Abstract
Synaptic strength can be highly variable from animal to animal within a species or over time within an individual. The process of synaptic plasticity induced by neuromodulatory agents might be unpredictable when the underlying circuits subject to modulation are themselves inherently variable. Serotonin (5-hydroxytryptomine; 5HT) and serotonergic signaling pathways are important regulators of animal behavior and are pharmacological targets in a wide range of neurological disorders. We have examined the effect of 5HT on electrical synapses possessing variable coupling strengths. While 5HT decreased electrical coupling at synapses with weak electrical connectivity, synapses with strong electrical coupling were less affected by 5HT treatment, as follows from the equations used for calculating coupling coefficients. The fact that the modulatory effect of 5HT on electrical connections was negatively correlated with the strength of electrical coupling suggests that the degree of electrical coupling within a neural network impacts subsequent neuromodulation of those synapses. Biophysical studies indicated that these effects were primarily due to 5HT-induced modulation of membrane currents that indirectly affect junctional coupling at synaptic contacts. In support of these experimental analyses, we created a simple model of coupled neurons to demonstrate that modulation of electrical coupling could be due solely to 5HT effects on H-channel conductance. Therefore, variability in the strength of electrical coupling in neural circuits can determine the pharmacological effect of this neuromodulatory agent.
Keywords: Helisoma, Serotonin, Neuromodulation, Electrical Coupling, Model Synapse
Introduction
Alterations of electrical and synaptic properties in response to neuromodulators are important cellular correlates of behavioral plasticity. Gap junctions present between electrically-coupled cells are potentially important sites of such neuromodulation (Bennett, 1997). Gap junctions are responsible not only for rapid electrical communication between cells, but also biochemical communication (Roerig and Feller, 2000). Extensive electrical coupling exists between neurons in mammalian nervous systems (Parker et al., 2009), especially during development (Peinado et al. 1993; Kandler and Katz 1995; Nadarajah et al. 1997). Alterations in electrical coupling have been examined in a wide range of vertebrate and invertebrate systems including: olfactory bulb (Maher et al., 2009), thalamic reticular nucleus (Parker et al., 2009), leech Retzius cells (Colombaioni and Brunelli 1988), Aplysia neurons (Bodmer et al. 1988; Carrow and Levitan 1989), lobster stomatogastric neurons (Kepler et al. 1990) and retinal cells from fish (DeVries and Schwartz 1989; Harsanyi and Mangel 1992; Qian et al. 1993; McMahon 1994). Furthermore, modulators as diverse as catecholamine neurotransmitters (Radu et al. 1982), peptide hormones (Wolinsky et al. 1985) and lipids (Guan et al. 1997) have been shown to influence electrical coupling between neurons.
Evidence has shown that electrical synapses can be plastic and subject to regulatory control by several mechanisms (for review see Pereda et al., 2004). Vertebrate gap junction proteins, connexins, can be directly regulated by many factors, including: pH, voltage, tyrosine kinases (reviewed in Bruzzone et al., 1996) and associated phospholipids (Locke and Harris, 2009). Indirect modulation of electrical coupling has also been demonstrated. Spira et al. (1980) demonstrated in Navanax that uncoupling can be explained by a simple circuit in which inhibitory synapses short circuit electrotonic spread, allowing motoneurons that are normally coupled to fire independently. Curti and Pereda (2004) demonstrated that a voltage-dependent enhancement of electrical coupling occurs between the Mauthner cell and VIIIth nerve terminals due to the presence of a subthreshold sodium current present at presynaptic terminals. This current amplifies the synaptic response, demonstrating that the strength of an electrical synapse can be modulated in a voltage-dependent manner by properties of the nonjunctional membrane. We should note that gap junctions containing innexins, invertebrate gap junction proteins, instead of connexins are from different gene families and that there are differences in covalent modifications within as well as across families (Phelan, 2005).
Alterations in cellular properties can also be a factor involved in regulating coupling between neurons. Identified neurons in simple neural networks which mediate specific circuit components can show considerable variation between animals in parameters such as modulator-evoked currents (Goaillard et al., 2009). This raises the question of whether neuromodulation can occur in a consistent manner in networks where underlying parameters are variable (Grashow et al., 2009). For example, during development neurons undergo changes in shape and size resulting in alterations in properties such as input resistance. Parker et al. (2009) demonstrated that neurons in the thalamic reticular nucleus maintain stability of electrical coupling strength despite these developmental changes in neuronal physiology. In contrast, Maher et al. (2009) demonstrated that coupling coefficients between mitral cell dendrites were relatively high in young mice, but decreased after postnatal day (P)10 due to a maturational increase in membrane conductance. Thus, modulation of cellular properties can have significant effects on electrical coupling.
Since electrical coupling within neural networks is a potentially important target for neuromodulation we examined the effect of serotonin on electrical coupling between identified Helisoma neurons in vivo and in vitro. Serotonin mediates a wide range of modulatory effects on neural circuit development and function. For example, serotonin can regulate coupling between developing rat cortical neurons (Rorig and Sutor, 1996), modulate rhythmic activity in leech neurons (Moss et al., 2005), and alter crustacean stomatogastric pattern generation (Johnson et al. 1993; Johnson et al. 1994). Serotonin has also been shown to regulate properties such as membrane input resistance which could alter the strength of electrical coupling (Antonsen and Edwards, 2007).
In the snail, Helisoma, many neuronal properties are modulated by 5HT, including overall development (Goldberg and Kater 1989), axonal outgrowth (Haydon et al. 1987; Murrain et al. 1990), post-inhibitory rebound (Quinlan and Murphy 1996), neuromuscular function (Zoran et al. 1989), and patterned neural activities (Quinlan and Murphy 1996). Motor neurons in the buccal ganglia demonstrated increases in neuronal excitability in response to serotonin application (Achee and Zoran, 1997). In buccal neuron 19, Price and Goldberg (1993) demonstrated that serotonin activates an inward sodium current via a cAMP-dependent mechanism which results in membrane depolarization, increased firing frequency and decreased input resistance, findings consistent with activation of Ih channels.
