Abstract
The “p53 signature” is a benign secretory cell outgrowth in the distal fallopian tube that shares properties with ovarian serous cancer – including p53 mutations - and is a putative serous cancer precursor. We expanded the precursor definition to all secretory cell outgrowths (SCOUTs) of 30 or more cells and scored normal (N) and altered (A) expression of both p53 and PAX2, a gene down-regulated in ovarian and endometrial cancer. SCOUTs were identified by BCL2/p73 staining in tubes from women with serous carcinoma, inherited mutations in BRCA1 or BRCA2, and controls. SCOUTs were prevalent in both proximal and distal tube and significantly associated with serous carcinoma versus the others (p <0.001). Eighty-nine percent were PAX2 (A); 26% were PAX2 (A)/p53 (A) (p53 signatures). PAX2 (A)/p53 (N) SCOUTs were free of p53 mutations; however, 12 of 13 p53 signatures were PAX2 (A). A tubal carcinoma and contiguous SCOUT were p53 (A)/PAX2 (A) and shared the same p53 mutation. SCOUTs are discretely localized alterations commonly containing altered expression of multiple genes within histologically benign tubal epithelium. Geographic distribution in the tube varies by genotype and immunophenotype, from widespread (PAX2) to confinement to a specific area (fimbria) of shared prevalence (PAX2 and p53). This study reveals, for the first time, an entity (SCOUT) that is associated with serous cancer, expands the topography of altered PAX2 expression in the female genital tract mucosa and highlights another potential pathway disturbance involved in early serous carcinogenesis in the fallopian tube.
Keywords: p53, PAX2, SCOUT, ovary, fallopian tube, serous, carcinoma, BRCA1, BRCA2
Introduction
Serous adenocarcinoma is the most common and lethal type of ovarian epithelial malignancy, comprising approximately 60–70% of ovarian cancer cases.[1] Currently, over 22 thousand women are diagnosed with ovarian cancer each year and over 15 thousand will not survive the disease. [2] Because the disease almost always presents clinically after regional spread has occurred, investigators have emphasized the importance of understanding the early phases of this disease, including precursor conditions.[3] Recently, studies of both asymptomatic women with germ-line mutations in BRCA 1 or 2 and those from the general population with pelvic serous carcinoma have proposed the tubal fimbria as a frequent site of origin.[4] [5] [6] [7] [8] [9] Two putative precursors in the distal fallopian tube have been described: serous tubal intraepithelial carcinoma, a focal cytologically altered lesion presumed to be an early and non-invasive but potentially lethal phase of malignancy, and the “p53 signature”, its histologically unremarkable precursor in benign mucosa. [10] [11] [12] [13] Variables supporting the “p53 signature” as a precursor to pelvic serous carcinoma include shared location (fimbria), presence of p53 mutations, continuity with early malignancy, shared risk factors, and an origin in the secretory cell.[14]
A major characteristic of the p53 signature is the absence of ciliated differentiation, resulting in homogeneous secretory cell outgrowth (SCOUT). This process is most conspicuous in the setting of p53 mutations and DNA damage, which is unique to the non-ciliated (secretory) tubal cell and detected by intense p53 nuclear staining (the p53 signature).12 [15] Whether the events leading to a p53 signature interrupt a process of normal ciliated differentiation in this population or occur only in cell types thatare not part of this differentiation pathway is unknown.
Because carcinogenesis is classically multigenic, we hypothesized that SCOUTs could emerge in the tube in the absence of p53 mutations via other pathways.[16] Recently, down-regulation of PAX2, a member of the pair box (PAX) gene family expressed in adult müllerian duct derivatives, has been documented as a frequent abnormality in early endometrial carcinoma (77%) and pelvic serous carcinomas (71%). [17] [18] [19] [20] PAX2 was selected for analysis based on three prior observations. 1) It is highly expressed in secretory cells of the fallopian tube; 2) it is down-regulated in high-grade serous carcinomas, based on both array and immunohistochemical data; and 3) in a recent study by Monte et al, PAX2 expression was found to be lost in not only endometrial carcinoma and its precursors (endometrial intraepithelial neoplasia), but also normal endometrial glands. 17, 20
The goal of this study was to determine if the pelvic serous cancer precursor model could be expanded through the study of SCOUTs. We determined 1) if SCOUTs could be identified and quantified in the fallopian tubes, 2) if they were associated with concurrent malignancies, 3) if they demonstrated dysregulation of PAX2 expression and if so, 4) did the alterations in PAX2 expression occurred independent of p53 inactivation in low and high risk populations.
