Abstract
Large animal models have been instrumental in advancing hematopoietic stem cell (HSC) gene therapy. Here we review the advantages of large animal models, their contributions to the field of HSC gene therapy, and recent progress in this field. Several properties of human HSCs including their purification, their cell-cycle characteristics, their response to cytokines, and the proliferative demands put on them after transplantation are more similar in large animal models than in mice. Progress in the development and use of retroviral vectors and ex vivo transduction protocols over the last decade has led to efficient gene transfer in both dogs and in nonhuman primates. Importantly, the approaches developed in these models have translated well to the clinic. Large animals continue to be useful to evaluate the efficacy and safety of gene therapy, and dogs with hematopoietic diseases have now been cured by HSC gene therapy. Nonhuman primates allow evaluation of aspects of transplantation as well as disease-specific approaches such as AIDS gene therapy that can not be modeled well in the dog. Finally, large animal models have been used to evaluate the genotoxicity of viral vectors by comparing integration sites in hematopoietic repopulating cells and monitoring clonality after transplantation.
Introduction
Hematopoietic stem cells (HSCs) are excellent targets for gene therapy due to the relative ease with which they can be manipulated and their ability to repopulate the entire hematopoietic system for the life of a patient. Early experiments showed that bone marrow (BM) transplantation is highly effective due to the ability to ablate the endogenous hematopoietic system with low-dose irradiation. Lethally irradiated mice that are infused with BM from an untreated mouse are rescued via repopulation with the donor's hematopoietic system.1 This approach lends itself to genetic modification since a modest number of donor cells can be easily harvested, exposed to a vector ex vivo, and then simply infused intravenously into an irradiated recipient. This is in contrast to in vivo or ex vivo gene therapy for solid organs, where the ability to deliver genes to a high percentage of a very large number of cells within a complex tissue structure is extremely challenging. The promise of HSC gene therapy has led to extensive experimentation in small and large animal models, and to successful clinical trials.
HSCs are defined by the ability to self renew, differentiate into all hematopoietic lineages, and reconstitute hematopoiesis in a lethally irradiated host long-term. This definition excludes the use of in vitro assays to evaluate gene transfer to HSCs, and necessitates the use of animal models. The progeny of long-term HSCs expand exponentially in vivo in a hierarchy resulting in multipotent progenitors, progenitors and ultimately billions of mature leukocytes. This imposes some criteria for efficient gene transfer. The HSC must be permissive for transduction by the proposed vector, the vector genome must be efficiently maintained in daughter cells, and transduction must not impair the ability of the HSC to renew, differentiate, or expand. To date only retroviral vectors including gammaretroviral, lentiviral, and foamy vectors have fulfilled these criteria in large animal models. These integrating vectors take advantage of mitosis to create a copy of the vector provirus in each daughter cell, ensuring transmission to all HSC progeny during hematopoiesis. Here we review the advantages of large animal models, contributions of large animal model studies to the field of HSC gene therapy, and recent progress in this field.
Limitations of mouse models for HSC gene therapy
The mouse model has been essential to advance HSC gene therapy, and early studies showed that self-renewing clones with both lymphoid and repopulation potential could be transduced by retroviral vectors.2-4 However, several aspects of gene transfer and transplantation are not modeled well in mice (Table 1). It is not possible to assess long-term engraftment in a short-lived animal model, and differences between mouse and human host cell receptors initially led to overestimates of gene transfer efficiency in the mouse model. Murine leukemia virus (MLV)-based vectors pseudotyped with the murine ecotropic envelope attained very high gene transfer efficiency to primitive mouse repopulating cells, estimated at 50% even with relatively low titers.2 Gene transfer using the ecotropic envelope is restricted to mouse cells, so the amphotropic envelope was used in early large animal and clinical studies.5,6 In these early studies, transient marking of less than 0.1% of repopulating cells was obtained in the dog, and in patients marking was also low, with an estimated average proviral copy number of 0.01 to 0.1. Transduction of dog and human progenitors with the amphotropic envelope is much less efficient than transduction of mouse progenitors with the ecotropic envelope, in part because of low expression of the amphotropic receptor on HSCs.7 This obstacle has been largely overcome by using envelope pseudotypes that efficiently transduce HSCs, including the vesicular somatitis virus glycoprotein (VSV-G).
Table 1.
Comparison of the suitability of animal models for HSC gene therapy
| Property | Mouse | NOD/SCID xenotransplant | Dog | Nonhuman Primate |
|---|---|---|---|---|
| Similarity of host cell receptors | + | +++ | + | ++ |
| Cycling of HSCs | + | +++ | +++ | +++ |
| Telomerase activity | + | +++ | +++ | +++ |
| Multilineage long term repopulation | + | + | +++ | +++ |
| Clinical translation of transplantation protocols | + | + | +++ | +++ |
| Relevance of genotoxicity assays | + | ++ | ++ | +++ |
| Overall ability to predict HSC gene transfer efficiency in humans | + | ++ | +++ | +++ |
| Cost | +++ | ++ | + | + |
However, other differences between mouse and human HSCs affect the transduction efficiency including their cell cycle status. Approximately 75% of mouse HSCs are outside of G0.8 Direct analysis of the cell cycle status of HSCs is not possible in humans or large animals because they cannot be purified to homogeneity. However, studies analyzing telomere length have estimated that mouse HSCs divide every 2.5 weeks, cat HSCs every 8-10 weeks, baboon and macaque HSCs approximately every 36 weeks, and human HSCs every 45 weeks.9-12 This is an important consideration for gene transfer since gammaretrovirus, lentivirus and foamy virus vectors do not transduce quiescent G0 cultures as efficiently as they transduce actively dividing cultures.13-15 In mouse studies, donor mice are typically treated with 5-fluorouracil to increase cell cycling and transduction.2-4 There is also a difference in the proliferative demand placed on repopulating cells of the mouse versus large animals or humans. A human makes a similar number of red blood cells in a day (2.5×1011) to what a mouse makes in a 2-year life span.11 A single cell can repopulate the entire hematopoietic system of a mouse,2,16 but to date engraftment in primates and dogs has always been polyclonal, with the exception of studies where leukemic or pre-leukemic expansion has been observed. Mice also have limitations for evaluating the genotoxicity of integrating viral vectors, since mouse cells are more readily transformed than human cells. It has been estimated that five distinct alterations are required to transform normal human primary cells but that only two or three events are necessary for mouse cells.17
Immunodeficient mouse models that allow transplantation of xenogeneic human repopulating cells have overcome some of these limitations. The latest generation of non-obese diabetic/severe combined immunodeficient (NOD/SCID) immune deficient mice do not express the IL-2 gamma receptor, and allow robust myeloid and lymphoid development in vivo.18,19 Evaluating gene transfer to human SCID repopulating cells (SRCs) in this model is powerful, since the harvest of donor cells, enrichment of HSCs, and ex vivo transduction conditions can be performed exactly as in clinical studies. However, there is some evidence that SRCs are not true long-term repopulating cells. Baboon CD34+ cells marked with a gammaretroviral vector were infused into both NOD/SCID mice and a baboon.20,21 Specific SRC clones were detected in baboon short-term (2-6-week), but not long-term (6-, 12-month) repopulating cells. Also, to date the relatively high levels of HSC gene transfer observed in NOD/SCID mice have not been observed in clinical trials.
