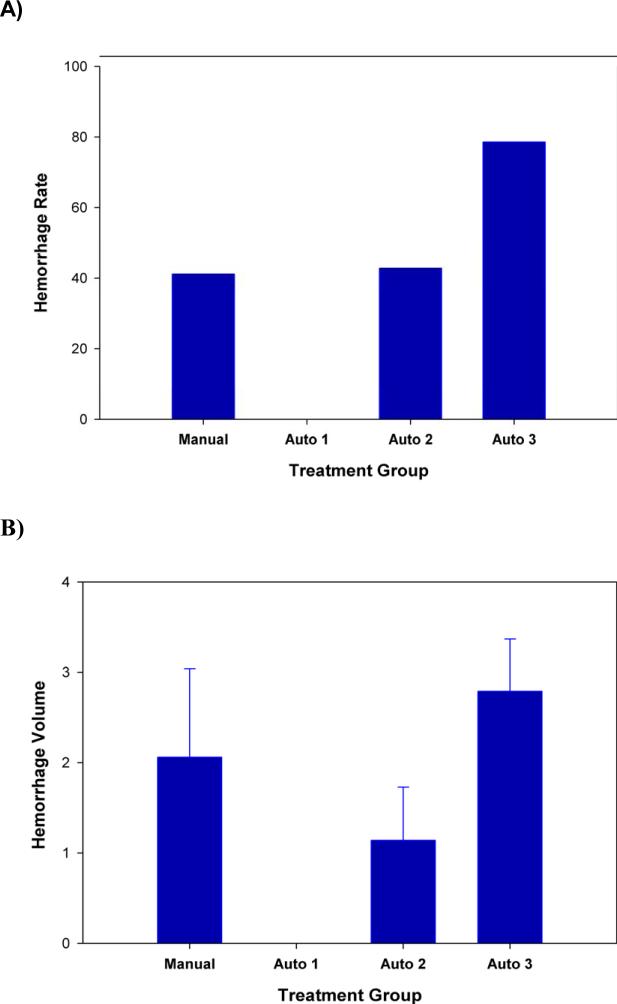
As described above, the hemorrhage rate in the manual embolization group was 41% with a hemorrhage volume of 2.06±0.98 faces. We compared the hemorrhage rate in pump-assisted embolized rabbits to the baseline achieved in manually embolized rabbits. Automated injection at 3ml/90 seconds did not result in hemorrhage (0%). However, as the injection rate was increased to 3 ml/45 seconds and 3 ml/30 seconds, the hemorrhage rate increased to 43% and 79%, respectively. In addition, the hemorrhage volume in the two groups were 1.14±0.59 and 2.79±0.58 faces, respectively.
2.4 Hemorrhage Subtypes
Table 3 shows the types of hemorrhage present in each of the experimental groups. Some of the animals had more than 1 type of hemorrhage present in the brain. For quantitative purposes, we treated each individual hemorrhage observed as a separate entity. Hemorrhages occurred throughout the brain and included the following structures: caudate putamen; thalamus; hippocampus; frontal, parietal, and occipital cortex; hypothalamus; suprachiasmatic area; cerebellum; pons; and midbrain. There were no apparent differences among the groups in the distribution of types or locations of hemorrhages.
Table 3.
Effect of Embolization on Hemorrhage Subtypes in Brain
| Hemorrhage Type |
Manual 3 ml/ 45 seconds |
Auto 1 3ml/ 90 seconds |
Auto 2 3 ml/ 45 seconds |
Auto 3 3 ml/ 30 seconds |
|---|---|---|---|---|
| Parenchymal | 7 | 0 | 1 | 4 |
| Hemorrhagic Infarction | 4 | 0 | 2 | 10 |
| Punctate Hemorrhage | 0 | 0 | 0 | 4 |
| Total ICH | 11 | 0 | 3 | 18 |
Note- In some cases, more than 1 type of hemorrhage was found in some embolized animals. This is shown in the representative Figure 1B. In addition, in the manual group there was 1 incidence of subarachnoid hemorrhage, which is not being counted as an ICH.