In this study, we have described the effects of serotonin on electrical coupling between two identical neurons, 19, in Helisoma. Serotonin significantly decreased electrical coupling at 19-19 synapses in vivo and in vitro and these results were consistent with effects of 5HT on H-current activation. In addition, these modulatory effects were negatively correlated with the strength of electrical connectivity, such that the 5HT-induced reduction in coupling was greatest at weakly coupled synapses. Furthermore, we created a two-cell model containing leak and H-current, in which the two cells were connected by gap junctions of varying strength that supported our results.
Results
Effects of 5HT on 19-19 synapses in buccal ganglia preparations
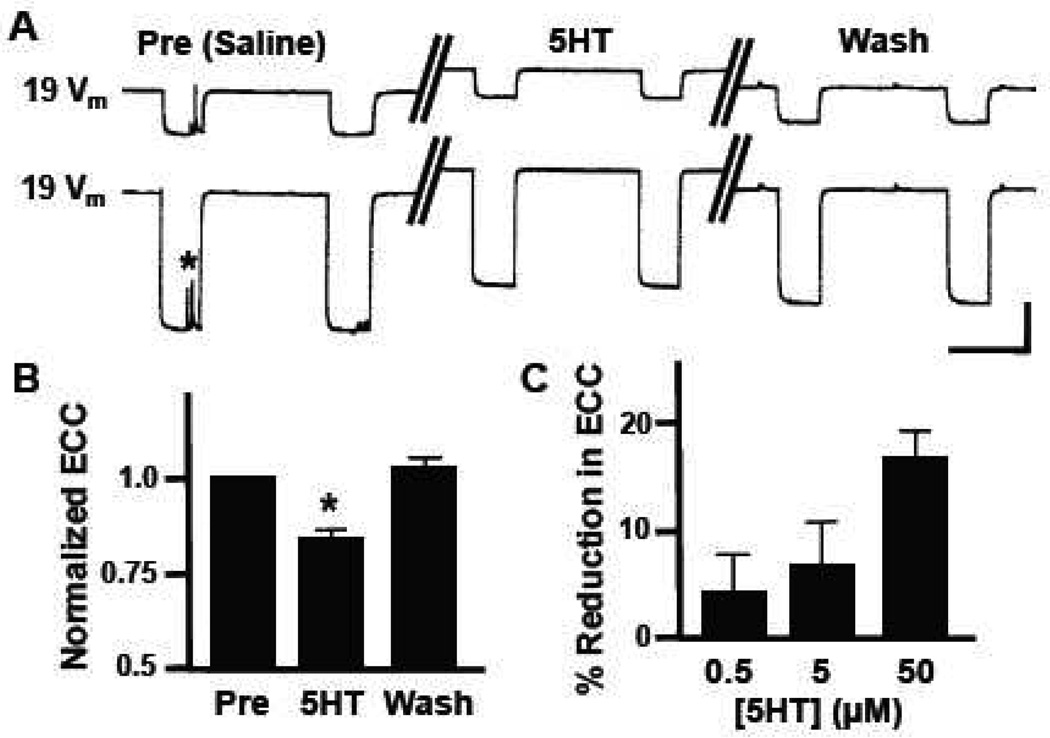
Electrical synapses have long been considered important regulators of cell-cell communication, but only recently have they gained recognition as important sites of synaptic plasticity (Hughes and Crunelli, 2006). The neurotransmitter serotonin (5HT) is a potent modulator of the Helisoma feeding neural circuit (Granzow and Kater 1977; Quinlan and Murphy 1996) and 5HT selectively prevents the formation of electrical connections while permitting chemical synaptogenesis between identified Helisoma neurons (Haydon and Kater 1988). Perfusion of isolated buccal ganglia with 50 µM 5HT caused a reversible depolarization in the membrane potential of bilaterally symmetrical motoneurons 19 (5.4 ± 1.2 mV; n=18; p<0.0001; Fig. 1A). Neuron 19-19 pairs had an average coupling coefficient of 0.22 ± 0.02, a value similar to that previously reported for this electrical connection (Goldberg and Kater, 1989) and the 5HT-induced depolarization was accompanied by a significant reduction in electrical coupling coefficient (ECC; n=18; p<0.0001; Fig. 1B). Electrical coupling was reduced 17.0 ± 2.4 % by 50 µM 5HT and this neuromodulatory effect was dosage-sensitive (Fig. 1C).
Figure 1.
Neuromodulatory effects of serotonin on 19-19 electrical coupling in buccal ganglia. (A) Representative current-clamp recordings from bilaterally symmetrical neurons 19. Hyperpolarizing current was injected into one neuron, while changes in membrane potential (Vm) were monitored in both the injected presynaptic neuron (lower trace) and the postsynaptic neuron (upper trace). Control responses (Pre, Saline) are represented by the set of traces on the left, responses obtained during bath perfusion with 50 µM serotonin (5HT) are in the middle, and post-treatment responses following saline wash (Wash) are shown on the right. Note the 5HT-induced membrane depolarization in both neurons. The asterisk represents spontaneous synaptic currents. (B) Bath perfusion with 50 µM 5HT caused a 17.0 ± 2.4 % (n=18) reduction in electrical coupling coefficient (ECC) between neurons 19 that recovered following wash (WASH). Data are normalized to pretreatment levels (PRE) and ECC in 5HT was significantly lower than the washout value (*, p<0.0001). (C) The 5HT-induced reduction in coupling coefficient between neurons 19 was dose-dependent (0.5 µM, n=7; 5.0 µM, n=7; 50 µM, n=18). Vertical scale bar in A equals 20 mV and horizontal scale bar equals 2 s.