The transplantation aspect of HSC gene therapy is also not modeled as well in mice. Early BM transplantation studies in mice were initially not successfully translated to the clinic, and subsequent studies in dogs and primates helped to define the obstacles to engraftment and to establish this now commonly employed therapeutic approach.22 In these studies inbred mouse strains did not predict the importance of HLA-matching for successful transplantation. HLA-matching is not a concern for autologous HSC gene therapy approaches, but other aspects of transplantation are also better modeled in large animals. For example, conditioning regimens with radiation and/or chemotherapy have translated well from the dog model.22 In contrast, in immunodeficient mice, non-myeloablative conditioning allows robust engraftment of even xenogeneic cells. Thus, conditioning regimens which are a critical component of HSC transplantation are not modeled well in the mouse. In non-immunodeficient mouse models the source of cells is different. CD34+ cells are used in large animal models and patients, but typically lineage−, Sca-1+, c-kit+ positive (LSK) cells are used in mice which are CD34low/− (ref16 due to differential expression of CD34 in mouse and human HSCs.23,24
The dog model for HSC gene therapy and the competitive repopulation assay
The dog has several advantages for HSC gene therapy studies. Dogs can be cared for with relative ease, have large litters, and are less expensive to procure and maintain than primates. Performing the many procedures required for HSC gene therapy studies including routine health exams, administering antibiotics, and performing peripheral blood draws and BM biopsies are also more easily performed on dogs than primates. Importantly, unlike in the mouse model, canine and human CD34+ bone marrow cells have similar in vitro and in vivo characteristics.25 Also, long-term studies can be performed in dogs, and repeated sampling of peripheral blood and bone marrow are easily performed. There are canine models of several hematopoietic diseases including alpha-l-iduronidase deficiency, X-linked severe combined immunodeficiency (X-SCID), canine leukocyte adhesion deficiency (CLAD), and pyruvate kinase deficiency, which allow preclinical testing in a disease setting. Another advantage of the dog for allogeneic gene transfer studies is that characterization of the dog leukocyte antigen (DLA) type I and II loci26,27 allows for DLA-matched transplants.
As described above, early studies for canine HSC gene transfer met with limited success as did early clinical studies. However, subsequent studies in the dog established means to efficiently mobilize HSCs, culture these cells ex vivo, transduce them with retroviral vectors, and achieve efficient engraftment in vivo.28-33 Importantly, these conditions have translated well to the clinical setting. The competitive repopulation assay34,35 was key to establishing these conditions since variability in outbred dog populations would have otherwise necessitated the use of many animals. By comparing two or more experimental conditions directly in the same animal, inter-animal variability is eliminated, and a small number of animals can identify the best approach (Figure 1). In early experiments, retroviral vectors with sequences that differed by only a few bp were used to compare different envelope pseudotypes from different retroviral packaging cell lines28 or to evaluate gene transfer to different sources of HSCs.36 The two vectors could be differentiated by PCR amplification and electrophoresis to determine the best conditions for HSC gene transfer. Fluorescent markers such as enhanced green/yellow fluorescent protein (EGFP/EYFP) can also be used which allow accurate evaluation of transgene expression in leukocytes of all hematopoietic lineages by flow cytometry.
Figure 1.

The competitive repopulation assay using fluorescent reporter genes in the dog model. a) The ex vivo transduction is divided into two experimental arms with equivalent numbers of CD34+ cells. A vector expressing enhanced green fluorescent protein (EGFP) is used for one experimental arm, and a different vector different expressing enhanced yellow fluorescent protein (EYFP) is used for the other experimental arm. The two experimental arms can differ in a number of ways including vector type, vector envelope pseudotype, ex vivo transduction protocol, source of CD34+ cells, or route of infusion. By directly comparing one of these variables in the same dog, inter-animal variability is eliminated, and the best approach can be determined with a small number of animals. b) The use of EG/YFP fluorescent reporter genes allows easy and accurate evaluation of transgene expression by flow cytometry. c) Transgene expression can be evaluated long-term in both myeloid and lymphoid lineages.
Gammaretroviruses pseudotyped with either gibbon ape leukemia virus (GALV), feline endogenous retrovirus (RD114) or vesicular stomatitis virus glycoprotein (VSV-G) envelopes can efficiently transduce dog HSCs if the ex vivo transduction is of sufficient length, includes an appropriate cytokine cocktail for canine cells during ex vivo culture, and includes the use of CH-296 fibronectin fragment (Retronectin). CH-296 contains the cell binding and heparin binding domains of fibronectin and enhances gene transfer by co-localizing vector and target cells, inhibiting apoptosis during ex vivo culture, and increasing engraftment.37-39 Primed BM is an excellent source of CD34+ cells, but steady-state marrow and mobilized peripheral blood can also be used.36 Canine granulocyte colony stimulating factor (cG-CSF) and canine stem cell factor (cSCF) are typically used to prime BM, or to mobilize PB CD34+ cells. More recently AMD3100, an antagonist of CXCR4 has been shown effective for mobilization of HSCs40 and AMD3100-mobilized canine HSCs can be efficiently transduced with lentiviral vectors (HPK, unpublished data). Our standard HSC gene transfer protocols have been previously published,41 and efficient gene transfer can been achieved using either gamma, lentivirus or foamy retroviruses (Figure 2). For foamy and lentiviral vectors, pre-stimulation is not required, and a single overnight exposure is sufficient for efficient gene transfer, thereby limiting ex vivo culture which can decrease engraftment.42,43 Interestingly, when foamy and lentiviral vectors were directly compared in a competitive repopulation assay at the same MOI (5) with a short 18-hour ex vivo transduction protocol, the efficiency of long-term marking was remarkably similar for both vector types.44 Canine HSC gene transfer is also efficient in the allogeneic setting, where one promising therapeutic application is chemoprotection of transplanted cells to allow higher dose chemotherapy for lymphoma or leukemia.45
Figure 2.