3. Discussion and Conclusions
This study shows that a range of hemorrhage rates and stroke rates can be achieved by altering the method of injection of large blood clots into brain. There are numerous interpretations of the data and uses for different levels of hemorrhage following embolic strokes.
First, using manual injection with an estimated rate of approximately 3 ml/45 seconds (variable pressure), the stroke success rate was 65%, whereas, the initial behavioral response to emboli and saline injection was 89%. There was a somewhat of a mismatch between stroke success measured using the amount of 57Co microspheres and behavioral response. Since we have noted this mismatch in previous studies(Lapchak et al., 2000; Lapchak et al., 2002a; Lapchak et al., 2002b; Lapchak, 2007; Lapchak et al., 2008; Lapchak and Han, 2009), we used strict inclusion criteria to ensure that all animals included in the study had bonafide strokes related to the presence of radiolabel in brain. In 41% of manually embolized rabbits with a bonafide stroke, we measured an incidence of one or more hemorrhages. This baseline is consistent with previous studies using the RLCEM(Chapman et al., 2001; Lapchak et al., 2000; Lapchak, 2007; Lapchak et al., 2008). In previous studies, we have shown that it is possible to modify or increase hemorrhage rate with the infusion of a thrombolytic such as tPA or TNK(Chapman et al., 2001; Lapchak et al., 2000). It is interesting to note, that in order to achieve a statistically significant effect (using chi square) of a thrombolytic or other pharmacological agent on hemorrhage rate in a population of rabbits, an approximate increase in rate corresponding to 80-100% is required(Lapchak et al., 2002b; Lapchak, 2007). Thus, with a baseline of 41%, a statistically significant increase is achievable with a mean population of 20-25 rabbits per group. Moreover, with a baseline of 41% it may be possible to study pharmacological agents to attenuate embolism-induced hemorrhage(Lapchak et al., 2000; Lapchak and Han, 2009), but this may be nearing the limit of sensitivity to produce statistically significant effects.
In the second aim of this study, we used automated or pump-assisted injection of emboli at various rates to determine the effect on stroke success rate and hemorrhage rate. When clots were injected at a rate of 3ml/90 seconds using a constant rate, rabbits presented the lowest hemorrhage rate (0%) and the lowest stroke success rate (19%) suggesting that the pump pressure used to move a clot through the cannula and carotid vascular pathway was insufficient. The mid-level injection rate using 3ml/45 seconds, which is similar to the rate used for the manual injection increased the stroke success rate (31%) and also increased the hemorrhage rate (43%). These results should be compared to the manual injection group. Even though the pump-assisted injection increased both parameters, the observation that 69% of all animal embolized with this method must be excluded from a study because they do not meet minimum inclusion criteria ( >10% of injected 57Co must be present in brain post-mortem) indicates that this is a very inefficient method to conduct translational hemorrhage research. Thus, although the hemorrhage rate of 43% in this group is similar to the manual injection group (41%), in order to conduct a basic Power analysis driven study requiring 20-25 to be included would require 60-75 animals to undergo surgery and embolization. This type of preclinical study would be cost prohibitive and scientifically unjustified.
In the final group, emboli were injected at a rate of 3ml/30 seconds, which presented the highest hemorrhage rate (79%) of all groups and a stroke success rate (63%) that was similar to the stroke success rate for manual injection group (65%). This method of producing embolism-induced hemorrhage may have great utility in preclinical hemorrhage research. Since the stroke success rate is relatively high compared to other pump-assisted groups and this study documents that almost 50% of injected radiolabel is present in brain post-mortem, a model with a high baseline hemorrhage rate is optimal for pharmacological studied aimed at discovering methodologies to reduce hemorrhage and the deleterious consequences of hemorrhage.
