Abstract
Objective
To determine current rates of and trends in hospitalizations for community-acquired pneumonia (CAP) and CAP - associated complications in children.
Methods
We performed a cross-sectional retrospective cohort study using the 1997, 2000, 2003, and 2006 Kids’ Inpatient Database. National estimates for CAP and CAP - associated local and systemic complications were calculated for children ≤ 18 years of age using complex survey statistics. Patients with comorbid conditions or in-hospital birth status were excluded. Percent change was calculated using 1997 (pre-PCV7) and 2006 (post-PCV7) data.
Results
There were a combined 619,102 discharges for 1997, 2000, 2003, and 2006 after inclusion and exclusion criteria were applied. Overall rates of CAP discharges did not change substantially between 1997 and 2006, but stratification by age revealed a 22% decrease for children < 1 year, minimal change for children 1–5 years, and an increase in rates for children 6–12 years (22%) and ≥ 13 years (41%). Rates of systemic complications were highest among children < 1 year but decreased by 36%. In all other age groups, systemic complication rates remained stable. Rates of local complications increased 78% overall, from 5.4 to 9.6 per 100,000. Children ages 1–5 years had the highest rate of local complications (16.5 per 100,000).
Conclusions
Since the introduction of PCV7 in 2000, rates of CAP-associated systemic complications decreased only in children < 1 year of age. Rates of pediatric CAP-associated local complications are increasing in all age groups. More research is needed to determine the factors underlying these trends.
Keywords: Pneumonia, empyema, pleural, epidemiology, heptavalent pneumococcal vaccine
BACKGROUND
Streptococcus pneumoniae is the most commonly identified bacterial cause of community-acquired pneumonia (CAP) in children. In February 2000 a heptavalent pneumococcal conjugate vaccine (PCV7) was licensed in the U.S. and subsequently added to the routine childhood vaccination schedule. Since then, overall rates of invasive pneumococcal disease (i.e. bacteremia, meningitis) have declined in both children1–7 and adults8–10, largely due to significant reductions in the burden of disease caused by vaccine serotype isolates. However, reductions in the incidence of pediatric CAP appear less dramatic and have been limited to young children. In pre-licensure randomized controlled trials, the risk of radiographically-confirmed pneumonia was approximately 20% lower in PCV7 recipients < 2 years of age compared with non-recipients.11 Post-licensure epidemiologic studies revealed decreases in all-cause pneumonia incidence of 39–52% in children less than 2 years of age,12, 13 but no change in older children.13, 14
The impact of PCV7 vaccination on the severity of pediatric CAP is less clear. Although vaccination with PCV7 has reduced the incidence of invasive pneumococcal disease (IPD) in children, several authors have reported regional increases in pediatric empyema, a CAP-associated complication, following widespread PCV7 uptake.15–17 Studies examining national trends in pneumonia-associated complications have also focused solely on empyema18, 19 and were limited to infants and preschool children.18 Among adults hospitalized with CAP, prior recipients of a 23-valent polysaccharide pneumococcal vaccine (PPV23) had lower all-cause mortality rates, risk of respiratory failure, sepsis syndrome, and cardiac arrest compared with vaccine non-recipients.20 To our knowledge, there have been no studies evaluating the impact of PCV7 introduction on the severity of illness in children hospitalized with CAP.
We conducted a retrospective cohort study using a national database to determine the rate of hospitalizations for CAP and CAP-associated complications in otherwise healthy children in the U.S. and to describe changes in rates, if any, following the introduction of PCV7.
METHODS
Study Design and Data Source
We performed a cross-sectional analysis of pediatric hospitalizations in the U.S. using the 1997, 2000, 2003, and 2006 Kids’ Inpatient Database (KID). KID is part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality (AHRQ). It is the only dataset on hospital use and outcomes specifically designed to study children’s use of hospital services in the U.S. The KID samples pediatric discharges from all community non-rehabilitation hospitals, including academic medical centers, in states participating in HCUP, using a complex stratification system, across pediatric discharge type and hospital characteristics. Discharge-level weights assigned to discharges within the stratum permit calculation of national estimates. Each dataset contains approximately 3 million discharges (unweighted) and is released every three years, beginning with 1997. The 2006 KID is the most recently available dataset and contains hospital administrative data from 38 states, representing 88.8% of the estimated U.S. population.21
Study Participants
Inclusion Criteria
Patients ≤ 18 years of age were eligible for inclusion if they required hospitalization for CAP in 1997, 2000, 2003, or 2006.