Effects of 5HT on 19-19 synapses in vitro
During 5HT neuromodulation in buccal ganglia, we observed that serotonin had a more potent effect on 19-19 electrical synapses possessing relatively weak electrical coupling, as is predicted by the equation used for calculating coupling coefficients. To test the idea that 5HT might affect electrical synapses differentially depending on their strength of coupling, 19-19 electrical connections were studied in cell culture where the magnitude of electrical coupling could be experimentally manipulated. Hadley et al. (1985) demonstrated that Helisoma neuronal pairs extending neurites in culture displayed an inverse relationship between coupling coefficient and intersomatic distance. Their studies indicated that both electrotonic decay and decreased cell-cell contact contributed to lower coupling in pairs whose somata were widely spaced. Therefore, we cultured neurons using two configurations that maximized differences in intersomatic distance and the extent of cell-cell contact. The use of neurite-bearing cultures (NB, see Experimental Procedures) provided neuronal pairs with minimal neuritic contact at considerable distance from the paired somata. Soma-soma cultures (SS, see Experimental Procedures), on the other hand, consisted of neurite-free neurons with total cell-cell contact at the paired somata.
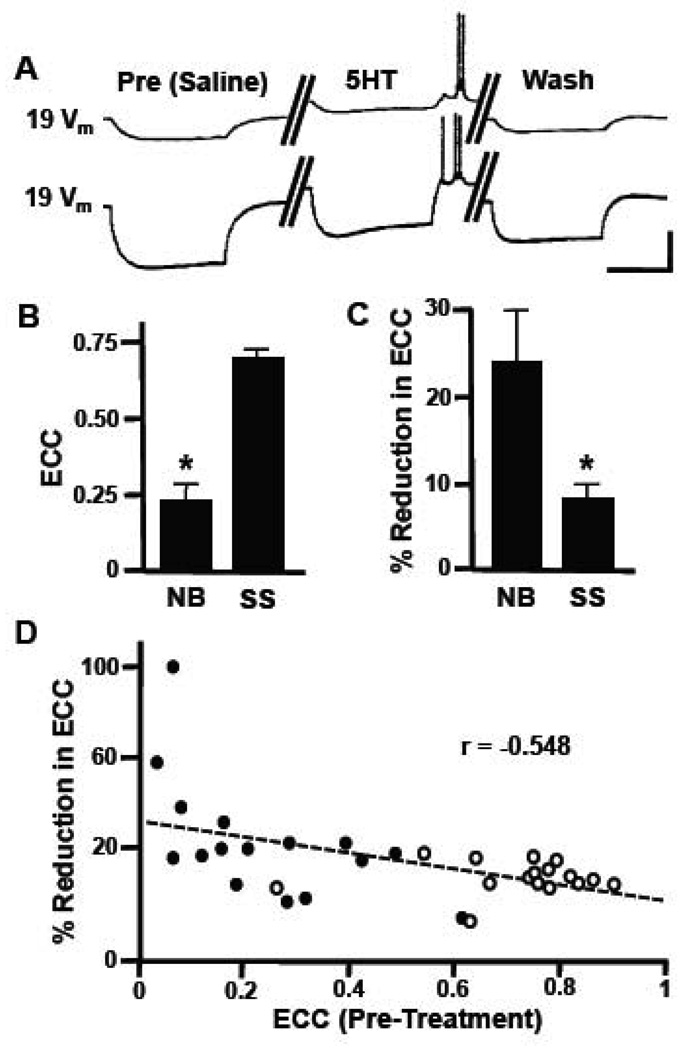
Similar to isolated ganglia, electrical coupling was again detected between neurons 19-19, and 5HT induced changes in neuronal parameters similar to those seen previously (Fig. 2A). The average electrical coupling coefficient in 19-19 neurite-bearing pairs (n=16) was significantly lower than that measured for soma-soma pairs (n=16; p<0.001; Fig. 2B), demonstrating that manipulation of cell-cell contact provided the necessary extremes to test the hypothesis that 5HT differentially affects synapses demonstrating weaker vs. stronger electrical coupling. Following exposure to 50 µM 5HT, the weaker coupling between neurite-bearing neurons was reduced by 24.4 ± 6.2 %, which represented a significantly greater reduction in ECC values than that detected at soma-soma electrical synapses with stronger coupling (8.1 ± 2.0 %; p<0.001; Fig. 2C). Control 19-19 pairs exposed to vehicle had negligible changes in coupling following this perfusion protocol (1.9 ± 0.7 % reduction in ECC; n=10) and both neurite-bearing and soma-soma synapses demonstrated a dose-responsiveness following exposure to 0.5, 5.0, 50.0 µM 5HT (data not shown).
Figure 2.
Serotonin-induced reduction in electrical coupling is greatest at weakly coupled electrical synapses in vitro. (A) Representative traces illustrate the modulatory effects of 50 µM serotonin on a 19-19 soma-soma cultured pair. Hyperpolarizing current was injected into one neuron, while changes in membrane potential (Vm) were monitored in both this presynaptic neuron (lower trace) and the postsynaptic neuron (upper trace). Control responses (Pre, Saline) are represented by the set of traces on the left, responses obtained during bath perfusion with 50 µM serotonin (5HT) are in the middle, and post-treatment responses following saline wash (Wash) are shown on the right. (B) Electrical coupling coefficients (ECC) measured between neurite-bearing (NB, n=16) and soma-soma (SS, n=93) pairs of neuron 19 were significantly different (*, p<0.001, 2-tailed t-test). (C) Bath perfusion with 50 µM 5HT caused a greater reduction in electrical coupling coefficient (ECC) in neurite-bearing pairs (NB) than in soma-soma cultures (*, p<0.001, 2-tailed t-test). Data were normalized to pretreatment levels. (D) Linear regression analysis shows an inverse relationship between the 5HT-induced reduction in coupling and initial coupling strength (ECC, Pre-Treatment). The correlation coefficient was statistically significant (r=−0.548; p<0.0005; n=36). SS pairs are indicated by open circles and NB pairs indicated by closed circles. Vertical scale bar in A equals 20 mV and horizontal scale bar equals 2 s.