Thus, from a preclinical drug development perspective, the manual injection method is appropriate for studying the attenuation of either baseline or thrombolytic-induced hemorrhage(Lapchak et al., 2000; Lapchak et al., 2002b; Lapchak, 2007; Lapchak and Han, 2009), whereas the highest pump-assisted rate method may be best to study effects of therapeutics on non-thrombolytic-induced hemorrhage. It is interesting to note that the 24 and 48 hour survival rate in those 2 groups were not statistically different even though the pump method had a 92% higher hemorrhage rate. That may be due to the presence of more hemorrhagic infarctions and punctate hemorrhages in the pump-assisted method compared to the manual-injection method. Thus, even with the use of 50% higher pump pressure to embolize rabbits, the increased hemorrhage rate was not attributed to increased parenchymal hemorrhages and did not appear to be simply pressure related.
In addition, in this study besides using standard histological methods to visualize 3 types of hemorrhage in brain post-mortem(Chapman et al., 2001; Lapchak et al., 2000; Lapchak et al., 2008; Lapchak and Han, 2009; Lyden et al., 1987; Lyden et al., 1990), we prepared a second set of rabbits for study using TTC staining, a method usually applied to infarct analysis(Bederson et al., 1986; Benedek et al., 2006; Liu et al., 2009) to measure viable cells with functional mitochondria. As shown in Figure 1B, standard histochemical analysis allowed us to visualize parenchymal hemorrhages, hemorrhagic infarctions and punctate hemorrhages, the hemorrhagic infarction being a hemorrhage in an ischemic zone. In Figure 2, when TTC is used to visualize hemorrhage, blood is present with a grey/brown pigment. Interestingly, in the rabbit embolic stroke model, surrounding the hemorrhage is a clearly defined area of TTC-negative tissue. There are also independent areas of TTC-negative tissue not associated with hemorrhages. As expected, this indicates that embolization results in both brain ischemia and hemorrhage where there is also ischemic tissues. Thus, in future work with the large clot model for purpose of reducing hemorrhage and attenuating embolism-induced neurodegeneration, it would be advantageous to include measures of infarct volume and hemorrhage using TTC staining. This methodological change would allow for the precise measurement of infarct volumes independent or associated with brain hemorrhage. Since we have shown that in the rabbit embolic stroke model, hemorrhages are surrounded by areas of TTC-negative tissue, areas which are normally associated with infarcted or ischemic tissue, it is possible that therapeutics which are neuroprotective may also be useful to limit the evolution of ischemia damage associated with a hemorrhage. This hypothesis can be tested using standard TTC staining methodology using the rabbit embolic stroke model as a test system.
In all of our embolism-induced hemorrhage studies to date(Chapman et al., 2001; Lapchak et al., 2000; Lapchak et al., 2008; Lapchak and Han, 2009; Lyden et al., 1987; Lyden et al., 1990), there has been one consistent finding, that is the inability of the embolism to produce a specific form of ICH in a population of animals. The evolution of an ICH in embolized rabbits is sensitive to a variety of factors including MMP activity(Lapchak et al., 2000), free radicals(Lapchak et al., 2002a), platelets(Lapchak et al., 2002b), immune mediators(Lapchak, 2007) and thrombolysis(Chapman et al., 2001; Lapchak et al., 2008), but we have yet to find a method to directly control the type of hemorrhage to be produced following embolization as evidenced by the data presented in Table 3. Nevertheless, our previous studies with the manual injection model have shown that pharmacological intervention can attenuate or increase overall hemorrhage independent of subtype. However, given the high morbidity and mortality rate of ICH, in particular parenchymal hemorrhages in hemorrhagic stroke patients,(Lapchak, 2002; Lapchak and Araujo, 2007; Lyden and Zivin, 1993; Mayer and Rincon, 2005; Ogasawara et al., 2007) additional studies targeting parenchymal hemorrhages are required.
In conclusion, we have shown that we can modify hemorrhage incidence in the RLCEM without directly affecting survival rate, by adjusting the rate at which an emboli is introduced into the brain vasculature, in particular the middle cerebral artery. In addition, we have provided evidence for distinct area of ischemic tissue (TTC-negative) adjacent to hemorrhages, indicating separation of ischemia and hemorrhage, areas that may be targets for neuroprotective agents to limit the extent of hemorrhage damage.