Definition of Pneumonia
Using a previously validated algorithm, patients were considered as having CAP if they met one of two criteria: 1) International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9 CM) primary diagnosis code indicating pneumonia (480–483, 485–486), empyema (510), or pleurisy (511.0–1, 511.9), or 2) Primary diagnosis of pneumonia-related symptom (e.g. cough, fever, tachypnea; see Table A of Appendix for ICD-9 CM codes) and secondary diagnosis of pneumonia, empyema or pleurisy.22
Exclusion Criteria
Patients with the following comorbid conditions were excluded as these comorbidities are characterized by risk factors not reflective of the general pediatric population: acquired and congenital immunologic disorders, malignancy, collagen vascular disease, sickle cell disease, cystic fibrosis, organ transplant, congenital heart defects, and heart failure. (Table B, Appendix) Patients identified as in-hospital births were excluded to minimize the inclusion of perinatally-acquired and neonatal nosocomial infections. Patients with a secondary diagnosis code indicating trauma were also excluded, as a diagnosis of pneumonia in this population likely reflects nosocomial etiology. (Table C, Appendix). CAP-associated complications were identified using ICD-9 CM diagnosis and procedure codes (Table D, Appendix). Complications were classified as local (empyema, lung abscess, necrotizing pneumonia, broncho-pleural fistula), systemic (acute respiratory failure, sepsis, extracorporeal membrane oxygenation, hemolytic-uremic syndrome), and metastatic (meningitis, central nervous system abscess, mastoiditis, pericarditis, endocarditis, osteomyelitis, septic arthritis).
Statistical Analysis
Subjects were characterized by age, race, sex, presence and type of complication, and discharge status (in-hospital death). Analyses were subsequently stratified by age and race. Age groups were defined as <1 year (infant), 1–5 years (pre-school age), 6–12 years (school-age), and ≥13 years (adolescent) to capture differences in CAP and CAP-associated complications related to age-influenced factors such as PCV7 vaccination status, CAP microbiological etiology, and age-based sociological factors.
Race was recorded as a single variable (white, black, other, and missing). Rate estimates for each race category were calculated; patients with missing race data were included as a separate variable to preserve the integrity of our estimates. Rate ratios of CAP discharges and CAP-associated complications for black and white study participants were calculated using same-year data.
Categorical variables were summarized by frequencies and percents. Rate calculations were performed using weighted observations as numerators and annual age-specific population estimates obtained from the U.S. Census Bureau as denominators.23 Point estimates with 95% confidence intervals were calculated using Taylor Series estimates. Percentage change for CAP and CAP-associated complications was calculated using 1997 (pre-PCV7 period) and 2006 (post-PCV7 period) rates. Calculations were performed using STATA 10 statistical analysis software (StataCorp LP, College Station, TX). This study was considered exempt from review by the institutional review board of The Children’s Hospital of Philadelphia.
RESULTS
The 1997, 2000, 2003, and 2006 KID contained a combined 28,916,332 weighted pediatric discharges. Of these, 619,102 cases (2.1%) remained after inclusion and exclusion criteria for CAP were applied.
Subject Characteristics
Table 1 presents subject characteristics by year. Age category proportions remained stable over time, with children 1–5 years comprising the largest age category. There was a slight predominance of male participants in all years. White children were the largest represented race category in all years (35–44%); race data were missing from 18–32% of discharges. Mortality rates were low at 0.01% in 1997–2003, and 0.001% in 2006 for all pediatric CAP discharges.
Table 1.
Characteristics of discharges with community-acquired pneumonia, 1997–2006
| Year | 1997 (N=148702) | 2000 (N=157847) | 2003 (N=157743) | 2006 (N=156810) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Percent | No. | Percent | No. | Percent | No. | Percent | |
| Race | ||||||||
| White | 56348 | 38 | 68643 | 44 | 54903 | 35 | 56108 | 36 |
| Black | 22864 | 15 | 22580 | 14 | 17960 | 11 | 18800 | 12 |
| Other | 22203 | 15 | 38448 | 24 | 39138 | 25 | 40803 | 26 |
| Missing | 47287 | 32 | 28175 | 18 | 45588 | 29 | 41099 | 26 |
| Age Category | ||||||||
| < 1 Year | 43851 | 29 | 44470 | 28 | 37798 | 24 | 37705 | 24 |
| 1–5 Years | 75033 | 50 | 76385 | 48 | 77530 | 49 | 79519 | 51 |
| 6–12 Years | 19372 | 13 | 21403 | 14 | 23126 | 15 | 23494 | 15 |
| 13–18 Years | 10446 | 7 | 15589 | 9 | 19289 | 12 | 16092 | 10 |
| Sex | ||||||||
| Male | 83291 | 56 | 88256 | 56 | 86034 | 55 | 85508 | 55 |
| Died | 334 | 0.01 | 394 | 0.01 | 270 | 0.01 | 193 | 0.001 |
Rate of Community-Acquired Pneumonia
Overall
The rate of CAP discharges by year is shown in Table 2. Overall rates of pediatric CAP discharges peaked in 2000 and then returned to pre-PCV7 levels.
Table 2.