As mentioned above, increasing the distance between neuronal cell bodies plated into culture and, therefore, sites of synaptic contact along the neurite affects the strength of electrical coupling. To determine whether graded differences in coupling, rather than simply two extremes, showed the same 5HT-induced reductions in ECC, we plated neurons 19 into culture at varying intersomatic distances from essentially 0 µm (soma-soma contacts, with no neurites) to cultures with neurites greater than 500 µm in length (neurite-neurite contacts). In this way, coupling coefficients in 19-19 cell pairs ranged from less than 0.05 to greater than 0.95 (n=36). Linear regression analysis determined an inverse relationship between 5HT-induced reduction in coupling and initial electrical coupling coefficient (Fig. 2D), with a correlation coefficient (r) of −0.55 (p<0.0005). Thus, electrical synapses demonstrating weaker coupling were more potently modulated by serotonin and this modulatory effect was graded over a physiological range of synaptic strengths. Note that this result is expected from the formula for coupling coefficient (see Experimental Procedures); i.e., when coupling is weak changes in the ratio of nonjunctional resistance to coupling resistance have a greater effect on coupling coefficient.
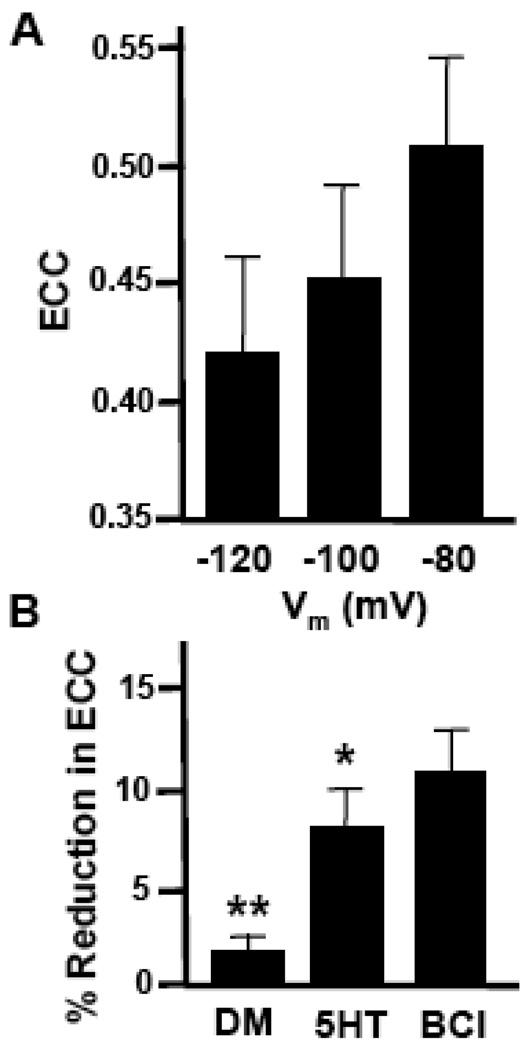
It is well known that many cellular currents are only active within a certain membrane potential range. For example, potassium currents due to the inward rectifier potassium ion channel, Kir, are generally not active when the membrane potential becomes more depolarized than resting membrane potential. Since 5HT depolarized neurons 19, we next asked whether alterations in resting membrane potential were responsible for alterations in coupling seen in the presence of 5HT. To examine this we utilized the soma-soma configuration, where membrane voltage near sites of synaptic contact could be more faithfully manipulated by current injection. Isopotential membrane voltages of −120, −100 and −80 mV were generated using hyperpolarizing current injections. More depolarized voltages were not used because increases in neuronal firing interfered with coupling measurements. 19-19 pairs exhibited a voltage-dependent increase in ECC as the neuronal membrane became less polarized (Fig. 3A). Thus, since 5HT causes a depolarization in membrane potential and a simultaneous decrease in electrical coupling, it is unlikely that changes in membrane potential alone are directly responsible for the modulatory effects of 5HT on coupling. This conclusion was further supported by experiments where membrane potential was manually adjusted to resting values following 5HT application. Instead of decreasing, the 5HT-induced reduction in electrical coupling was in fact enhanced during these current injections which returned the membrane potential to baseline levels (base current injection, BCI) (p<0.05; Fig. 3B). To rule out the possibility that the amplitude of the injected current affected electrical coupling, for example recruitment of a voltage-activated conductance, current was injected at variable amplitudes from 0.2 to 3.0 nA before, during and after 5HT perfusion. For a given cell pair, coupling coefficients remained constant over this range of injection steps regardless of the extent of membrane hyperpolarization elicited and 5HT induced a consistent reduction in coupling (data not shown). Taken together, these results support the notion that membrane depolarization alone is not responsible for the 5HT-induced reduction in electrical coupling.
Figure 3.
5HT-induced changes in membrane potential do not account for coupling modulation. A. Histogram illustrates the effect of membrane voltage manipulation on electrical coupling at 19-19 somatic synapses (n=14). Although the mean ECC increases with membrane depolarization, the differences between −120 and −80 mV are not statistically significant (p=0.375; one-way ANOVA, Kruskal-Wallis). B. Histogram of the percent reduction in coupling between soma-soma pairs of neurons 19 in the presence of vehicle (DM), 50 µM 5HT (5HT), or 50 µM 5HT followed by base current injections (BCI, n=22). A greater reduction in coupling was present following serotonin treatment plus BCI, than in the presence of serotonin alone (*, p<0.05). Treatment with DM alone caused significantly less reduction in coupling than the 5HT-treated synapses (**, p<0.001).