4. Experimental Procedure
4.1 Animals and Animal Welfare
Male New Zealand white rabbits weighing 2 to 2.5 Kg were purchased from Rabbit Source, Ramona, CA. Rabbits were supplied food (alfalfa cubes) and water ad libitum while under quarantine in an enriched environment for at least 5 days prior to experimental use. Surgery was done in a sterile controlled environment with a room temperature between 22.8–23.2°C. All surgical, manual embolization and histological procedures were done as described previously(Lapchak et al., 2000; Lapchak, 2007; Lyden et al., 1989), except for the modifications described below. Institutional Animal Care and Use Committee (IACUC) approved the surgical and treatment procedures used in this study. Per the IACUC-approved protocol, rabbits were euthanized if they were in pain, showed extreme discomfort, or if they were unable to reach food or water.
4.2 Surgery and Embolization
Rabbits were anesthetized with halothane via face mask, 5% in 3 l/min at induction, and 3% in 3 l/min oxygen as a maintenance dose. The right internal carotid artery was exposed, and the external carotid artery and the common carotid artery were ligated. If any other branches were seen originating from the internal carotid artery, these were also ligated. A Becton, Dickinson and Company (B-D) plastic catheter oriented toward the brain was inserted into the common carotid artery and secured with ligatures. The incision was closed around the catheter so that the distal end was accessible outside. The catheter was filled with 0.2 ml of heparinized saline (33 units/ml) and plugged with injection caps. The animals were allowed to recover from anesthesia for at least 2 hours before embolization(Lapchak et al., 2008).
Non-autologous emboli were prepared by withdrawing 2-4 ml of arterial blood from a single donor rabbit. Because the studies had a 48 hour endpoint, there was no concern of immunogenicity of blood clots prepared from a single donor rabbit that were subsequently used in experiment for a group of rabbits on a particular experiment day. The blood was mixed with a tracer quantity of 57Co labeled plastic NEN-Trac microspheres (New England Nuclear Inc. MA), and allowed to clot for at least 3 hours at 39oC. The clot was cut into small cubes weighing approximately 2.5-3.5 mg and they were suspended in phosphate-buffered saline containing 0.1% bovine serum albumin. The amount of radiolabel present in each blood clot was measured using a mobile gamma counter. Prior to embolization, each animal was placed in a Plexiglas restrainer and the injection cap of the catheter was removed to allow the rabbit's blood to fill the catheter and wash out the heparinized saline. The line was then filled with heparin-free normal saline and a pre-flush was done to ensure catheter patency.
4.3 Emboli Injection
For embolization, one of the clot cubes was placed inside the injection port of the indwelling carotid catheter and injected with 3 ml of saline flush, followed immediately by an additional 3 ml flush. For manual injection, the clot was injected over 45 seconds (i.e. 3ml/45 seconds). For pump-assisted constant rate injection, we used a KDS100 infusion pump (KD Scientific, PA) at a setting of 120, 240 or 360 ml/hr with appropriate B-D syringes to inject 3 ml for embolization, followed by a 3 ml flush. Thus, with the pump settings of 120, 240 or 360 ml/hr, the actual injections were as follows: 3ml in 90 seconds, 45 seconds and 30 seconds, respectively. Automated blood clot injection was compared to manual injection using the same injection volumes. Care was taken during both flushes to be sure that no air bubbles were present in the catheter or syringe. If the animal did not have a behavioral response reaction, which could include nystagmus, leaning, kicking, rolling, loss of balance, hemiparesis, vocalization or seizure, then another blood clot was injected in the same way 3 minutes after the first embolization. If there was no behavioral response reaction to either embolization, no further blood clots were administered. Animals that had no behavioral response reaction after administration of two clots were treated in the same manner as animals responding to emboli. Inclusion or exclusion of animals from the study was based upon the criteria described below. After the embolization process was completed, the catheter was ligated close to the neck and the rest of the catheter and injection port were heat-sealed. Animals that died prior to sacrifice were included in this study; the brains were fixed and sectioned as below. The surviving animals were euthanized 24 hours post-embolization with 1-1.5 ml of Beuthanasia-D via the marginal ear vein.