Rates of community-acquired pneumonia and associated complications, 1997–2006*
| Year | |||||
|---|---|---|---|---|---|
| 1997 | 2000 | 2003 | 2006 | % Change, 1997 vs. 2006 | |
| Rate of CAP discharges (95% CI) | 199.1 (198.1, 200.1) | 207.6 (206.6, 208.6) | 204.3 (203.1, 205.3) | 201.2 (200.2, 202.2) | 1.1% |
| Rate of Any Complication (95% CI) | 11.8 (11.6, 12.1) | 14.6 (14.3, 14.8) | 15.8 (15.3, 15.8) | 15.1 (14.8, 15.3) | 28.0% |
| Proportion of CAP (%) | 5.9 | 7.0 | 7.7 | 7.5 | |
| Rate of Local Complications (95% CI) | 5.4 (5.2, 5.6) | 7.4 (7.2, 7.6) | 8.9 (8.6, 9.0) | 9.6 (9.4, 9.9) | 77.8% |
| Proportion of CAP (%) | 2.7 | 3.6 | 4.4 | 4.8 | |
| Rate of Systemic Complications (95% CI) | 6.8 (6.6, 7.0) | 7.7 (7.5, 7.9) | 7.5 (7.3, 7.7) | 6.2 (6.0, 6.3) | −8.8% |
| Proportion of CAP (%) | 3.4 | 3.7 | 3.7 | 3.1 | |
Reported as rate per 100,000 age-specific U.S. population.
Abbreviations: CI, confidence interval
By Age
The rate of CAP discharges varied inversely with age, with the highest rates occurring in children <1 year. The rate of CAP discharges in children < 1 year decreased by 21.9% between 1997 and 2006, with 90% of the decrease occurring by 2003. In children 1–5 years, there was minimal interval change in rates of CAP discharges, while in children 6–12 years rates increased by 21.9% and in children ≥13 years there was a 40.5% increase. (Table 3)
Table 3.
Rates of community-acquired pneumonia, 1997–2006, stratified by age.*
| Age (years) | 1997 | 2000 | 2003 | 2006 | % Change, 1997 vs. 2006 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | ||
| < 1 | 43851 (30) | 1169.0 (1158.2, 1179.9) | 44691 (28) | 1159.1 (1148.4, 1169.8) | 37798 (24) | 937.6 (928.2, 947.0) | 37705 (24) | 912.9 (903.8, 922.1) | −21.9% |
| 1–5 | 75033 (50) | 383.1 (380.3, 385.8) | 76775 (48) | 397.7 (394.9,400.5) | 77530 (49) | 395.6 (392.8, 398.4) | 79519 (51) | 390.4 (387.7, 393.2) | 1.9% |
| 6–12 | 19372 (13) | 69.3 (68.3, 70.2) | 21531 (14) | 74.1 (73.1, 75.1) | 23126 (15) | 80.9 (79.9, 82.0) | 23494 (15) | 84.5 (83.4, 85.6) | 21.9% |
| 13–18 | 10446 (7) | 44.7 (43.8, 45.5) | 15663 (10) | 64.7 (63.6, 65.7) | 19289 (12) | 77.1 (76.1, 78.2) | 16092 (10) | 62.8 (61.9, 63.8) | 40.5% |
Reported as rate per 100,000 age-specific U.S. population.
Abbreviations: CI, confidence interval
By Race
Rates of CAP discharges in black children were greater than white children in all years studied. (Table 4) However, this difference decreased over time, from a rate ratio of 2.0 in 1997, to 1.6 in 2006.
Table 4.
Rate* and Rate Ratios of Community Acquired Pneumonia & Community Acquired Pneumonia-Associated Complications, 1997–2006, Stratified by Race
| Year | 1997 | 2000 | 2003 | 2006 | 1997–2006 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | % Change | |
| Community-acquired pneumonia | |||||||||
| White | 56348 (38) | 96.0 (95.2, 96.8) | 68975 (43) | 115.5 (114.7, 116.4) | 54903 (35) | 91.3 (90.5, 92.1) | 56108 (36) | 92.7 (92.0, 93.5) | −3.4% |
| Black | 22864 (15) | 190.0 (187.5, 192.5) | 22694 (14) | 182.6 (180.3, 185.0) | 17960 (11) | 142.6 (140.6, 144.7) | 18800 (12) | 147.6 (145.5, 149.8) | −22.3% |
| Black-White Rate Ratio | 2.0 (1.94, 2.01) | 1.58 (1.56, 1.60) | 1.56 (1.54, 1.59) | 1.59 (1.56, 1.62) | |||||
| Any complication | |||||||||
| White | 3567 (40) | 6.1 (5.9, 6.3) | 4757 (43) | 8.0 (7.7, 8.2) | 4578 (38) | 7.6 (7.4, 7.8) | 4609 (39) | 7.6 (7.4, 7.8) | 24.6% |
| Black | 1108 (13) | 9.2 (8.7, 9.8) | 1394 (13) | 11.2 (10.6, 11.8) | 1207 (10) | 9.6 (9.1, 10.1) | 1356 (12) | 10.7 (10.1, 11.2) | 16.3% |
| Black-White Rate Ratio | 1.51 (1.42, 1.62) | 1.41 (1.33, 1.49) | 1.26 (1.18, 1.34) | 1.40 (1.31, 1.49) | |||||
| Local complications | |||||||||
| White | 1831 (46) | 3.1 (3.0, 3.3) | 2771 (49) | 4.6 (4.5, 4.8) | 2839 (42) | 4.7 (4.6, 4.9) | 3100 (41) | 5.1 (4.9, 5.3) | 64.5% |
| Black | 491 (12) | 4.1 (3.7, 4.5) | 719 (13) | 5.8 (5.4, 6.2) | 714 (11) | 5.7 (5.3, 6.1) | 871 (12) | 6.8 (6.4, 7.3) | 65.9% |
| Black-White Rate Ratio | 1.32 (1.18, 1.45) | 1.26 (1.15, 1.35) | 1.21 (1.11, 1.30) | 1.33 (1.24, 1.44) | |||||
| Systemic complications | |||||||||
| White | 1854 (37) | 3.2 (3.0, 3.3) | 2175 (37) | 3.6 (3.5, 3.8) | 1959 (34) | 3.3 (3.1, 3.4) | 1750 (36) | 2.9 (2.8, 3.0) | −9.4% |
| Black | 650 (13) | 5.4 (5.0, 5.8) | 730 (12) | 5.9 (5.5, 6.3) | 553 (10) | 4.4 (4.0, 4.8) | 559 (12) | 4.4 (4.0, 4.8) | −18.5% |
| Black-White Rate Ratio | 1.69 (1.56, 1.87) | 1.61 (1.48, 1.75) | 1.35 (1.22, 1.48) | 1.52 (1.38, 1.67) | |||||
Rates reported as rate per 100,000 U.S. population ≤ 18 years.