Materials and Methods
This study was approved by the institutional review board at Brigham and Women’s Hospital.
Case Material and Screening for SCOUTs by immunohistochemistry
The study material consisted of twenty-four consecutive cases from 2009 diagnosed as “serous adenocarcinoma, high grade”, 25 consecutive cases from 2008–2009 of unilateral or bilateral salpingo-oophorectomy for non-malignant indications, including endometriosis, benign ovarian cysts and fibroids, and 75 cases consecutive cases of prophylactic bilateral salpingo-oophorectomy specimens of patients with BRCA 1 or 2 mutations (BRCA+) from 2005–2006. The slides were collected from the archives at Brigham and Women’s Hospital.
A SCOUT was defined as a discrete expansion of at least 30 epithelial cells of secretory type (BCL2 expressing, p73 non-expressing) that were distinct against a heterogeneous background of tubal secretory and ciliated cells (Figure 1). A p53 signature was defined as a discrete expansion of at least 12 secretory type cells with intense p53 nuclear staining, as described before. Although a higher cell number was used to discriminate SCOUTs from background epithelium, in the great majority of cases a p53 signature was viewed as a SCOUT that had strong p53 imunostaining. Thus a P53A SCOUT was the same as a p53 signature. The number of cells required for a designation of SCOUT was empirically set at 30 to exclude more common variations in secretory and ciliated differentiation associated with hormonal fluctuations. Fimbrial and more proximal sections of tube from each case were analyzed using antibodies to BCL2 (Clone 124, code M0887, LOT: 00054759, DAKO Denmark), a marker for secretory cells, and p73, a marker for ciliated cells.12 The sections were scored by two observers (MM, KM) with confirmation by a third (EYC) required for inclusion. Sections were reviewed without knowledge of the case type, tabulated and entered into a database for statistical analysis. Frequencies of SCOUTs in the three groups were compared and analyzed by the Fisher’s exact test.
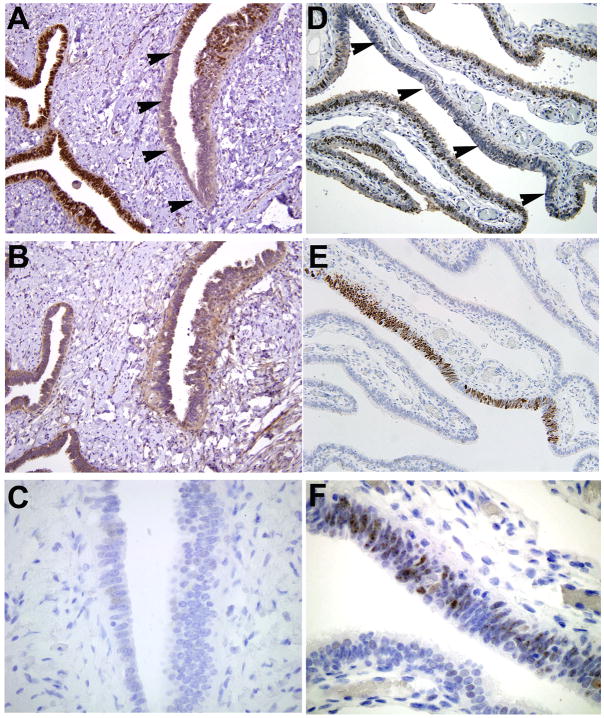
Figure 1.
A&B) BCL2 immunostained fallopian tube, showing a heterogeneous population of ciliated (non-staining) and secretory (staining) cells. C) Nuclear staining with p73 highlights ciliated cells. D&E) secretory cell outgrowths (SCOUTs), seen as uninterrupted linear outgrowths of BCL2 positive cells (arrows). F) Absence of staining for p73.