Efficient long-term HSC gene transfer in the canine model using gamma-, lenti- and foamy retroviral vectors. Transgene expression was detected by flow cytometry for EG/YFP. MLV-based gammaretroviral vector gene transfer (top panel), HIV-based lentiviral vector gene transfer (middle panel), and foamy virus vector gene transfer (bottom panel) to canine myeloid and lymphoid cells.
Therapeutic gene transfer to HSCs in the dog model
Several hematopoietic diseases were first cured in the dog model using conventional marrow allografts, setting the stage for HSC gene therapy. Pyruvate kinase deficiency in Basenji dogs is associated with severe hemolytic anemia, where affected dogs suffer from chronic, regenerative, hemolytic anemia, and red blood cell survival is shortened from one month to a few days.46 Studies in the 1970s showed that marrow allografts from healthy littermates in three young PK-deficient dogs re-established normal hematopoiesis46 and ameliorated the disease phenotype long term.47 Allogeneic transplantation also has cured canine X-SCID,48 CLAD,49 and diminished the severity of lesions in the canine alpha-l-iduronidase deficiency model.50
The first demonstrations of effective HSC gene therapy in the dog model were achieved for CLAD51 and for X-SCID.52 In the CLAD study, ex vivo gene transfer using a gammaretrovirus vector pseudotyped with GALV and expressing the canine leukocyte integrin ITGB2 gene (CD18) resulted in 6 of 11 treated dogs achieving therapeutic levels of corrected leukocytes. In the X-SCID study, in vivo gene delivery to neonates using a RD114-pseudotyped gammaretroviral vector expressing canine interleukin-2 common gamma chain (γc) was used rather than ex vivo transduction. In the setting of X-SCID, corrected cells have a strong selective advantage and the low levels of transduced cells obtained by in vivo delivery were sufficient to correct the disease phenotype. The CLAD phenotype has since also been corrected using foamy vectors.53
Nonhuman primate models for HSC gene therapy
There are some limitations of the dog model that necessitate the use of nonhuman primates for HSC gene therapy studies. The closer genetic relationship of nonhuman primates to humans means that primate cells respond to many human cytokines used for mobilization and ex vivo transduction. Also, for AIDS the dog cannot model the disease, and the nonhuman primate model has been developed.54 The choice of primate species depends on several criteria including ethical considerations, cost, and availability. The highly sentient nature, high cost and low availability of chimpanzees outweigh the advantage of their close phylogenetic relationship to humans. Baboons and macaques are considered to be a more appropriate from an ethical perspective, and are more readily available and thus inexpensive, although the relative expense is still high. As with the dog model, BM transplantation studies established the utility of nonhuman primates,55 and CD34+ cells are used as the stem cell source.56,57
As in the dog model the efficiency of HSC gene transfer to nonhuman primates was initially very low, typically less than 1% (reviewed in58). A number of conclusions were drawn from these early studies as outlined by van Beusechem and Valerio; 1) primate HSCs were more refractory to transduction with gammaretroviral vectors than mouse HSCs, 2) co-culture with packaging cell lines during ex vivo culture of primate HSCs could have deleterious effects on engraftment, 3) appropriate growth factors including SCF, IL-3 and IL-6 could increase engraftment, and 4) the poor results in nonhuman primates were not due to large differences in the size of the graft. In particular, it was recognized that in primates, using the amphotropic receptor did not yield results similar to using the ecotropic receptor in mouse studies. Following these studies, alternate envelope pseudotypes and transduction protocols were evaluated, and efficient gene transfer to nonhuman primate HSCs was established. Comparison of gene transfer using the amphotropic and GALV envelopes in a competitive repopulation assay in the baboon showed that GALV was superior to amphotropic envelope, and that marking between 1 and 5% could be established in vivo as detected by Southern blot.35 In this study large inter-animal variation in gene transfer efficiency highlighted the importance of the competitive repopulation assay. The use of CH-296 fragment and the addition of MGDF and Flt-3 ligand to the ex vivo transduction further improved gene transfer into the 5-20% range,59 and stable long-term marking in over 60% of PB cells can be obtained when GALV-pseudotyped vectors are used with CH-296 and a cytokine cocktail of SCF, IL-3, IL-6, MGDF, Flt3-L, and G-CSF.60
Lentiviral vectors also mediate efficient HSC gene transfer in nonhuman primates, but host restriction mediated by TRIM5 alpha must be taken into account when using lentiviral vectors.61 In baboons, highly variable gene transfer was observed using VSV-G pseudotyped HIV-derived lentiviral vectors, but in one animal marking of up to 8.6% of long-term repopulating cells was achieved. In this study a direct comparison of lentiviral and gammaretroviral marking using a short ex vivo transduction protocol designed to maintain the engraftment potential of HSCs showed that lentiviral vectors were superior. However, very high MOIs (54-156) were required for efficient lentiviral transduction. Also, the addition of cytokines was necessary for efficient transduction in this study, as has also been observed for efficient transduction of human hematopoietic progenitors, and SRCs.62,63
In the rhesus macaque, transduction with HIV-derived lentiviral vectors is potently inhibited by TRIM5 alpha,61-66 and long-term marking with HIV-based vectors was typically lower than 3%, with one animal having marking levels of approximately 10% and 2% in granulocytes and lymphocytes, respectively.67,68 Efficient transduction of approximately 18% of rhesus macaque repopulating cells69 can be achieved using SIV-based vectors, and expression is maintained long-term.70 However, SIV vectors do not efficiently transduce human CD34+ cells69 which would limit the utility of the rhesus macaque model for preclinical studies. This limitation has been overcome in two ways. Unlike the rhesus macaque, the pigtailed macaque (Macaca nemestrina) is permissive for HIV-1 replication, although in vivo replication is inefficient.71 We thus examined HSC gene transfer in the pigtailed macaque using HIV-derived lentiviral vectors and observed highly efficient gene transfer with marking levels of 20–23% in granulocytes and 12–23% in lymphocytes.72 Efficient gene transfer was attained at MOIs of only 5–10 using a short ex vivo transduction protocol. Concurrent studies identified truncated forms of TRIM5 alpha in pigtailed macaques that do not restrict HIV-1 infection,73 likely explaining why their HSCs can be efficiently transduced by HIV-derived vectors at a low MOI. Recently, another approach was developed to overcome the limitations of the rhesus model. A lentiviral vector capable of transducing both human and rhesus blood cells was developed by combining components of both HIV-1 and SIV, including the SIV capsid (sCA) and SIV-Vif.74 As would be expected, this chimeric vector compared favorably to an HIV-1 vector in rhesus macaque hematopoietic repopulating cells, and high marking levels were attained in vivo (7–30%). Importantly, the chimeric vector efficiently transduced human CD34+ cells, albeit not as efficiently as HIV-based vectors at higher MOIs.74
HIV gene therapy in the macaque large animal model
For HIV gene therapy studies, primates are particularly advantageous. Despite substantial efforts to generate a vaccine for HIV-AIDS, there is still no clearly effective vaccine available,75 and alternative therapies have been explored including treating AIDS by transplanting genetically modified HSCs. In this approach, HIV-resistant HSCs might reconstitute the entire hematopoietic system, including the targets of HIV; T cells, macrophages dendritic cells and microglial cells. Allogeneic transplantation with naturally resistant CCR5-negative HSCs has controlled HIV replication in a patient with AIDS, offering proof-of-principle that this is a viable therapeutic approach.76 However, inefficient delivery of anti-HIV transgenes to HSCs in clinical trials has been an obstacle. In two clinical studies using gammaretroviral vectors, long-term marking was less than 0.01%.53,77 More recently, in a phase 2 clinical trial, marking was very low, and vector DNA did not reach the quantifiable range of the assay (0.38% of cells analyzed) in any blood cell sample at any time point.78 The authors concluded that the approach was promising, but that improvements to increase engraftment were needed.