Abbreviations: CI, confidence interval
Community-Acquired Pneumonia-Associated Complications
Overall
Between 1997 and 2006 the rate of discharges with any CAP-associated complication increased by 28% (11.8 and 15.1 per 100,000, respectively), while the rate of local complications increased by 77.8% (5.4 and 9.6 per 100,000, respectively). Empyema accounted for > 97% of all local complications. Systemic complication rates decreased by 8.8% (6.8 and 6.2 per 100,000, respectively). (Table 2) The proportion of discharges with any associated complication increased from 5.9% to 7.5%, while the proportion with local complications increased from 2.7% to 4.8%. The proportion resulting in systemic complications remained relatively stable at 3.1–3.7%. In 1997, 2000, 2003, and 2006 there were an estimated 75, 100, 72, and 98 discharges, respectively, with CAP-associated metastatic complications. There were so few metastatic complications as to preclude us from presenting meaningful rates or subset analysis.
By Age
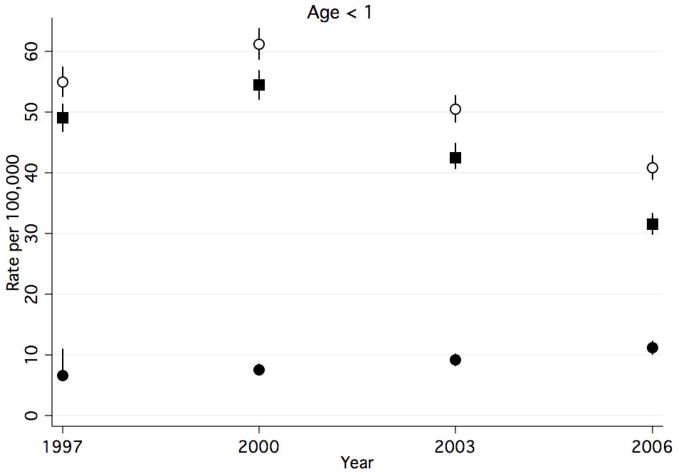
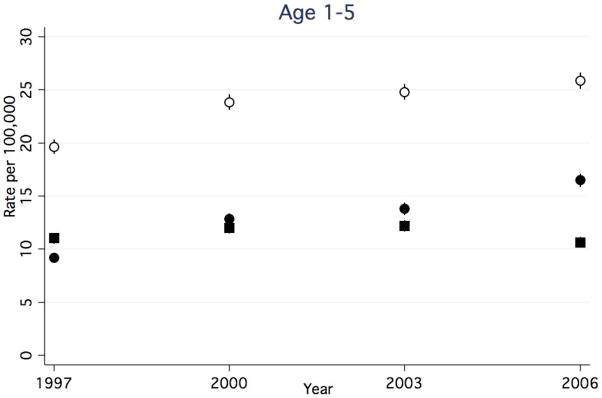
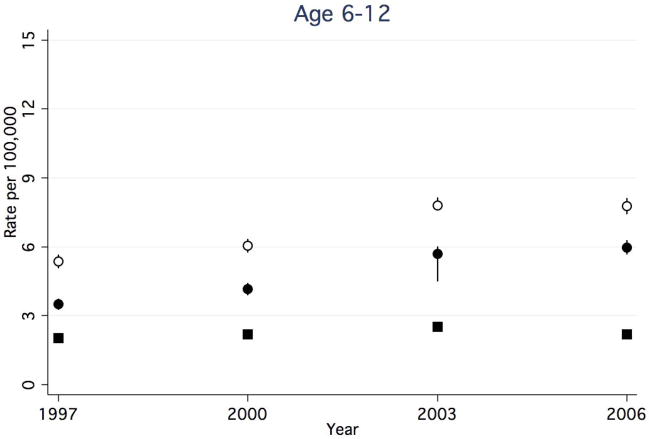
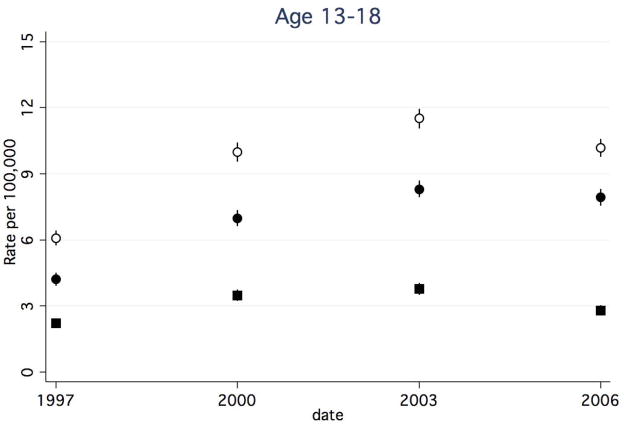
Between 1997 and 2006, rates of any CAP-associated complication in children <1 year decreased (Figure 1a), while rates in all other age groups increased (Figure 1b–d). The rate of any CAP-associated complication was highest at each time point for children <1 year, however, this group experienced a 25.5% decrease between 1997 and 2006 (from 54.9 to 40.9 per 100,000, respectively). Among children 1–5 years, the rate of any complication increased 31.5%, from 19.7 to 25.9 per 100,000. Among children 6–12 years, the rate of any complication increased by 44.4%, from 5.4 to 7.8 per 100,000. Children ≥13 years had the highest rate increase, with a 67.2% increase between 1997 and 2006 (6.1 and 10.2 per 100,000, respectively).