Mechanism of 5HT-induced reduction in coupling
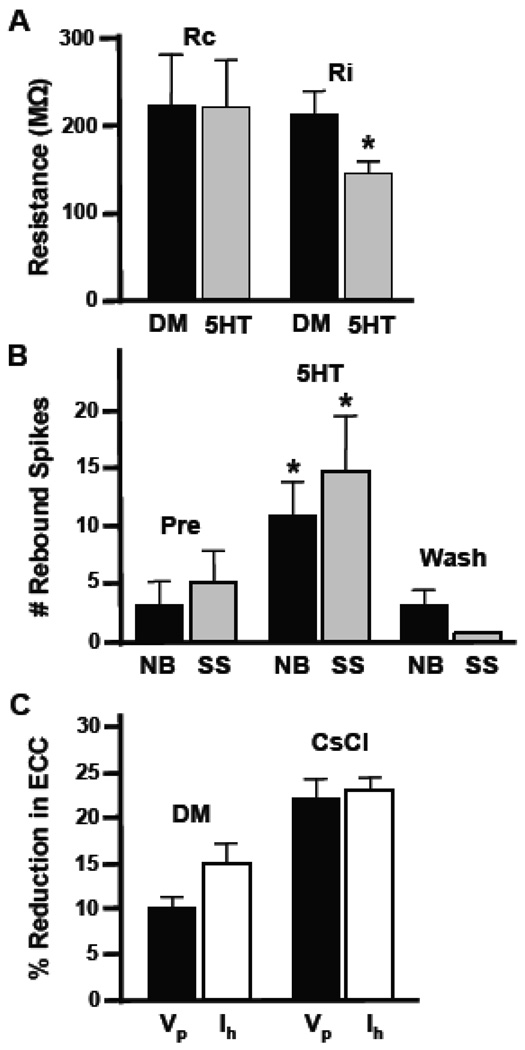
As mentioned earlier, application of serotonin has been shown to alter input resistance in Helisoma neurons and turn on a voltage-gated depolarizing current (Price and Goldberg, 1993). To determine whether 5HT-induced reductions in coupling were 1) indirect, and due to alterations in neuronal membrane input resistance (Ri) or 2) direct, due to alterations in coupling resistance (Rc) at the electrical synapse, we experimentally recorded input resistance and then calculated coupling resistance for electrical synapses in these soma-soma pairs (Table 1; also see Experimental Procedures). Although input resistance decreased significantly in the presence of 5HT (32%, p<0.03; n=12) from 215.52 MΩ to 147.14 MΩ, little change in coupling resistance was seen (p=0.49; Table 1; Fig. 4A). Thus, the major factor responsible for modulation of electrical coupling was likely a change in extrajunctional conductance.
Table 1.
ECC, Ri, and Rc values for experimental B19-B19 electrical synapses.
| Coupling Coefficient (DM) |
Coupling Coefficient (5HT) |
Ri (MΩ) (DM) |
Ri (MΩ) (5HT) |
Rc (MΩ) (DM) |
Rc (MΩ) (5HT) |
|---|---|---|---|---|---|
| 0.50 | 0.44 | 100 | 87 | 100 | 110 |
| 0.65 | 0.48 | 170 | 149 | 92 | 161 |
| 0.67 | 0.58 | 276 | 230 | 136 | 167 |
| 0.68 | 0.67 | 186 | 166 | 88 | 82 |
| 0.34 | 0.27 | 180 | 159 | 349 | 430 |
| 0.62 | 0.48 | 273 | 170 | 167 | 184 |
| 0.24 | 0.21 | 140 | 117 | 443 | 439 |
| 0.44 | 0.32 | 459 | 155 | 584 | 329 |
| 0.17 | 0.13 | 113 | 90 | 553 | 602 |
| 0.81 | 0.68 | 200 | 106 | 47 | 50 |
| 0.78 | 0.76 | 237 | 178 | 67 | 56 |
| 0.80 | 0.72 | 252 | 160 | 63 | 62 |
Figure 4.
Mechanism of neurotransmitter-induced reductions in coupling. (A) Histogram showing alterations in input resistance (Ri), but not coupling resistance (Rc) caused by treatment of neurons with 50µM 5HT. Ri was significantly reduced (*, p<0.03; n=12), while no change in coupling resistance was calculated (p=0.49). (B) Histogram illustrates post-inhibitory rebound (PIR) in 19-19 pairs during bath application of 50 µM 5HT. PIR was increased in both neurite-bearing (NB, n=17; p<0.005) and soma-soma 19-19 pairs (SS, n=6; p<0.005). These effects were reversed upon washout. (C) Coupling coefficients were calculated during hyperpolarizing current injection at early, peak voltage changes (Vp) as well as at later time points during the “sag” (Ih). Bars on the left (DM) show the normalized percent reduction in electrical coupling coefficient (ECC) induced by 5HT at both time points in the same preparations (n=11; p=0.23). The culture medium in this experiment contained only defined medium (DM). Bars on the right represent data from an experiment conducted in medium containing 5 mM CsCl to block Ih (CsCl).
Serotonin also induced a significant enhancement of post-inhibitory rebound (PIR) in neurons of 19-19 cell pairs (Fig. 4B). The mean number of rebound spikes in neurite-bearing neurons prior to 50 µM 5HT application was 3.3 ± 1.9 action potentials (APs; n=6) as compared to 11.0 ± 2.9 APs following 5HT exposure (p<0.005; Fig. 5B). Somatic synapses also possessed a 188% (n=17) increase in post-inhibitory rebound spiking following serotonin perfusion (p<0.005; Fig. 4B). Enhanced post-inhibitory rebound during treatment with serotonin is consistent with enhancement of a hyperpolarization-activated inward current, Ih. This is seen as a voltage compensation ("sag"; e.g., Fig. 2A) during prolonged hyperpolarizing current injection that was enhanced in the presence of 5HT, suggesting that serotonin might selectively enhance Ih, thereby causing a reduction in coupling. To determine whether this was the case, we compared the difference in the 5HT-induced reduction in coupling coefficient at the peak voltage change elicited by current injection (Vp) to the reduction observed during the “sag” at the end of the hyperpolarizing current injection (Ih; Fig. 4C). There was a 13% increase in the effect of 5HT on coupling during Ih compared to Vp (n=7; p=0.23) which was eliminated in the presence of 5 mM cesium chloride (CsCl) which has been shown to block Ih (Corotto and Michel, 1998; p=0.42, Fig. 4C). This indicates 5HT’s effects on electrical coupling are greatest when Ih is greatest; i.e., during the voltage “sag”.