Given these limitations, the nonhuman primate is an excellent model to explore methods to efficiently deliver anti-HIV genes into long-term repopulating cells. Macaques can be infected with HIV-SIV (SHIV) chimeras,54 allowing in vivo testing of the efficacy of transgenes that target HIV-1. SHIV chimeras contain HIV-1 envelope, tat and rev, so transgenes that inhibit fusion79,80 and that target tat and rev81 can be evaluated in this model. There are limitations of the SHIV-macaque model since some components of SHIV are based on SIV, and the pathology and viral replication in vivo do not exactly replicate HIV-1 infection.82,83 However, many aspects of the pathology are well-modeled including profound CD4+ T-cell loss, immunodeficiency and other organ-specific disease including encephalitis.54 Continued improvements to the SHIV-macaque model may overcome some of these limitations.84 In a landmark study, An et al. showed that a CCR5-specific shRNA could be delivered to rhesus macaque HSCs using SIV-derived vectors, and that CD4+ lymphocytes derived in vivo were protected from SIV infection in an ex vivo challenge.85 We have also established efficient transduction of pigtailed macaque HSCs using an HIV-based vector that expresses a transmembrane bound HIV fusion inhibitor,79 and shown protection of macaque CD4+ lymphocytes after ex vivo challenge (manuscript under consideration).
Other large animal models for HSC gene therapy
Sheep have also been used for xenogeneic transplantation of human repopulating cells.86 Stem cell kinetics are better modeled in cats than in mice10 and cats have also been used for gene therapy studies.87
In vivo selection in large animal models
To date, therapeutic efficacy in HSC gene therapy trials has been limited to immunodeficiencies, where corrected cells have a selective advantage. However, diseases such as beta-thalassemia require relatively high levels of gene transfer, estimated at 20%.88 One approach to consistently achieve high marking levels is to add a transgene that can mediate in vivo selection in a bicistronic design. Several approaches have been developed for this purpose, but to date selection using methylguanine methyltransferase (MGMT) has been the only method to effect stable selection in large animal HSCs (reviewed in89). In this approach HSCs are transduced with a mutant form of MGMT that is resistant to O6-benzylguanine (O6BG) which inhibits endogenous MGMT.90
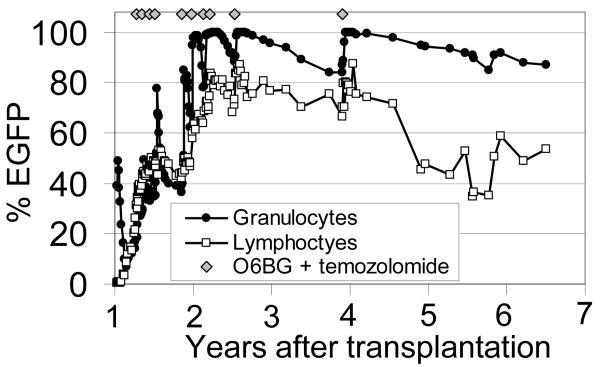
In the canine model marking can be stably increased using MGMT with over 80% of repopulating granulocytes stably expressing the transgene of interest (Figure 3 and references45,91). A recent report of selection in rhesus macaques resulted in only transient selection in vivo,92 but we have obtained efficient and stable long-term selection to over 60% in the pigtailed macaque using HIV-based vectors and 80% in the baboon model using gammaretroviral vectors (manuscript under consideration). One important difference in these two studies, was that animals with stable increases had higher marking levels prior to initiating treatment with O6BG and the alkylating agent.
Figure 3.
Efficient and stable MGMTP140K-mediated in vivo selection in the dog model. A gammaretroviral vector expressing the P140K mutant MGMT mediates efficient selection after treatment with temozolomide and O6BG. Treatments were well tolerated, and resulted in chemoprotection. This research was originally published in Blood. Brian C. Beard, Reeteka Sud, Kirsten A. Keyser, Christina Ironside, Tobias Neff, Sabine Gerull, Grant D. Trobridge and Hans-Peter Kiem. Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients. Blood. 2009; Vol. 113, pp. 5094-5103. © the American Society of Hematology.