4.4 Intracerebral Hemorrhage and Radioactivity Quantitation
The brains were removed and immersion fixed in 4% paraformaldehyde (Fisher Scientific, CA) for at least 7 days, and then examined by an observer naïve to the treatment groups (technician on duty DS). The surface blood vessels were then stripped from the right hemisphere of each brain. The cerebellum was also removed from the brainstem. Hemispheres were cut into six 5mm-thick coronal slices, each having two faces for a total of 12 faces. We recorded the size of hemorrhage as the number of section faces showing hemorrhage. After evaluation for hemorrhage, the total radioactivity in tissues was measured using a gamma counter. The amount of radiolabel present in the brain (including the right hemisphere vessels) was compared to that contained in the labeled blood clot at embolization. If fewer than 10% of the counts were found in the brain and vessels, it was assumed that the labeled blood clot had not reached the brain and the data from these animals was excluded from further analyses. Thus, only animals with greater than 10% of the label were included in the study.
Three major types of intracerebral hemorrhage can be identified in fixed brain:
parenchymal hemorrhages are large homogenous masses of blood within tissue;
hemorrhagic infarction consists of red speckling of an area, usually surrounded by soft infarcted tissue, and
punctate hemorrhages which are small isolated red marks within tissue that do not extend through the tissue like a blood vessel would.
4.5 TTC staining of rabbit brain tissue
Using a separate set of manually embolized rabbits, we used the vital 2,3,5-triphenyltetrazolium (TTC) purchased from Sigma (St. Louis, MO) to determine the pattern of infarcts and hemorrhages in brain. Following euthanasia, brains were removed from the skull within 1 minute and 5 mm coronal slices were immersed in 2% fresh TTC dissolved in saline at 37°C for 10 minutes. Cells that are metabolically active convert TTC into a red stain, whereas damaged tissue, the area which correlates with an infarct, is pale in color(Bederson et al., 1986) . After complete development of the red color, each coronal slice was digitally photographed, given a dated code and stored to prepare a compiled figure.
4.6 Statistical Analysis
For all experiments in this study, rabbits were randomly allocated into treatment groups before the embolization procedure, with concealment of the randomization guaranteed by using an independent party (PAL). The randomization sequence was not revealed until all postmortem analyses were complete. In the present study, 20-25 rabbits were included in each group based upon Power Analysis and historical data showing that a minimum of 20 rabbits is required for statistical significance (Lapchak et al., 2000; Lapchak et al., 2002a; Lapchak et al., 2002b; Lapchak, 2007; Lapchak et al., 2008; Lapchak and Han, 2009). Statistical analyses were done as described previously(Lapchak et al., 2008). Data were analyzed using MedCalc Version 9.4.1.0 (www.Medcalc.be) for comparison of proportions for behavioral response rates, stroke success rates and hemorrhage rates. Analysis of variance followed by the t-test was used for the analysis of differences among radioactive counts and hemorrhage volume using SigmaStat 3.5.
Figure 3. Intracerebral Hemorrhage Rate and Volume Following Embolization.
Hemorrhage Rate (A) and Hemorrhage volume (B) following large clot embolization using 4 different embolization methods: Manual (manual investigator-assisted injection with a variable pressure flow rate 3 ml/45 seconds), Auto 1 (KDS pump-assisted injection flow rate 3ml/90 seconds), Auto 2 (KDS pump-assisted injection flow rate 3 ml/45 seconds), Auto 3 (KDS pump-assisted injection flow rate 3 ml/ 30 seconds). The injection volume was maintained constant at 3 ml using all methods.