Figure 5.
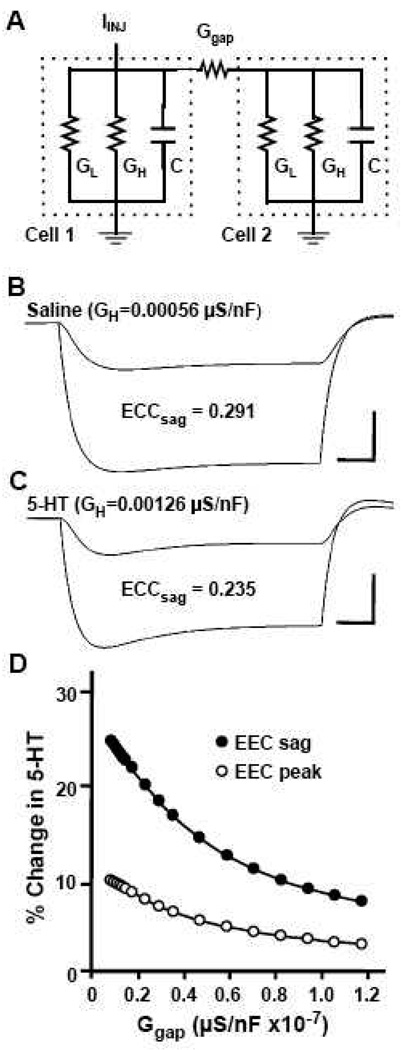
Effects of 5HT modulation on a model electrical synapse. (A) Equivalent circuit diagram for a two-cell neuronal network coupled by a gap junction conductance. This two-cell network is modeled as two resistance/capacitance (RC) circuits with identical cells each having resistive currents based on two conductances: leak (GL) and H (GH). Cell 1 is subjected to current injection (IINJ) and is connected to Cell 2 by a single electrical synapse (Ggap). (B) Voltage traces for the two cells produced by the equivalent circuit model following a 1 nA current injection into Cell 1. H-channel conductance (GH) was modeled after B19 electrical properties in saline and generated an ECC of 0.291 at maximal voltage sag. (C) Voltage traces depicting electrical coupling between the model cells following modulation by 5HT: H-channel conductance (GH) was adjusted to mimic the effects seen in B19 during 5HT application. Increased GH generated a larger sag during the sustained voltage change and an ECC calculated at maximal sag of 0.235, indicating that changes in H-current alone can affect modulation of electrical coupling. (D) Percent reduction in electrical coupling coefficient (% Change in 5HT) calculated at both the sag (ECC sag) and peak time periods (ECC peak) under 5HT conditions (increased GH) as compared with saline conditions, are plotted against gap junction conductance (Ggap). The effects of 5HT were greatest, with both calculations, when gap junctional conductance was low. Furthermore, the effect was pronounced when sag voltage changes were measured, particularly when the model synapse was weakly coupled.
Modeling the effects of 5HT on electrical coupling due to Ih
To determine whether Ih could be responsible for 5HT’s effect on coupling, we created a two-cell model according the equivalent circuit shown in Figure 5A. Cells contained leak and H-currents and were connected by an electrical synapse. H-current was modeled to mimic control (saline) levels in our cultured neurons (H=0.00056 µS/nF; Fig. 5B). To mimic the effect of 5HT, H-current was increased (H=0.00126 µS/nF; Fig. 5C) and the effect on neuronal properties and electrical coupling was examined. Increasing H-current resulted in a depolarization at rest in both model cells from −70.3 mV to 67.2 mV, an increased “sag” during hyperpolarizing current injection, and a reduction in electrical coupling. Further increases in H-current resulted in greater reductions in electrical coupling (Table 1; peak vs. “sag”). In addition, when gap junction conductance was varied at the model electrical synapse, the modulatory effects of 5HT on coupling were greatest at the weaker synapses (Figure 5D). Thus, our model demonstrates that 5HT activation of H-current alone can mimic the effects of serotonin on electrical coupling at cultured neurons.
Discussion
In the present study, we demonstrated 1) that the effects of serotonin on electrical coupling are likely due to increased activation of the H-current, and 2) that serotonin decreases electrical coupling at mature electrical synapses both in vivo and in vitro in a manner negatively correlated with the strength of electrical connectivity at these synapses. Serotonin plays an important role in the modulation of feeding behavior in a wide range of invertebrate animals (Rosen et al. 1983; Beltz et al. 1984; Lent et al. 1989; Quinlan and Murphy 1996). While it has previously been shown that modulation of extrajunctional channels can regulate the strength of electrical coupling, this is the first study to demonstrate that H-current alone could be responsible for these changes.
Electrical synapses formed far from the soma on the distal portion of long neurites were generally weak and serotonin virtually abolished their coupling. However, synapses formed near or at the soma were strong and more resistant to the 5HT-induced reduction in coupling. While this result might in part be due to differences in transfer resistance in the two preparations, it also follows from the equation for calculations of electrical coupling, that the greater the coupling the less the effect of alterations in input resistance. Gap junctional proteins in invertebrates (innexins) and vertebrates (connexins) have variable forms that can produce electrical synapses of different coupling strength (Phelan et al., 2008). Thus the nature of gap junctions and the identity of specific electrical connections with a network might contribute to their susceptibility to neuromodulation. These results suggest that variability in modulatory effects on mature and developing neural circuits is, at least in part, a product of the inherent variation in electrical coupling within those neural networks.