Genotoxicity studies in large animal models
After clonal expansion leading to frank leukemia occurred in the French X-linked SCID gene therapy trial,93 a comprehensive study of large animals that received gammaretroviral transduced HSCs was performed.94 In this study there was no evidence of progression towards oligoclonal or monoclonal hematopoiesis in 42 rhesus macaques, 23 baboons, and 17 dogs that had significant levels of gammaretroviral-mediated gene transfer. An exception was observed in a rhesus macaque where a fatal myeloid sarcoma, a type of acute myeloid leukemia, occurred in the kidney five years after transplantation of CD34+ cells transduced with a murine stem cell virus (MSCV)-based gammaretroviral vector.95 This animal was unusual in that marking during the first year after transplant was very high, with up to 80% marking in myeloid cells, and most marked cells were derived from one clone. Analysis of the tumor showed two clonal vector insertions, and one was in the anti-apoptotic gene BCL2-A1. Overall, these studies suggested that although gammaretroviral vector-mediated leukemias can occur, disease or transgene-specific factors likely contributed to the leukemogenesis frequently observed in the X-SCID trial.
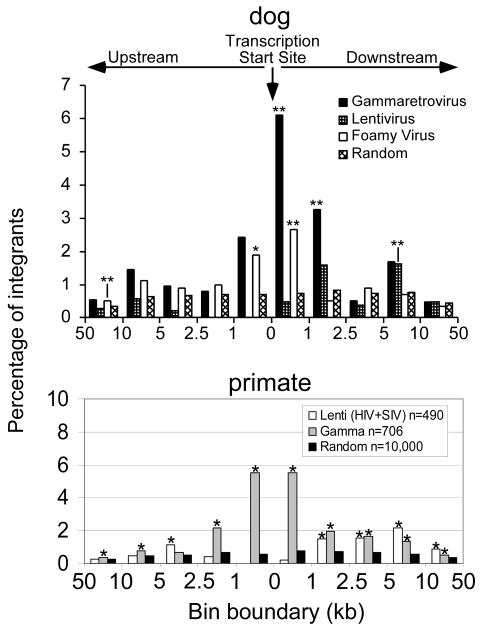
A comparison of MLV-based gammaretroviral, lentiviral and foamy virus integrants in canine repopulating cells showed that the unique integration profiles observed in vitro for all three vector classes were also observed in dog long-term repopulating cells (Figure 4 and reference96). MLV vector proviruses were found more frequently within and close to proto-oncogene transcription sites than lentiviral or foamy vectors. In primate repopulating cells, the distinct integration profile for lentiviral and MLV vectors observed in vitro was also seen in repopulating cells (Figure 4 and references97,98). Both vector types were found more frequently in and near proto-oncogenes in repopulating cells than in a random dataset. Analysis of functional classes of genes with integrants within 100 kilobases (kb) of their transcription start sites showed an over-representation of genes involved in growth or survival near both lentiviral and MLV integrants. Microarray analysis showed that both MLV and lentiviral vectors were found close to genes with high expression levels in primitive cells enriched for hematopoietic stem cells,98 consistent with the observation of an over-representation of primate integrants near the MDS1/EVI1 locus in repopulating cells.99 However, in these studies there was no evidence of progression to leukemia. Avian sarcoma leukosis virus (ASLV) gammaretroviral vectors were also analyzed in rhesus repopulating cells.100 ASLV vectors did not favor gene-rich regions, transcription start sites, or CpG islands. Importantly, they were not enriched within or near proto-oncogenes, and no insertions were observed close to, or within, the MDS1/EVI1 locus.
Figure 4.
Retroviral integration site profiles in dog and nonhuman primate long term repopulating sites. The top panel shows the location of integration sites relative to the transcription start sites of Refseq genes for 114 gamma-, 327 lenti- and 341 foamy retroviral intergrants in dog long-term repopulating cells. The bottom panel shows MLV-based gammaretroviral integration sites and lentiviral integration sites (HIV and SIV) in nonhuman primate repopulating cells. Asterisks mark significant differences from a random data set, * is p<0.01 and ** is p<0.001. Frequencies are normalized as the percentage of total integrants per kilobase. This research was originally published in Human Gene Therapy. Brian C. Beard, Kirsten A. Keyser, Grant D. Trobridge, Laura J. Peterson, Daniel G. Miller, Michael Jacobs, Rajinder Kaul, Hans-Peter Kiem. Unique integration profiles in a canine model of long-term repopulating cells transduced with gammaretrovirus, lentivirus, or foamy virus. Human Gene Therapy. 2007; Vol. 18, pp. 423-434, and in Molecular Therapy. Brian C. Beard, David Dickerson, Kate Beebe, Christina Gooch, James Fletcher, Tulin Okbinoglu, Daniel G Miller, Michael A Jacobs, Rajinder Kaul, Hans-Peter Kiem, and Grant D. Trobridge. 2007; Vol. 15 pp. 1356–1365.
Summary
Large animal models have been instrumental in developing effective HSC gene therapy approaches and continue to play an important role in translating HSC gene therapy approaches to the clinic. Improved immunodeficient mice strains have extended the utility of mouse models, but the ability to mediate efficient long-term gene transfer into large animal HSCs with multi-lineage repopulating ability is still the best way to predict clinical efficacy. Currently, there are several disease-specific models in large animals, and with improvements in dog and primate embryonic stem cell and transgenic technology, it is likely that additional hematopoietic disease-specific large animal models will be developed.
Acknowledgments
This work was supported in part by grants DK077806, HL53750, AI061839, AI063959, and DK56465 from the National Institutes of Health, Bethesda, MD. H.P.K. is a Markey Molecular Medicine Investigator and the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research. We also acknowledge the assistance of Bonnie Larson, Helen Crawford and Christina Ironside in preparing the manuscript.