Assessment of frequency of PAX2 and p53 expression in SCOUTs
Immunohistochemical analysis was carried out in two phases. In phase one, altered PAX2 (loss of) and p53 (increased) expression in the secretory cells of previously identified BCL2/p73 defined localized SCOUT lesions were designated PAX2 (A) (Invitrogen; Cat# 71-6000) and p53 (A) (clone DO-1; ImmunoTech, Westbrook, ME), signifying presumed down-regulation and loss of function of the two genes, respectively. In phase two, additional p53 signatures and tubal intraepithelial carcinomas were obtained from the archive for analysis of p53 and PAX2.
Relationship between PAX2(A) and p53(A)
To determine whether alterations in PAX2 and p53 expression were linked or independent, three parameters were assessed.
The tubal location (fimbrial vs more proximal) of p53(N) SCOUTs was tabulated and compared to p53(A) SCOUTs (p53 signatures).
Sequencing of the p53 gene was conducted in a subset of PAX2 (A)/p53 (N or A) SCOUTs to evaluate p53 mutations. Selected tissue blocks containing foci of interest were serially sectioned and confirmed by immunostaining. Laser capture microdissection (LCM) was used to isolate SCOUT and control somatic DNA from selected cases using the PALM microbeam instrument. Genomic DNA was extractedas previously described and amplified by polymerase chain reaction (PCR), using tailed primers designed to amplify exons 2,3,5–9 and 11 of p53. A secondary amplification was performed using T3 and T7 primers specific to the tail sequence used in the primary amplification. PCR products were then sequenced from both strands using T3 and T7 primers. Data was analyzed using the Mutation Surveyor program (Soft Genetics, State College, PA). Candidate mutations found by the software were compared to a reference database for cancer-associated p53 mutations (Universal mutation database, http://www.umd.be:2072/IFAMTP53A.shtml). For each tissue set, duplicate samples were run to confirm the presence/absence of a given mutation. 12
p53(N) SCOUTs were immunostained for nuclear localization of γ-H2AX (clone JBW301, Upstate Cell Signaling Solution, Charlottesville, VA) to confirm or exclude evidence of a DNA damage response. These were compared to p53 signatures, which are known to be γ-H2AX-positive. 12 [21]
Results
Detection and frequency of SCOUTs in the three groups
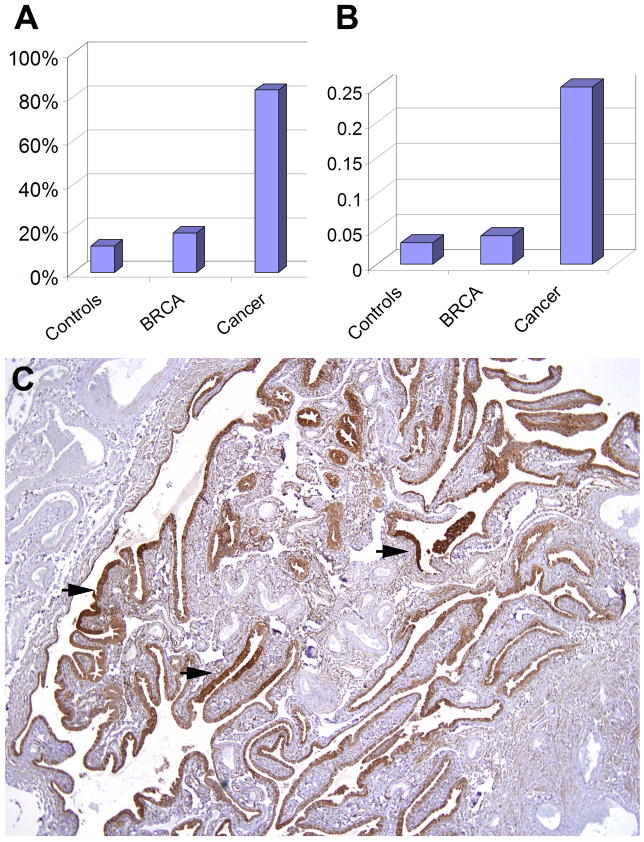
Figure 1 illustrates the heterogeneous population of secretory and ciliated cells in a normal fallopian tube stained for BCL2 (panels A&B) and p73 (panel C). SCOUTs were conspicuous by homogeneous staining of a linear contiguous population of BCL2 positive cells (panels D&E), lacking the distinctive p73 staining that characterizes ciliated cells (panel F). Figure 2 summarizes the absolute frequency of SCOUTs in the three groups. Twelve, 18 and 83% of fallopian tubes from controls, BRCA+ healthy women and serous carcinomas harbored SCOUTs (p < 0.001). To allow for sampling differences across the different groups, the total number of histologic cross sections of tube examined was tallied for each group and the SCOUT index expressed as a ratio of SCOUTs detected per cross section examined. This resulted in a SCOUT index of .03 (4/118), .04(22/569), and .25(36/144) SCOUTs seen per section in the three groups respectively (p < .001) (Figure 2).