In Helisoma, either stimulation of serotonergic cerebral neuron C1 or perfusion of ganglia with serotonin activates a patterned motor output of buccal neurons involved in the coordination of feeding behavior (Granzow and Kater 1977). Specifically, serotonin leads to a depolarization of many buccal motoneurons, including 19, as well as sustained spiking activity (Price and Goldberg 1993). Motoneurons with similar function are segregated into electrically-coupled units within the buccal ganglia of Helisoma (Kater 1974) and the behavioral consequences of serotonin-activation of the snail’s feeding central pattern generator (CPG) are to phase-lock radular retraction and hyper-retraction (Quinlan and Murphy 1996). Weak electrical coupling, with coefficients as weak as 0.02, exist at neuron 19 connections with premotor (cyberchron) neurons (Bulloch et al. 1980). On the other hand, coupling coefficients of 0.54 and 0.72 have been reported at other Helisoma electrical connections, respectively (Murphy et al. 1983). A differential neuromodulatory mechanism affecting weak electrical connections more than strong synapses would have a targeted impact on integration within neuronal circuit domains containing weakly coupled partners.
The current electrophysiological analyses indicate that 5HT-induced reductions in electrical coupling at 19-19 synapses are due to modulation of a non-junctional membrane conductance. Mapara et al. (2008) recently demonstrated that Helisoma possess receptors in the 5-HT1 and 5-HT7 families of serotonin receptors that are located exclusively on neuritis in ganglia in situ, and at the cell body in vitro in cultured neurons. Serotonin has been shown to modulate the activity of a variety of neuronal ion channels (Klein et al., 1982; Kirk et al., 1988; Taussig et al., 1989; Blumenfeld et al., 1990; Baster and Byrne, 1990). Price and Goldberg (1993) demonstrated that serotonin elicits a depolarization of membrane potential in Helisoma neuron 19 that is accompanied by an increase in firing frequency and a decrease in input resistance (Price and Goldberg 1993). They also demonstrated that 5HT-activated an inward current due mostly to sodium ions, and that this activation was due to a cAMP-dependent mechanism. In addition, they determined that 5HT activated an outward current as well. All of these findings are consistent with activation of Ih channels. Similar 5HT-activation of Ih has been seen in other systems (e.g., Kiehn et al., 2000). Studies have shown that Ih channels can carry both sodium and potassium currents or activate additional potassium currents in conjunction with Ih (e.g., Kiehn and Harris-Warrick, 1992; Yamaguchi et al., 2003). Thus, assuming that serotonin causes an equivalent increase in conductance through Ih channels in both weakly and strongly coupled pairs, it is likely that the greater effect of serotonin on more weakly coupled pairs is due to the proportionally greater shunting of current.
However, serotonin has previously been shown to have a direct effect on Helisoma gap junctions as well (Berdan et al. 1987; Guthrie et al. 1994). Other neuromodulators such as dopamine reduce gap junction channel open probability at zebrafish retinal cell electrical synapses two-to-three fold (McMahon 1994; McMahon and Brown 1994). Furthermore, neuromodulatory effects on gap junctional conductance often involve activation of intracellular second messengers. For example, cAMP has been implicated in the regulation of electrical coupling between leech Retzius cells (Colombaioni and Brunelli 1988), catfish and skate horizontal cells (DeVries and Schwartz 1989; Qian et al. 1993), and pancreatic beta-cells (Mears et al. 1995). As mentioned above, some of the electrophysiological effects of 5HT on neuron 19 have been shown to involve activation of cAMP-dependent mechanisms (Price and Goldberg 1993). The studies in this paper, however, indicate very little change in coupling resistance and a significant change in input resistance. Therefore, indirect effects appear to be largely at play in the 5HT-induced reduction of electrical coupling at these mature Helisoma synapses.
Transient electrical coupling is common during development (Budnik et al. 1989; Goldberg and Kater 1989; Diefenbach et al. 1995; Oland et al. 1995; Kandler and Katz, 1998) and regeneration (Haydon et al. 1984; Murrain et al. 1990; Szabo and Zoran, 2007). Serotonin has been shown to modulate many developmental processes in Helisoma including neuron-selective inhibition of neurite outgrowth and synaptogenesis (Goldberg and Kater, 1989). Goldberg (1998) further demonstrated that activation of a cAMP-regulated sodium conductance in neuron 19 opens voltage-gated calcium channels resulting in activation of a calcium/calmodulin-dependent pathway that inhibits growth cone motility. This sodium conductance activated by serotonin could be due to activation of the same Ih channels found in adult animals. Since the ability of neurons to communicate via gap junctions likely plays a fundamental role in determining patterns of neuronal connectivity (Bulloch and Kater 1981; Wolszon et al. 1994; Balice-Gordon et al. 1995; Kandler and Katz 1995; Maher et al., 2009; Parker et al., 2009), modulation of coupling at key time points in development could have far-reaching consequences and a mechanism of differential modulation based on strength of electrical coupling such as that demonstrated here would have implications for plasticity of developing neural networks.
Experimental Procedures
Experiments were conducted on laboratory stocks of albino (red) pond snails, Helisoma trivolvis, which were maintained in 20 gallon aquaria at 26°C. Aquaria were kept on a controlled photoperiod of 12 hour light/12 hour dark and snails were fed lettuce and trout chow daily.