Figure 1.
Figure 1a–d. Rate of Hospital Discharges for Community Acquired Complications, 1997–2006, Stratified by Age. (a). < 1year. (b). 1–5 years. (c). 6–12 years. (d).13–18 years. Open circles indicate any complication, solid squares indicate systemic complications, and solid circles indicate local complications. Bar lines indicate 95% confidence intervals. Rates are per 100,000 age-specific U.S. population.
Rates of CAP-associated systemic complications were highest in children < 1 year in all years studied, while the lowest rates occurred in children 6–12 years. Between 1997 and 2006 rates of systemic complications in children < 1 year declined from 49.0 to 31.6 per 100,000, representing a 35.5% decrease. Rates remained otherwise stable amongst other age groups.
Rates of CAP-associated local complications increased in all age groups. While rates of local complications were highest in children 1–5 years for all years studied, children ≥13 years had the greatest percent increase in rates over time. In children <1 year, rates increased 67.2%, from 6.7 to 11.2 per 100,000. (Figure 1a). Among children 1–5 years, rates increased 79.3%, from 9.2 to 16.5 per 100,000. (Figure 1b). Among children 6–12 years, rates increased 71.4%, from 3.5 to 6.0 per 100,000. (Figure 1c). Children ≥13 years experienced an 88.1% increase in the rates between 1997 and 2006 (4.2 and 7.9 per 100,000, respectively). (Figure 1d).
By Race
Rates of any, systemic, and local CAP-associated complications were higher in black children than in white children for all years studied (Table 4). Rate ratios between black and white children for any, systemic, and local complications showed a downward trend between 1997 and 2003, with a slight increase from 2003–2006. Between 1997 and 2006, rates of any CAP-associated complication increased by 24.6% in white children and 16.3% in black children. Systemic complication rates decreased 18.5% in black children, while rates in white children decreased by 9.4%. Both groups experienced similar increases in rates of local complications (64.5% and 65.9% for white and black children, respectively).
DISCUSSION
We describe national changes in discharge rates for pediatric CAP and CAP-associated complications in the pre- and post-PCV7 periods. Since the introduction of PCV7 in 2000, uptake has been rapid, with 68% of 19–35 month olds having received 3(+) doses by 2003, and 87% in 2006.24 We report that although overall rates of CAP discharges remained relatively unchanged, rates decreased for children < 1 year and increased in those 6 years of age and older. Overall rates of systemic complications were dramatically higher in infants than in any other age group, yet infants were the only age group to experience declines over time in this area. In contrast, local complication rates were found to be rising in all pediatric age groups and were highest among children 1–5 years. Race appeared to play a role, as black children had consistently higher discharge rates of CAP and CAP-associated complications than white children in all years studied.
While CAP hospitalization rates for the entire cohort were stable overall, there were differences by age group. Infants were the sole age category to experience a decline in CAP discharge rates, a finding consistent with other studies showing post-PCV7 reductions in rates of all-cause pneumonia for children < 2 years11–13, 25. While previous post-licensure studies have not shown CAP rate changes for children > 2 years13, 14, we report that CAP discharge rates increased in children > 5 years. We may have been able to find a difference in rates for these older age groups due to the larger sample size of our cohort compared with previous studies13, 14. However, the reason for the increase in CAP discharges is unclear. Pneumococcal serotype replacement has been occurring since the introduction of PCV71, 5–7, 15 and may contribute to the increase in CAP discharge rates in older children, although data suggest that serotype replacement is more commonly seen in young children and older adults.5, 6, 15 It is also possible that changes in the epidemiology of other pathogens such as methicillin-resistant Staphylococcus aureus (MRSA)26, 27 or atypical organisms, rather than changes in rates of IPD, are responsible for this trend.