References
- 1.Lorenz E, Uphoff D, Reid TR, Shelton E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst. 1951;12:197–201. [PubMed] [Google Scholar]
- 2.Dick JE, Magli MC, Huszar D, Phillips RA, Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985;42:71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- 3.Williams DA, Lemischka IR, Nathan DG, Mulligan RC. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984;310:476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- 4.Keller G, Paige C, Gilboa E, Wagner EF. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985;318:149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- 5.Stead RB, Kwok WW, Storb R, Miller AD. Canine model for gene therapy: Inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988;71:742–747. [PubMed] [Google Scholar]
- 6.Brenner MK, Rill DR, Holladay MS, Heslop HE, Moen RC, Buschle M, et al. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993;342:1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Girard LJ, Jordan CT, Anderson SM, Cline AP, Bodine DM. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. PNAS. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shepherd BE, Kiem HP, Lansdorp PM, Dunbar CE, Aubert G, Larochelle A, et al. Hematopoietic stem-cell behavior in nonhuman primates. Blood. 2007;110:1806–1813. doi: 10.1182/blood-2007-02-075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abkowitz JL, Catlin SN, Guttorp P. Evidence that hematopoiesis may be a stochastic process in vivo. Nat Med. 1996;2:190–197. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- 11.Abkowitz JL, Persik MT, Shelton GH, Ott RL, Kiklevich JV, Catlin SN, et al. Behavior of hematopoietic stem cells in a large animal. PNAS. 1995;92:2031–2035. doi: 10.1073/pnas.92.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttorp P, Newton MA, Abkowitz JL. A stochastic model for haematopoiesis in cats. IMA Journal of Mathematics Applied in Medicine & Biology. 1990;7:125–143. doi: 10.1093/imammb/7.2.125. [DOI] [PubMed] [Google Scholar]
- 13.Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. erratum appears in Mol Cell Biol 1992 Jan;12(1):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 15.Trobridge G, Russell DW. Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors. J Virol. 2004;78:2327–2335. doi: 10.1128/JVI.78.5.2327-2335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 17.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. erratum appears in N Engl J Med. 2003 Feb 13;348(7):674 Review. [DOI] [PubMed] [Google Scholar]
- 18.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, et al. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 19.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor [gamma] chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem HP. Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates. Blood. 2003;102:4329–4335. doi: 10.1182/blood-2003-01-0082. [DOI] [PubMed] [Google Scholar]
- 21.Mezquita P, Beard B, Kiem HP. NOD/SCID repopulating cells contribute only to short-term repopulation in the baboon. Gene Ther. 2008;15:1460–1462. doi: 10.1038/gt.2008.108. [DOI] [PubMed] [Google Scholar]
- 22.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–15. [PubMed] [Google Scholar]
- 23.Drew E, Merkens H, Chelliah S, Doyonnas R, McNagny KM. CD34 is a specific marker of mature murine mast cells. Exp Hematol. 2002;30:1211. doi: 10.1016/s0301-472x(02)00890-1. [DOI] [PubMed] [Google Scholar]
- 24.Okuno Y, Iwasaki H, Huettner CS, Radomska HS, Gonzalez DA, Tenen DG, et al. Differential regulation of the human and murine CD34 genes in hematopoietic stem cells. PNAS. 2002;99:6246–6251. doi: 10.1073/pnas.092027799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suter SE, Gouthro TA, McSweeney PA, Nash RA, Haskins ME, Felsburg PJ, et al. Isolation and characterization of pediatric canine bone marrow CD34+ cells. Veterinary Immunology & Immunopathology. 2004;101:31–47. doi: 10.1016/j.vetimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Wagner JL, Burnett RC, Storb R. Organization of the canine major histocompatibility complex: current perspectives. J Hered. 1999;90:35–38. doi: 10.1093/jhered/90.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Venkataraman GM, Stroup P, Graves SS, Storb R. An improved method for dog leukocyte antigen 88 typing and two new major histocompatibility complex class I alleles, DLA-88*01101 and DLA-88*01201. Tissue Antigens. 2007;70:53–57. doi: 10.1111/j.1399-0039.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 28.Kiem HP, McSweeney PA, Bruno B, Goerner M, Buron G, Morris J, et al. Improved gene transfer into canine hematopoietic repopulating cells using CD34-enriched marrow cells in combination with a gibbon ape leukemia virus–pseudotype retroviral vector. Gene Ther. 1999;6:966–972. doi: 10.1038/sj.gt.3300925. [DOI] [PubMed] [Google Scholar]
- 29.Goerner M, Bruno B, McSweeney PA, Buron G, Storb R, Kiem HP. The use of granulocyte colony-stimulating factor during retroviral transduction on fibronectin fragment CH-296 enhances gene transfer into hematopoietic repopulating cells in dogs. Blood. 1999;94:2287–2292. [PubMed] [Google Scholar]
- 30.Goerner M, Horn PA, Peterson L, Kurre P, Storb R, Rasko JEJ, et al. Sustained multilineage gene persistence and expression in dogs transplanted with CD34+ marrow cells transduced by RD114-pseudotype oncoretrovirus vectors. Blood. 2001;98:2065–2070. doi: 10.1182/blood.v98.7.2065. [DOI] [PubMed] [Google Scholar]
- 31.Horn PA, Keyser KA, Peterson LJ, Neff T, Thomasson BM, Thompson J, et al. Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood. 2004;103:3710–3716. doi: 10.1182/blood-2003-07-2414. [DOI] [PubMed] [Google Scholar]
- 32.Kiem HP, Darovsky B, von Kalle C, Goehle S, Graham T, Miller AD, et al. Long-term persistence of canine hematopoietic cells genetically marked by retrovirus vectors. Hum Gene Ther. 1996;7:89–96. doi: 10.1089/hum.1996.7.1-89. [DOI] [PubMed] [Google Scholar]
- 33.Kiem HP, Allen J, Trobridge G, Olson E, Keyser K, Peterson L, et al. Foamy virus-mediated gene transfer to canine repopulating cells. Blood. 2007;109:65–70. doi: 10.1182/blood-2006-04-016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 35.Kiem HP, Heyward S, Winkler A, Potter J, Allen JM, Miller AD, et al. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 36.Thomasson B, Peterson L, Thompson J, Goerner M, Kiem HP. Direct comparison of steady-state marrow, primed marrow, and mobilized peripheral blood for transduction of hematopoietic stem cells in dogs. Hum Gene Ther. 2003;14:1683–1686. doi: 10.1089/104303403322542329. [DOI] [PubMed] [Google Scholar]