Figure 2.
A) Graphical display of absolute SCOUT frequency in controls, asymptomatic women with BRCA+ and women with serous carcinoma; B) normalized to allow for differences in sampling by showing SCOUT frequency as a function of tissue section examined. C) Multiple BCL2 positive SCOUTs in a section of normal tube (arrows) from a subject with serous carcinoma (See Table 2, samples 04).
Expression of p53 and PAX-2 in SCOUTs, p53 signatures and early tubal carcinomas
PAX2 expression is frequently lost in SCOUTs: Immunohistochemical stains for p53 and PAX-2 were performed on 42 SCOUTs identified in the three groups. Of 36 SCOUTs, nine were p53 (A)/PAX2 (A) and fulfilled the criteria for p53 signatures; 23 were p53 (N)/PAX2 (A)). In all, 32 of 36 SCOUTs (89%) were PAX2 (A) (Figure 3A–C)(Table 1).
Figure 3.
PAX2, p53 and H2AX immunostaining of SCOUTs. A–C) PAX2 (A)/p53 (N)/H2AX (−); D–F) PAX2 (A)/p53 (A)/H2AX (+) (p53 signature).
Table 1.
Coordination of abnormal (A) PAX2 and p53 immunostaining in SCOUTs, p53 signatures, tubal intraepithelial carcinomas and advanced serous carcinoma.
| SCOUTs (N = 36) | p53 SIGNATURES (N=13) | STIC (N = 12) | Serous Carcinoma (N = 16) | |
|---|---|---|---|---|
| P53(A) | 9 (25%) | 13 (100%) | 11 (92%) | 16 (100%) |
| PAX2(A) | 32 (89%) | 12 (92%) | 12 (100%) | 11 (69%) |
SCOUT = secretory cell outgrowth, STIC = serous tubal intraepithelial carcinoma; P53(A), PAX2(A) = abnormal expression (up and down-regulation respectively).
Loss of PAX2 expression is coordinated with abnormal p53 expression in p53 signatures, serous tubal intraepithelial carcinomas (STICs) and serous carcinomas: Although just 9 (25%) SCOUTs analyzed from the three groups were p53(A) and fulfilled the criteria for p53 signatures, all but one of these and four additional cases from the pathology files were PAX2(A) (Table 1)(Figure 3). Twelve STICs examined were p53 (A), 11 with increased and 1 with absent p53 staining, consistent with accumulation of mutated p53 protein or deletion respectively, as previously documented (Table 1). Similar to the p53 signatures, nearly all STICs were PAX2 (A) (92%). By comparison, all of 16 cases of high-grade serous carcinomas examined were p53 (A); 11 (69%) were PAX2 (A), the remainder showed heterogeneous expression of PAX-2, consistent with variable regulation of PAX2 expression in these malignancies, as described previously. 19
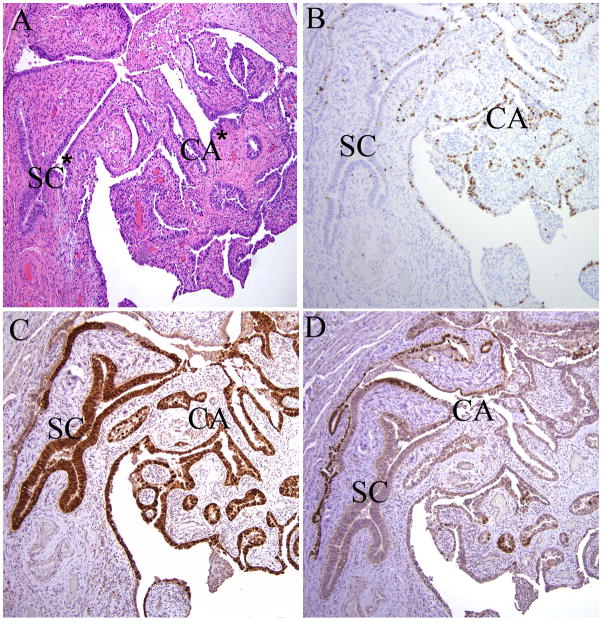
We identified three cases of p53 (A) SCOUTs in close proximity with serous carcinoma; all PAX2 (A). One p53 (A)/PAX2 (A) SCOUT was contiguous with an invasive carcinoma of the same immunophenotype (Figure 4A–C; Case 10, Table 2).