This is the first national study to examine rates of systemic CAP-associated complications in the pre- and post-PCV7 era. Rates of systemic complications varied inversely with age, with infants having the highest rates and children ≥6 years having the lowest rates. The decline in systemic complications for the entire cohort was largely attributable to the decrease in infant rates, and might be explained in part by the fact that infants have been the primary recipients of PCV7.28 Adult data suggest that pneumococcal vaccination can modify the severity of illness in those hospitalized with CAP and may reduce the occurrence of CAP-associated complications.20 A plausible mechanism may be the reduction of concomitant pneumococcal bacteremia among PPV23 recipients.29 Experimental models have shown that cell-wall components of killed pneumococci are capable of triggering an inflammatory cascade response in the host, resulting in death. 30 The reduction of pneumococcal bacteremia may prevent the initiation of these inflammatory processes, reducing the severity of illness in those requiring hospitalization for CAP. Large declines in rates of IPD, including bacteremia, occurred in children < 2 years after PCV7 licensure,6, 7, 15, 31–35 possibly explaining why, similar to adult recipients of PPV23, PCV7 may reduce the frequency of CAP-associated systemic complications.
In contrast to trends in CAP-associated systemic complications, rates of local complications increased for all age groups, with the highest rates occurring in pre-school age children. In addition, the presence of any CAP-associated complication in children 1–18 years was largely attributable to local complications. It is unclear, however, if this trend can be attributed fully to the changing epidemiology of IPD after introduction of PCV7. Two studies reported increasing regional rates of empyema in children prior to PCV7 licensure,27, 36 raising the possibility that the current increase in rate of local complications is a continuation of a previous trend. Rates of local complications may also be influenced by the increasing prevalence of community-acquired MRSA, which has become the most commonly isolated pathogen from empyema in several centers.26, 27 The limitations of standard microbiological isolation techniques have made etiology and incidence studies of empyema problematic. Bacteria are infrequently isolated from blood or pleural fluid cultures of children with empyema17, 26, 27, and at one center, culture-negative empyema accounted for most of the increase in empyema frequency.17 Small studies using PCR-based assays have identified S. pneumoniae in 75–87.5% of culture-negative empyema.37–40 Empyema may be an overlooked major category of IPD in the post-PCV7 era.
Black race is an independent risk factor for IPD,6, 41 and racial disparities in children with IPD are greatest for those <2 years.42, 43 Discharge rates of CAP and CAP-associated complications are higher in black versus white children. Our findings suggest that the gap in rates of CAP discharges and systemic complications between black and white children has narrowed over time. Post-licensure data demonstrated a similar reduction of racial disparity in IPD rates in children after the first two years of widespread PCV7 vaccination. 42–44 Rates of CAP discharges and systemic complications in black children have appeared to plateau, with no further declines after 2003. Both groups saw similar increases in rates of local complications over time. Further efforts will be required to ensure that reductions in racial disparity can be maintained as the epidemiology of IPD changes.
This study has several limitations. First, KID is an administrative database of discharge-level data without clinical information beyond that captured in ICD-9 CM codes. The identification of CAP discharges depends on the accuracy of ICD-9 coding, thus, miscoding of patients with CAP and CAP-associated complications is possible. We may have underestimated the rate of complications, because patients with CAP and a CAP-associated metastatic complication such as osteomyelitis may have received a primary discharge diagnosis of osteomyelitis rather than CAP, resulting in the exclusion of such patients from our study. This may explain the extremely low incidence of metastatic complications found in our study. We expect that such misclassification would not occur disproportionately from one year to the next, so the observed trend in CAP-associated complication rates likely reflects a true trend. Second, while ICD-9 discharge diagnosis codes identify CAP with high sensitivity, ICD-9 codes have poor sensitivity in identifying patients with pneumonia caused by specific pathogens. Therefore, we could not identify whether changes in rates of pneumonia-associated complications were attributable to specific pathogens. Third, patients with hospital-acquired pneumonia may have been included as there is no specific ICD-9 CM code for CAP. We minimized the likelihood of such misclassification by using a previously validated approach to identify patients with CAP.22 Using this approach, patients with pneumonia listed as a secondary rather than primary diagnosis must have had a pneumonia-related symptom (i.e., cough, tachypnea) listed as the primary diagnosis. For example, a patient hospitalized following traumatic injury who develops ventilator-associated pneumonia is likely to have trauma, rather than pneumonia or a pneumonia-related symptom, listed as the primary diagnosis. Fourth, we were unable to determine the vaccination status of our study population to assess the efficacy of PCV7 in preventing CAP, and could only infer the impact of PCV7 introduction on the general pediatric population. Last, the inclusion of every third year into the KID may have impacted our ability to interpret trends accurately, given year-to-year epidemiological fluctuations in incidence caused by factors unrelated to pneumococcal vaccination.
CONCLUSIONS
Since the introduction of PCV7 in 2000, rates of CAP hospitalizations have decreased in children < 1 year, but appear to be increasing in children > 5 years. Rates of systemic complications have decreased in children < 1 year, but rates of local complications are increasing in all pediatric age groups. Further studies are needed to determine the underlying epidemiological factors associated with these changes.