- 37.Donahue RE, Sorrentino BP, Hawley RG, An DS, Chen IS, Wersto RP. Fibronectin fragment CH-296 inhibits apoptosis and enhances ex vivo gene transfer by murine retrovirus and human lentivirus vectors independent of viral tropism in nonhuman primate CD34+ cells. Mol Ther. 2001;3:359–367. doi: 10.1006/mthe.2001.0269. [DOI] [PubMed] [Google Scholar]
- 38.Dao MA, Hashino K, Kato I, Nolta JA. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood. 1998;92:4612–4621. [PubMed] [Google Scholar]
- 39.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 40.Burroughs L, Mielcarek M, Little MT, Bridger G, MacFarland R, Fricker S, et al. Durable engraftment of AMD3100-mobilized autologous and allogeneic peripheral blood mononuclear cells in a canine transplantation model. Blood. 2005;106:4002–4008. doi: 10.1182/blood-2005-05-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beard BC, Kiem HP. Canine models of gene-modified hematopoiesis. Methods in Molecular Biology. 2009;23:341–361. doi: 10.1007/978-1-59745-409-4_23. [DOI] [PubMed] [Google Scholar]
- 42.Peters SO, Kittler ELW, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 43.Tisdale JF, Hanazono Y, Sellers SE, Agricola BA, Metzger ME, Donahue RE, et al. Ex vivo expansion of genetically marked Rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 44.Trobridge GD, Allen JM, Peterson L, Ironside CG, Russell DW, Kiem HP. Foamy and lentiviral vectors transduce canine long-term repopulating cells at similar efficiency. Hum Gene Ther. 2009;20:519–523. doi: 10.1089/hum.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beard BC, Sud R, Keyser KA, Ironside C, Neff T, Gerull S, et al. Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients. Blood. 2009;113:5094–5103. doi: 10.1182/blood-2008-09-176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiden PL, Storb R, Graham TC, Schroeder ML. Severe hereditary haemolytic anaemia in dogs treated by marrow transplantation. Br J Haematol. 1976;33:357–362. doi: 10.1111/j.1365-2141.1976.tb03551.x. [DOI] [PubMed] [Google Scholar]
- 47.Weiden PL, Hackman RC, Deeg HJ, Graham TC, Thomas ED, Storb R. Long-term survival and reversal of iron overload after marrow transplantation in dogs with congenital hemolytic anemia. Blood. 1981;57:66–70. [PubMed] [Google Scholar]
- 48.Felsburg PJ, Somberg RL, Hartnett BJ, Suter SF, Henthorn PS, Moore PF, et al. Full immunologic reconstitution following nonconditioned bone marrow transplantation for canine X-linked severe combined immunodeficiency. Blood. 1997;90:3214–3221. [PubMed] [Google Scholar]
- 49.Creevy KE, Bauer TR, Jr, Tuschong LM, Embree LJ, Silverstone AM, Bacher JD, et al. Mixed chimeric hematopoietic stem cell transplant reverses the disease phenotype in canine leukocyte adhesion deficiency. Vet Immunol Immunopathol. 2003;95:113–121. doi: 10.1016/s0165-2427(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 50.Breider MA, Shull RM, Constantopoulos G. Long-term effects of bone marrow transplantation in dogs with mucopolysaccharidosis I. Am J Pathol. 1989;134:677–692. [PMC free article] [PubMed] [Google Scholar]
- 51.Bauer TR, Hai M, Tuschong LM, Burkholder TH, Gu YC, Sokolic RA, et al. Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex vivo hematopoietic stem cell gene therapy. Blood. 2006;108:3313–3320. doi: 10.1182/blood-2006-03-006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ting-De Ravin SS, Kennedy DR, Naumann N, Kennedy JS, Choi U, Hartnett BJ, et al. Correction of canine X-linked severe combined immunodeficiency by in vivo retroviral gene therapy. Blood. 2006;107:3091–3097. doi: 10.1182/blood-2005-10-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer G, Selander D, Engel B, Carbonaro D, Csik S, Rawlings S, et al. Gene therapy for pediatric AIDS (Review) Ann NY Acad Sci. 2000;918:318–329. doi: 10.1111/j.1749-6632.2000.tb05501.x. [DOI] [PubMed] [Google Scholar]
- 54.Joag SV. Primate models of AIDS (Review) Microbes and Infection. 2000;2:223–229. doi: 10.1016/s1286-4579(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 55.van Bekkum DW. The rhesus monkey as a preclinical model for bone marrow transplantation. Transplant Proc. 1978;10:105–111. [PubMed] [Google Scholar]
- 56.Andrews RG, Bryant EM, Bartelmez SH, Muirhead DY, Knitter GH, Bensinger W, et al. CD34+ marrow cells, devoid of T and B lymphocytes, reconstitute stable lymphopoiesis and myelopoiesis in lethally irradiated allogeneic baboons. Blood. 1992;80:1693–1701. [PubMed] [Google Scholar]
- 57.Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, et al. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Beusechem VW, Valerio D. Gene transfer into hematopoietic stem cells of nonhuman primates [Review] Hum Gene Ther. 1996;7:1649–1668. doi: 10.1089/hum.1996.7.14-1649. [DOI] [PubMed] [Google Scholar]
- 59.Kiem HP, Andrews RG, Morris J, Peterson L, Heyward S, Allen JM, et al. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 60.Horn PA, Topp MS, Morris JC, Riddell SR, Kiem HP. Highly efficient gene transfer into baboon marrow repopulating cells using GALV-pseudotype oncoretroviral vectors produced by human packaging cells. Blood. 2002;100:3960–3967. doi: 10.1182/blood-2002-05-1359. [DOI] [PubMed] [Google Scholar]
- 61.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 62.Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G, et al. Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zielske SP, Gerson SL. Cytokines, including stem cell factor alone, enhance lentiviral transduction in nondividing human LTCIC and NOD/SCID repopulating cells. Mol Ther. 2003;7:325–333. doi: 10.1016/s1525-0016(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 64.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79:3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.An DS, Kung SK, Bonifacino A, Wersto RP, Metzger ME, Agricola BA, et al. Lentivirus vector-mediated hematopoietic stem cell gene transfer of common gamma-chain cytokine receptor in rhesus macaques. J Virol. 2001;75:3547–3555. doi: 10.1128/JVI.75.8.3547-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An DS, Wersto RP, Agricola BA, Metzger ME, Lu S, Amado RG, et al. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34(+) cells. J Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Kim YS, Larochelle A, Renaud G, Wolfsberg TG, Adler R, et al. Sustained high-level polyclonal hematopoietic marking and transgene expression 4 years after autologous transplantation of rhesus macaques with SIV lentiviral vector-transduced CD34+ cells. Blood. 2009;113:5434–5443. doi: 10.1182/blood-2008-10-185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, et al. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992;257:103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- 72.Trobridge GD, Beard BC, Gooch C, Wohlfahrt M, Olsen P, Fletcher J, et al. Efficient transduction of pigtailed macaque hemtopoietic repopulating cells with HIV-based lentiviral vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brennan G, Kozyrev Y, Kodama T, Hu SL. Novel TRIM5 isoforms expressed by Macaca nemestrina. J Virol. 2007;81:12210–12217. doi: 10.1128/JVI.02499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uchida N, Washington KN, Hayakawa J, Hsieh MM, Bonifacino AC, Krouse AE, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cold shower for AIDS vaccines (Editorial) Nat Med. 2007;13:1389–1390. doi: 10.1038/nm1207-1389. [DOI] [PubMed] [Google Scholar]