Figure 4.
p53 (A) SCOUT (SC; p53 signature) juxtaposed with a serous carcinoma in the fallopian tube (CA). A) Hematoxylin and eosin stain; B) Mib-1 highlights absence of proliferative activity in the SCOUT; C) intense nuclear staining for p53 in both; D) PAX2 staining, present in the adjacent normal tubal secretory cells (left), is lost in the SCOUT and serous carcinoma, paralleling the p53 staining. * Denotes loci sharing a missense mutation in p53 codon 220 detailed in Table 2 (samples 10SC, 10Ca).
Table 2.
Analysis of SCOUTs for p53 mutations
| Sample* | Source | Exons tested | WT/Mut | Nucleotide | Amino acid | Effect | Comment |
|---|---|---|---|---|---|---|---|
| 02N | Normal | E2–E9, E11 | WT | ||||
| 02SC | SCOUT | E2–E11 | WT | Remote to carcinoma | |||
| 02Ca | CA | E2–E4. E6, E8–E11 | Mut | c.1079G>T | G360V | missense | |
| 04N | Normal | E2–E11 | WT | ||||
| 04SC1 | SCOUT | E2–E11 | WT | Remote to carcinoma (Figure 3C) | |||
| 04SC2 | SCOUT | E2–E11 | WT | Remote to carcinoma | |||
| 04SC3 | SCOUT | E2–E11 | WT | Remote to carcinoma | |||
| 04SC4 | SCOUT | E2–E11 | WT | Remote to carcinoma | |||
| 06N | Normal | E2–E5, E7, E9–E11 | WT | ||||
| 06SC | SCOUT | E2–E4, E6, E7, E9–E11 | WT | ||||
| 10N | Normal | E2–E11 | WT | ||||
| 10SC | SCOUT | E2–E11 | Mut | c.659A>C | p.Y220S | missense | Contiguous with carcinoma (Figure 4) |
| 10T | STIC | E2–E11 | Mut | c.659A>C | p.Y220S | missense | |
| 10Ca | Serous carcinoma | E2–E11 | Mut | c.659A>C | p.Y220S | missense | Contiguous with SCOUT (Figure 4) |
Sample numbers are from the original laboratory datasheet. Samples with inadequate DNA amplification were excluded. SCOUT = secretory cell outgrowth; STIC = serous tubal intraepithelial carcinoma; WT = wild type p53 DNA sequence; Mut = p53 mutation detected.
Relationship between abnormalities in p53 and PAX2 expression
SCOUTs are prevalent in both the proximal and distal fallopian tubes: Table 3 summarizes the locations of p53 (A) (p53 signatures) and p53 (A) SCOUTs from the study by Lee et al and the data from the current study. 12 The ratio of fimbrial/proximal location for p53 signatures in the study by Lee et al and the current study was 4.2/1 and 5/1. In contrast the ratio for p53 (N) SCOUTs in the current study was 1.3/1. The difference in distribution between the first two observations combined and the last was significant at p <.001 and indicates that p53 (N) SCOUTs are not as regionally restricted as p53 (A) SCOUTs (Table 3). 12
Table 3.