Acknowledgments
Sources of funding: Drs. Lee and Kronman are both recipients of a Young Investigator Award from the Academic Pediatric Association. Dr. Shah received support from the National Institute of Allergy and Infectious Diseases (K01 AI73729) and the Robert Wood Johnson Foundation under its Physician Faculty Scholar Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations Used in Manuscript
- CAP
Community acquired pneumonia
- PCV7
Heptavalent pneumococcal conjugate vaccine
- PPV23
Twenty-three valent polysaccharide pneumococcal vaccine
- IPD
Invasive pneumococcal disease
- KID
Kids’ Inpatient Database
- HCUP
Healthcare Cost and Utilization Project
- AHRQ
Agency for Healthcare Research and Quality
- ICD-9 CM
International Classification of Diseases, 9th Revision, Clinical Modification
- MRSA
Methicillin-resistant Staphylococcus aureus
APPENDIX
Table A.
ICD-9CM Diagnosis Codes Used to Identify Pneumonia-Related Symptoms
| Symptom | ICD-9 CM |
|---|---|
| Fever | 780.6 |
| Respiratory Abnormality, unspecified | 786.00 |
| Shortness of Breath | 786.05 |
| Tachypnea | 786.06 |
| Wheezing | 786.07 |
| Cough | 786.2 |
| Hemoptysis | 786.3 |
| Abnormal Sputum | 786.4 |
| Chest Pain | 786.5 |
| Precordial Pain | 786.51 |
| Painful Respiration | 786.52 |
| Abnormal Chest Sound (rales, friction, abnormal percussion) | 786.7 |
Table B.
ICD-9CM Diagnosis Codes Used to Identify Excluded Comorbidities
| Disease Category | ICD-9 CM Diagnosis Code |
|---|---|
| Human Immunodeficiency Virus | 042 |
| Malignancy | 140.x - 208.x |
| Cystic Fibrosis | 277 |
| Immune Mechanism Disorder | 279, 334.8 |
| Sickle Cell | 282.6x |
| Diseases of White Blood Cells | 288 |
| Other Lung Conditions | 507, 517 |
| Congenital Heart Defects | 745–747 |
| Encounter for Radiation, Chemotherapy, Transplant | V42, V58.0, V48.1x |
X = digits 0–9, where applicable
Table C.
ICD-9 CM External Causes of Injury and Poisoning (E-Codes) Used to Identify Trauma
| Category | ICD-9 CM E-Code |
|---|---|
| Railway Accidents | E800–807 |
| Motor Vehicle Traffic Accidents | E810–819 |
| Motor Vehicle Non-Traffic Accidents | E820–825 |
| Other Road Vehicle Accidents | E826–829 |
| Water Transport Accidents | E830–838 |
| Accidental Falls | E880–888 |
| Accidents Caused by Fire, Flame | E890–899 |
| Accidents Caused by Submersion, Suffocation | E910–915 |
| Other Accidents | E916–925 |
| Suicide, Self-Inflicted Injury | E953–959 |
| Homicide/Injury Purposely Inflicted | E960–969 |
| Legal Intervention | E970–977 |
| Undetermined Accident or Purposely Inflicted | E983–989 |
Table D.
ICD9-CM Diagnosis and Procedure Codes for Identification of Community Acquired Pneumonia-Associated Complications
| Complication Subcategory | Complication Description | ICD-9 CM Diagnosis and/or Procedure Code |
|---|---|---|
| Local | Emypema | 510.x, 511.0, 511.1, 511.9 |
| Lung abscess | 513.x | |
| Broncho-pleural Fistula | 33.42†, 510.0 | |
| Necrotizing Pneumonia | 32.x‡ | |
| Systemic | Acute Respiratory Failure | 518.8x, 799.1 |
| SIRS/Sepsis | 995.9x | |
| ECMO | 39.6x† | |
| Hemolytic Uremic Syndrome | 283.11 | |
| Metastatic | Meningitis | 320.0, 320.1, 320.2, 320.3, 320.8x |
| CNS Abscess | 324.x | |
| Mastoiditis | 383.0x | |
| Pericarditis | 420.x | |
| Endocarditis | 421.x | |
| Osteomyelitis | 730.0x, 730.2x | |
| Septic Arthritis | 711.x |
x = digits 0–9, where applicable
= ICD-9CM procedure code
= ICD-9CM procedure code for lung resection as proxy
References
- 1.Messina AF, Katz-Gaynor K, Barton T, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr Infect Dis J. 2007;26:461–7. doi: 10.1097/INF.0b013e31805cdbeb. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics. 2004;113:443–9. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 4.Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485–9. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 5.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 6.Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569–76. doi: 10.1086/518149. [DOI] [PubMed] [Google Scholar]
- 7.Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan KL. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin Infect Dis. 2006;42:907–14. doi: 10.1086/500941. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis. 2008;46:1664–72. doi: 10.1086/587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 10.Shah SS, Ratner AJ. Trends in invasive pneumococcal disease-associated hospitalizations. Clin Infect Dis. 2006;42:e1–5. doi: 10.1086/498745. [DOI] [PubMed] [Google Scholar]
- 11.Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810–5. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med. 2007;161:1162–8. doi: 10.1001/archpedi.161.12.1162. [DOI] [PubMed] [Google Scholar]
- 13.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JC, Jackson M, Yu O, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–54. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 16.Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A, Mason EO. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006;25:250–4. doi: 10.1097/01.inf.0000202137.37642.ab. [DOI] [PubMed] [Google Scholar]