Acknowledgments and Funding
This work was supported by a U01 Translational research grant NS060685 to PAL. J. Wei and D. Song were the technicians on duty during this study and are thanked for their contribution and diligence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–9. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Benedek A, Moricz K, Juranyi Z, Gigler G, Levay G, Harsing LG, Jr., Matyus P, Szenasi G, Albert M. Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res. 2006;1116:159–65. doi: 10.1016/j.brainres.2006.07.123. [DOI] [PubMed] [Google Scholar]
- Chapman DF, Lyden P, Lapchak PA, Nunez S, Thibodeaux H, Zivin J. Comparison of TNK with wild-type tissue plasminogen activator in a rabbit embolic stroke model. Stroke. 2001;32:748–52. doi: 10.1161/01.str.32.3.748. [DOI] [PubMed] [Google Scholar]
- Hu B, Liu C, Zivin JA. Reduction of intracerebral hemorrhaging in a rabbit embolic stroke model. Neurology. 1999;53:2140–5. doi: 10.1212/wnl.53.9.2140. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–40. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM. Reducing bleeding complications after thrombolytic therapy for stroke: clinical potential of metalloproteinase inhibitors and spin trap agents. CNS Drugs. 2001;15:819–29. doi: 10.2165/00023210-200115110-00001. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. Pharmacological effects of the spin trap agents N-t-butyl-phenylnitrone (PBN) and 2,2,6, 6-tetramethylpiperidine-N-oxyl (TEMPO) in a rabbit thromboembolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2001;32:147–53. doi: 10.1161/01.str.32.1.147. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:38–43. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Song D, Wei J, Purdy R, Zivin JA. Effects of the spin trap agent disodium- [tert- butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit Large clot embolic stroke model: combination studies with tissue plasminogen activator. Stroke. 2002a;33:1665–70. doi: 10.1161/01.str.0000017145.22806.aa. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Song D, Zivin JA. The nonpeptide glycoprotein IIb/IIIa platelet receptor antagonist SM- 20302 reduces tissue plasminogen activator-induced intracerebral hemorrhage after thromboembolic stroke. Stroke. 2002b;33:147–52. doi: 10.1161/hs0102.100530. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Tumor necrosis factor-alpha is involved in thrombolytic-induced hemorrhage following embolic strokes in rabbits. Brain Res. 2007;1167:123–8. doi: 10.1016/j.brainres.2007.06.072. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM. Advances in hemorrhagic stroke therapy: conventional and novel approaches. Expert Opin Emerg Drugs. 2007;12:389–406. doi: 10.1517/14728214.12.3.389. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Han MK, Salgado KF, Streeter J, Zivin JA. Safety Profile of Transcranial Near-Infrared Laser Therapy Administered in Combination With Thrombolytic Therapy to Embolized Rabbits. Stroke. 2008;39:3073–3078. doi: 10.1161/STROKEAHA.108.516393. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Han MK. The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor simvastatin reduces thrombolytic-induced intracerebral hemorrhage in embolized rabbits. Brain Res. 2009;1303:144–50. doi: 10.1016/j.brainres.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Translational Stroke Research. 2010;1:96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27:627–37. doi: 10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden PD, Zivin JA, Soll M, Sitzer M, Rothrock JF, Alksne J. Intracerebral hemorrhage after experimental embolic infarction. Anticoagulation. Arch Neurol. 1987;44:848–50. doi: 10.1001/archneur.1987.00520200052018. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Zivin JA, Clark WA, Madden K, Sasse KC, Mazzarella VA, Terry RD, Press GA. Tissue plasminogen activator-mediated thrombolysis of cerebral emboli and its effect on hemorrhagic infarction in rabbits. Neurology. 1989;39:703. doi: 10.1212/wnl.39.5.703. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Madden KP, Clark WM, Sasse KC, Zivin JA. Incidence of cerebral hemorrhage after treatment with tissue plasminogen activator or streptokinase following embolic stroke in rabbits [corrected]. Stroke. 1990;21:1589–93. doi: 10.1161/01.str.21.11.1589. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Zivin JA. Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc Brain Metab Rev. 1993;5:1–16. [PubMed] [Google Scholar]
- Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol. 2005;4:662–72. doi: 10.1016/S1474-4422(05)70195-2. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–72. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, Sakai C, Nagata I, Ogawa A. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg. 2007;107:1130–6. doi: 10.3171/JNS-07/12/1130. [DOI] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–30. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12:833–42. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res. 1995;703:151–5. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–6. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–12. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–6. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–33. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–52. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]