Topographical distribution of SCOUTs with normal (N) and abnormal (A) p53 immunostaining in the fallopian tubes
| Study | SCOUT | Fimbria (F) | Proximal (P) | Ratio (F/P) |
|---|---|---|---|---|
| Lee et al 12 | P53(A) | 25 | 6 | 4.2/1 |
| Current | P53(A) | 10 | 2 | 5/1 |
| Current | P53(N) | 48 | 37 | 1.3/1 |
Difference in regional frequency of p53 signatures and p53(−) SCOUTs is significant at p <.001.
SCOUT = secretory cell outgrowth; P53(A) = p53 signature.
SCOUTs with wild-type p53 function are devoid of p53 mutations: Table 2 summarizes a small series of SCOUTs from 4 cases in which most or all of the p53 exons could be fully analyzed. No p53 mutations were identified in five PAX2 (A)/p53 (N) SCOUTs that were either unassociated with or remote to serous carcinoma (03, 04, 06), three of which are illustrated in Figure 2, panel C. One PAX2 (A)/p53 (A) SCOUT (p53 signature) in continuity with a serous carcinoma (Figure 4) was fully analyzed. Sequencing of this SCOUT and the contiguous serous carcinoma revealed an identical p53 mutation at codon 220. A tubal intraepithelial carcinoma (not illustrated) also contained the same mutation.
Evidence of DNA damage (H2AX) is limited to p53 (A) SCOUTs: SCOUTs were analyzed for evidence of DNA damage via examination of γ-H2AX expression. Punctate staining for γ-H2AX was consistently absent in p53 (N) SCOUTs in contrast to p53 (A) SCOUTs (p53 signatures) as previously described (Figure 3C&F).12
Discussion
In the reproductive age woman, the fallopian tube mucosa consists of both secretory and ciliated cells arranged in alternating small groups punctuated by stretches of one or the other cell type. Although the two cell types are morphologically distinct, it is assumed that a subset of the secretory cell population is capable of undergoing ciliated cell differentiation or maturation, as shown previously in estradiol-exposed cultured human fallopian tube epithelium.[22] In the fallopian tubes, the prevailing pattern is thus one in which secretory cells alternate with ciliated cells on a regular basis (Figure 1A&B). In contrast, expansion of the secretory cell population to 30 or more cells to form a linear secretory cell outgrowth (SCOUT) that is not interrupted by ciliated differentiation is unusual (Figure 1D&E). Whether this “secretory” cell expansion signifies a loss of ability to undergo ciliation or a unique susceptible cell type that is defined by gene dysregulation is unclear, but merits further study, particularly in light of the apparent up-regulation of BCL2 staining by immunohistochemical analysis. We postulated that these expanded arrays of secretory-type cells signified alterations in gene function analogous to p53 signatures. Based on prior experience with p53 signatures, which are in essentially a subset of SCOUTs with altered p53 expression and (frequently) p53 mutations, we initiated a search for functional alterations in other genes.12
In the uterus, loss of PTEN expression has been shown to be one of the earliest events in endometrial carcinogenesis, presumably beginning as a common clonal aberration in morphologically normal glands (latent precursors) of continuous lineage with both cancer precursors (endometrial intraepithelial neoplasia) and malignancy.[23] This model of endometrial carcinogenesis has continued to evolve. Recently, sporadic loss of PAX2 expression was identified in isolated glands of normal endometrium and shown to occur commonly and independent of PTEN loss, but to coexist with loss of PTEN in neoplasia.17 The precise role of PAX2 in this process is unclear. This gene not only plays an essential role in development e.g. urogenital morphogenesis, but has also been implicated to play a role in various types of malignancy such as breast, kidney, prostate and ovary, consistentwith diverse context-sensitive roles in cell proliferation, growth and differentiation. [24] The role of PAX2 in upper genital tract neoplasia would seem different, in as much as it was initially described as a marker for mullerian or urothelial differentiation. However, the expression of this gene in serous carcinomas has been observed to be low, both in a prior report and a recent one from our group. Of particular interest was the near complete loss in early serous carcinomas in the distal fallopian tube and a similar albeit less restricted reduction in expression in malignancies. In the latter, the expression PAX2, when exceeding 5% of tumor cells, tended to be heterogeneous, suggesting variable gene regulation in more advanced malignancies (19).