- 17.Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatric Infectious Disease Journal. 2008;27:1030–32. doi: 10.1097/INF.0b013e31817e5188. [DOI] [PubMed] [Google Scholar]
- 18.Grijalva CG, Nuorti JP, Zhu Y, Griffin MR. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis. 2010;50:805–13. doi: 10.1086/650573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li ST, Tancredi DJ. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2010;125:26–33. doi: 10.1542/peds.2009-0184. [DOI] [PubMed] [Google Scholar]
- 20.Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006;42:1093–101. doi: 10.1086/501354. [DOI] [PubMed] [Google Scholar]
- 21.HCUP Kids’ Inpatient Database (KID) Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality; Rockville, M.D: 2006. [Google Scholar]
- 22.Whittle J, Fine MJ, Joyce DZ, et al. Community-acquired pneumonia: can it be defined with claims data? Am J Med Qual. 1997;12:187–93. doi: 10.1177/0885713X9701200404. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics Bridged Race Estimates 1990–2006. Centers for Disease Control and Prevention; (Accessed at http://wonder.cdc.gov/bridged-race-v2006.html) [Google Scholar]
- 24.Centers for Disease Control and Prevention. [Accessed Oct. 26, 2010];National Immunization Survey - Children 19–35 months. at http://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm#nis.
- 25.Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine--United States, 1997–2006. MMWR Morb Mortal Wkly Rep. 2009;58:1–4. [PubMed] [Google Scholar]
- 26.Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004;113:1735–40. doi: 10.1542/peds.113.6.1735. [DOI] [PubMed] [Google Scholar]
- 27.Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003;22:499–504. doi: 10.1097/01.inf.0000069764.41163.8f. [DOI] [PubMed] [Google Scholar]
- 28.Advisory Commitee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49 (RR-9):1–35. [PubMed] [Google Scholar]
- 29.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–55. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 30.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–4. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 31.Black S, France EK, Isaacman D, et al. Surveillance for invasive pneumococcal disease during 2000–2005 in a population of children who received 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:771–7. doi: 10.1097/INF.0b013e318124a494. [DOI] [PubMed] [Google Scholar]
- 32.Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–24. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 33.Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children’s Hospital Emergency Department and Urgent Care Center. Arch Pediatr Adolesc Med. 2004;158:671–5. doi: 10.1001/archpedi.158.7.671. [DOI] [PubMed] [Google Scholar]
- 34.Herz AM, Greenhow TL, Alcantara J, et al. Changing epidemiology of outpatient bacteremia in 3- to 36-month-old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2006;25:293–300. doi: 10.1097/01.inf.0000207485.39112.bf. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson M, Bulloch B, Smith M. Prevalence of occult bacteremia in children aged 3 to 36 months presenting to the emergency department with fever in the postpneumococcal conjugate vaccine era. Acad Emerg Med. 2009;16:220–5. doi: 10.1111/j.1553-2712.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 36.Tan TQ, Mason EO, Jr, Wald ER, et al. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002;110:1–6. doi: 10.1542/peds.110.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004;59:522–5. doi: 10.1136/thx.2003.016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eltringham G, Kearns A, Freeman R, et al. Culture-negative childhood empyema is usually due to penicillin-sensitive Streptococcus pneumoniae capsular serotype 1. J Clin Microbiol. 2003;41:521–2. doi: 10.1128/JCM.41.1.521-522.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarrago D, Fenoll A, Sanchez-Tatay D, et al. Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin Microbiol Infect. 2008;14:828–34. doi: 10.1111/j.1469-0691.2008.02028.x. [DOI] [PubMed] [Google Scholar]
- 40.Obando I, Munoz-Almagro C, Arroyo LA, et al. Pediatric parapneumonic empyema, Spain. Emerg Infect Dis. 2008;14:1390–7. doi: 10.3201/eid1409.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–35. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 42.Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197–203. doi: 10.1001/jama.291.18.2197. [DOI] [PubMed] [Google Scholar]
- 43.Talbot TR, Poehling KA, Hartert TV, et al. Elimination of racial differences in invasive pneumococcal disease in young children after introduction of the conjugate pneumococcal vaccine. Pediatr Infect Dis J. 2004;23:726–31. doi: 10.1097/01.inf.0000133046.60555.de. [DOI] [PubMed] [Google Scholar]
- 44.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 45.Chu BHR, Elixhauser A, Ross D. HCUP Method Series Report #2007-02 Online. U.S. Agency for Healthcare Research and Quality; Jan 10, 2007. Using the KIDS’ Inpatient Database (KID) to Estimate Trends. [Google Scholar]