- 76.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 77.Amado RG, Mitsuyasu RT, Rosenblatt JD, Ngok FK, Bakker A, Cole S, et al. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum Gene Ther. 2004;15:251–262. doi: 10.1089/104303404322886101. [DOI] [PubMed] [Google Scholar]
- 78.Mitsuyasu RT, Merigan TC, Carr A, Zack JA, Winters MA, Workman C, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15:285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egelhofer M, Brandenburg G, Martinius H, Schult-Dietrich P, Melikyan G, Kunert R, et al. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol. 2004;78:568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hildinger M, Dittmar MT, Schult-Dietrich P, Fehse B, Schnierle BS, Thaler S, et al. Membrane-anchored peptide inhibits human immunodeficiency virus entry. J Virol. 2001;75:3038–3042. doi: 10.1128/JVI.75.6.3038-3042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li MJ, Bauer G, Michienzi A, Yee JK, Lee NS, Kim J, et al. Inhibition of HIV-1 infection by lentiviral vectors expressing Pol III-promoted anti-HIV RNAs. Molecular Therapy. 2003;8:196–206. doi: 10.1016/s1525-0016(03)00165-5. [DOI] [PubMed] [Google Scholar]
- 82.Korth MJ, Taylor MD, Katze MG. Interferon inhibits the replication of HIV-1, SIV, and SHIV chimeric viruses by distinct mechanisms. Virology. 1998;247:265–273. doi: 10.1006/viro.1998.9249. [DOI] [PubMed] [Google Scholar]
- 83.Hu SL. Non-human primate models for AIDS vaccine research (Review) Current Drug Targets - Infectious Disorders. 2005;5:193–201. doi: 10.2174/1568005054201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hatziioannou T, Ambrose Z, Chung NP, Piatak M, Jr, Yuan F, Trubey CM, et al. A macaque model of HIV-1 infection. PNAS. 2009;106:4425–4429. doi: 10.1073/pnas.0812587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.An DS, Donahue RE, Kamata M, Poon B, Metzger M, Mao SH, et al. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. PNAS. 2007;104:13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lucas ML, Seidel NE, Porada CD, Quigley JG, Anderson SM, Malech HL, et al. Improved transduction of human sheep repopulating cells by retrovirus vectors pseudotyped with feline leukemia virus type C or RD114 envelopes. Blood. 2005;106:51–58. doi: 10.1182/blood-2004-11-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Josephson NC, Sabo KM, Abkowitz JL. Transduction of feline hematopoietic cells by oncoretroviral vectors pseudotyped with the subgroup A feline leukemia virus (FeLV-A) Molecular Therapy. 2000;2:56–62. doi: 10.1006/mthe.2000.0090. [DOI] [PubMed] [Google Scholar]
- 88.Persons DA, Allay ER, Sabatino DE, Kelly P, Bodine DM, Nienhuis AW. Functional requirements for phenotypic correction of murine beta-thalassemia: implications for human gene therapy. Blood. 2001;97:3275–3282. doi: 10.1182/blood.v97.10.3275. [DOI] [PubMed] [Google Scholar]
- 89.Trobridge G, Beard BC, Kiem HP. Hematopoietic stem cell transduction and amplification in large animal models. Hum Gene Ther. 2005;16:1355–1366. doi: 10.1089/hum.2005.16.1355. [DOI] [PubMed] [Google Scholar]
- 90.Crone TM, Goodtzova K, Edara S, Pegg AE. Mutations in human O6-alkylguanine-DNA alkyltransferase imparting resistance to O6-benzylguanine. Cancer Res. 1994;54:6221–6227. [PubMed] [Google Scholar]
- 91.Neff T, Beard BC, Peterson LJ, Anandakumar P, Thompson J, Kiem HP. Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood. 2005;105:997–1002. doi: 10.1182/blood-2004-08-3169. [DOI] [PubMed] [Google Scholar]
- 92.Larochelle A, Choi U, Shou Y, Naumann N, Loktionova NA, Clevenger JR, et al. In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene. J Clin Invest. 2009;119:1952–1963. doi: 10.1172/JCI37506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hacein-Bey-Abina S, von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. erratum appears in Science 2003 Oct 24;302(5645):568. [DOI] [PubMed] [Google Scholar]
- 94.Kiem HP, Sellers S, Thomasson B, Morris JC, Tisdale JF, Horn PA, et al. Long-term clinical and molecular follow-up of large animals receiving retrovirally transduced stem and progenitor cells: no progression to clonal hematopoiesis or leukemia. Molecular Therapy. 2004;9:389–395. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 95.Seggewiss R, Pittaluga S, Adler RL, Guenaga FJ, Ferguson C, Pilz IH, et al. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood. 2006;107:3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beard BC, Keyser KA, Trobridge GD, Peterson LJ, Miller DG, Jacobs M, et al. Unique integration profiles in a canine model of long-term repopulating cells transduced with gammaretrovirus, lentivirus, and foamy virus. Hum Gene Ther. 2007;18:423–434. doi: 10.1089/hum.2007.011. [DOI] [PubMed] [Google Scholar]
- 97.Hematti P, Hong BK, Ferguson C, Adler R, Hanawa H, Sellers S, et al. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biology. 2004;2:e423. doi: 10.1371/journal.pbio.0020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beard BC, Dickerson D, Beebe K, Gooch C, Fletcher J, Okbinoglu T, et al. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Molecular Therapy. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- 99.Calmels B, Ferguson C, Laukkanen MO, Adler R, Faulhaber M, Hyeoung-Joon K, et al. Recurrent retroviral vector integration at the Mds1-Evi1 locus in nonhuman primate hematopoietic cells. Blood. 2005;106:2530–2533. doi: 10.1182/blood-2005-03-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu J, Renaud G, Gomes TJ, Ferris A, Hendrie PC, Donahue RE, et al. Reduced genotoxicity of avian sarcoma leukosis virus vectors in rhesus long-term repopulating cells compared to standard murine retrovirus vectors. Molecular Therapy. 2008;16:1617–1623. doi: 10.1038/mt.2008.135. erratum appears in Mol Ther 2008 Oct;16(10):1770. [DOI] [PMC free article] [PubMed] [Google Scholar]