The tubal model for pelvic serous carcinogenesis has up to this point, been based principally on loss of p53 function, a feature that has defined the p53 signature in addition to tubal intraepithelial carcinomas and advanced serous carcinomas.11, 12 The clonal expansion of benign secretory epithelium seen in p53 signatures is analogous to the PTEN-null glands in normal endometrium, designated “latent precancers” in the latter site.23 The distal tube is the most common site for the p53 signature, which in thoroughly sectioned tubes will be found in over one-half of cases and independent of genetic risk.[25] Evidence supporting the p53 signature as a serous cancer precursor includes not only the presence of p53 mutations, but a predilection for the fimbrial secretory-type cell, a DNA damage response (γ-H2AX+ nuclear foci), higher frequency in serous carcinomas, and low parity.12 [26] [27] Nevertheless, given the multigenic nature of carcinogenesis, other pathways would be expected to be involved.
This study expands the range of discrete epithelial outgrowths in the fallopian tube containing gene alterations associated with pelvic serous cancer, in this case defined by altered PAX2 expression. In this scenario, SCOUTs are more broadly defined than p53 signatures, i.e. they include this entity but usually do not display alterations in p53, either by immunostaining or p53 sequence analysis (Table 2). Most SCOUTs display loss of PAX2, and are significantly more widely distributed in the tube than p53 signatures, the latter more likely to be fimbrial in location (Table 3). Based on our observations, most SCOUTs do not have p53 mutations and can occur independently of this genetic event. However, although SCOUTs with normal p53 immunostaining do not display evidence of either DNA damage or p53 mutations, those with abnormal p53 expression are almost always PAX2 negative (Table 1, Figure 3). This coordination of abnormalities in both p53 and PAX2 expression occurs in the distal fallopian tube and characterized p53 signatures, early tubal (intraepithelial) and advanced pelvic serous carcinomas (Table 1). All three entities can, on occasion, be demonstrated in continuity and shown to share identical p53 mutations and loss of PAX2 staining (Figure 4). Precisely why SCOUTs that do not have p53 mutations do not predominate in the distal tube is unclear but this may reflect in part different mechanisms by which p53 mutations and loss of PAX2 develop. The latter, unlike p53 mutations, are relatively common in otherwise normal endometrial glands and do not exhibit evidence of DNA damage.
This study has revealed an entity (SCOUT) that occurs in a significantly higher frequency in the fallopian tubes of women with serous cancer. It remains to be determined whether the early loss of PAX2 function is integral to the development of serous cancer or is a marker for other, functionally relevant gene perturbations. The similar frequency of SCOUTs in both BRCA+ women and normal controls parallels that seen for p53 signatures, indicating that SCOUTs, like p53 signatures, are not influenced by BRCA status. Nevertheless, that SCOUTs harbor abnormalities in gene expression (p53 and PAX2) that are so tightly linked to pelvic serous cancer endorses further the fallopian tube as an important site of initiation. The topographic convergence of alterations in expression of these two genes is consistent with a scenario in which more widespread perturbations in gene function (PAX2) co-exist with more site-specific (p53) genetic events in locations prone to serous carcinogenesis. 17 The potential significance of SCOUTs lies in the fact that, like p53 signatures, they are a visible and evaluable precursor entity, the study of which might yield a greater understanding of both serous carcinogenesis and the tubal cell population that is involved in this process. Moreover, given the strong association between abnormal PAX2 and p53 expression and serous carcinomas, this immunophenotype can be addressed in other proposed precursor models, such as the ovarian and secondary Müllerian system, to determine their relevance to pelvic serous carcinogenesis.[28]
Table 4.
Variables in common between the tubal p53 signature and pelvic serous cancer
|
Acknowledgments
This work was supported by grants from the NCI 1R21CA124688-01A1 (CP Crum, PI) (P50 CA105009 [SPORE]: D. Cramer, PI), The Columbia Hospital for Women Research Foundation (CP Crum, PI), and the Francis Ward Paine and TSA Pemberton Funds from the Division of Women’s and Perinatal Pathology, Brigham and Women’s Hospital. The authors are grateful to Drs. Ross S. Berkowitz, Michael G. Muto, Colleen Feltmate and Judy Garber of Brigham and Women’s Hospital and the Dana Farber Cancer Institute for their clinical contributions.
Footnotes
The authors have no conflicts of interest.
Author contributions: EC organized the data, retrieved the cases, scored the stained slides and wrote the first draft. NM was responsible for developing the PAX2 immunoassay. KM verified all antibodies and scored the stains. MM and GN performed all of the immunostains. Ning performed the laser capture microfissections. AM and YY conducted all of the DNA sequencing. FMcK was involved in study planning, produced the p63 antibodies and edited the manuscript. BQ and GM contributed to study design via their experience with the Li Fraumeni and PAX2 models and edited the manuscript. WX supervised the selection of antibodies, performed surveys of existing expression data, and edited the manuscript. CC conceived of the concept of SCOUT, identified the questions to be addressed and wrote the final manuscript draft.
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 1993;329:1550–9. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Brewer MA, Johnson K, Follen M, et al. Prevention of ovarian cancer: intraepithelial neoplasia. Clin Cancer Res. 2003;9:20–30. [PubMed] [Google Scholar]
- 4.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 5.Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 6.Cass I, Holschneider C, Datta N, et al. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet Gynecol. 2005;106:1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 7.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvador S, Gilks B, Köbel M, Huntsman D, Rosen B, Miller D. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19:58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 10.Colgan TJ. Challenges in the early diagnosis and staging of Fallopian-tube carcinomas associated with BRCA mutations. Int J Gynecol Pathol. 2003;22:109–20. doi: 10.1097/00004347-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 13.Jarboe E, Folkins A, Nucci MR, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 14.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levanon K, Ng V, Piao HY, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–13. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Land H, Parada LF, Weinberg RA. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–8. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 17.Monte NM, Webster KA, Neuberg D, et al. Interaction of PAX2 and PTEN drive emergence of endometrial precancers from a preclinical latent phase. Cancer Research. AUTHOR WILL UPDATE AT PROOF In press. [Google Scholar]
- 18.Tung CS, Mok SC, Tsang YT, et al. PAX2 expression in low malignant potential ovarian tumors and low-grade ovarian serous carcinomas. Mod Pathol. 2009;22:1243–50. doi: 10.1038/modpathol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roh MH, Yassin Y, Miron A, et al. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX-2 expression. AUTHOR WILL UPDATE AT PROOF In press. [DOI] [PubMed] [Google Scholar]
- 20.Chivukula M, Dabbs DJ, O’Connor S, et al. PAX 2: a novel Müllerian marker for serous papillary carcinomas to differentiate from micropapillary breast carcinoma. Int J Gynecol Pathol. 2009;28:570–8. doi: 10.1097/PGP.0b013e3181a76fa2. [DOI] [PubMed] [Google Scholar]
- 21.Thiriet C, Hayes JJ. Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–22. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod. 1998;13:3114–20. doi: 10.1093/humrep/13.11.3114. [DOI] [PubMed] [Google Scholar]
- 23.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 24.Dressler GR. PAX-2, kidney development, and oncogenesis. Med Pediatr Oncol. 1996;27:440–4. doi: 10.1002/(SICI)1096-911X(199611)27:5<440::AID-MPO9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Mehra K, Chang M, Folkins A, et al. The impact of tissue block sampling on the detection of p53 signatures in fallopian tubes from women with BRCA 1 or 2 mutations (BRCA+) and controls. AUTHOR WILL UPDATE AT PROOF In press. [DOI] [PubMed] [Google Scholar]
- 26.Saleemuddin A, Folkins AK, Garrett L, et al. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol Oncol. 2008;111:226–32. doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw PA, Rouzbahman M, Pizer ES, et al. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22:1133–8. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 28.Dubeau L. The cell of origin of ovarian epithelial tumors. Lancet Oncol. 2008;9:1191–7. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]