Reduced ganglia preparations
Snails were deshelled and pinned to a Sylgard-coated dissecting dish. For studies of semi-intact ganglia preparations, a midline incision was made in the dorsal body wall. Removal of the buccal ganglia consisted of severing the cerebrobuccal connectives, as well as the heterobuccal, ventrobuccal, and posterior buccal nerves. In addition, the esophagus was cut from its site of connection to the buccal musculature. Electrical connections between paired Helisoma buccal motoneurons 19 and 19–110 were studied. These neurons innervate radular tensor muscle groups (Kater 1974; Zoran et al. 1989). When ganglia were pinned in appropriate configurations, neuronal cell bodies of 19 and 110 were readily identified. The right buccal neuron 110 might have a symmetrical partner in the left buccal ganglia but it has been difficult to consistently identify, possibly due to the slightly asymmetric arrangement of the posterior nerves at this site; therefore all experiments have utilized neuron 110 from the right ganglia (when the rostral side is up and neuron 110 is visible). Prior to recording, dissected buccal ganglia were stored briefly in defined medium (DM). DM consisted of Leibowitz-15 (L-15, Formula No. 82-5154EC Gibco Laboratories) containing Helisoma salts (40.0 mM NaCl, 1.7 mM KCl, 4.1 mM CaCl2, 1.5 mM MgCl2, and 10.0 mM HEPES) at pH 7.5.
Neuronal Cultures
For studies of neurons isolated into cell culture, excised buccal ganglia were placed into 0.2% trypsin (Sigma) in DM for 20 minutes to partially digest the neural sheath. Ganglia were pinned to a Sylgard dish containing 3 ml of high osmolarity DM (56.0 mM NaCl, 2.4 mM KCl, 5.7 mM CaCl2, 2.1 mM MgCl2, and 14.0 mM HEPES). The buccal commissure and the relevant nerve trunks, containing the axons of 19 and 110 neurons, were crushed with fine forceps. The sheath of each ganglion was cut along the dorsal surface, next to a neuronal soma, using an electrolytically sharpened microknife. Pressure applied to the ganglion forced the neuronal cell body through the incision and the neuron was collected into a fire-polished, non-adhesive (hemolymph-coated) micropipette using negative pressure produced by a microsyringe (Gilmont). Neurons were then transferred into specific culture conditions, as described below for each experiment.
For studies of neurons exhibiting neuritic growth, cells were transferred directly into adhesive 35 mm culture dishes (No. 3001 Falcon) containing 2 ml of conditioned medium (CM). The dishes were made adhesive by pretreatment with 0.1% poly-l-lysine (PLL) in a 0.15M Tris buffer. CM was generated by incubating 2 central ring ganglia per 1 ml of DM in these PLL-coated culture dishes for 3 days. Brain-derived factors in CM are required for neurite outgrowth in these cultures (Wong et al. 1981). Neurons were maintained in these culture conditions while neurites extended and established contacts.
For studies of neurons lacking neurite outgrowth, cells were transferred into non-adhesive, 35 mm culture dishes (No. 1008 Falcon) containing 2 ml of CM. CM was bulk-cultured in silicone-treated (Sigmacote) glass petri dishes before being transferred to culture dishes that had been made non-adhesive by pretreatment with a 0.5% solution of bovine serum albumin (BSA). Neurons were maintained in these culture conditions as single, spherical cells for 24 h before being transferred into fresh CM dishes and paired into contact. Cell pairs were cultured for an additional 24 h and then transferred to recording chambers (PLL-treated culture dishes containing 2 ml DM) for electrophysiological study.
General Electrophysiology
Electrophysiological properties of neurons were examined using intracellular recording techniques. Glass microelectrodes (borosilicate; FHC), possessing tip resistances ranging from 10–20 MΩ, were filled with 1.5 M KCl. Current-clamp recordings of neuronal membrane potentials were amplified using a bridge-balanced electrometer (Getting Instrumental Inc.) and records were viewed on a storage oscilloscope (Tektronix). In most experiments unless otherwise stated, neuronal membrane potential was maintained with base current injection (BCI) at approximately −70 mV. Electrical coupling was measured by injecting constant amplitude, hyperpolarizing current pulses (3 s in duration) into one neuron (0.2 – 3nA) while simultaneously recording membrane voltage changes in the presynaptic (injected) neuron (approximately 30–50 mV) and its synaptic partner. Coupling coefficients were determined as the ratio of postsynaptic to presynaptic voltage changes (Bennett 1977). Data analyses for coupling ratios and input resistance measurements were taken at the peak of the membrane hyperpolarization. Electrophysiological recordings were digitized by a MacLab A/D data acquisition system linked to a Macintosh Quadra 950 computer using Chart software. Records were archived onto a magneto-optical disk for later analysis and printing.
Analysis of Reduced Ganglia Preparations
Dual recordings were made from electrically coupled cell pairs. Preparations were pinned onto a Sylgard-coated glass petri dish containing 2 ml of 10x calcium saline (40.0 mM NaCl, 1.7 mM KCl, 41.0 mM CaCl2, 1.5 mM MgCl2, 10.0 mM HEPES). Increased levels of calcium were used to reduce general motor activity of the preparation. Neuronal pairs were then penetrated with glass microelectrodes and hyperpolarizing current injections were applied sequentially to each cell (0.3–1 nA for 3 s). Constant perfusion of the recording chamber (3 ml/min) was maintained throughout the experiment using a peristaltic pump (Pharmacia). Solutions of 0.5, 5.0 or 50 µM serotonin creatine sulfate (5HT; Sigma) in 10x calcium saline were perfused through the recording chamber for 2–3 minutes. Treatment was followed by a 20–30 min wash with 10x calcium saline. The modulatory effects of serotonin on motoneuronal electrical coupling were determined by injecting multiple hyperpolarizing current pulses into each neuron of the pair before, during, and after perfusion. Data measurements were taken at peak membrane potential changes associated with hyperpolarizing current injections unless otherwise indicated. Analysis of the data determined that these electrical connections were non-rectifying. Thus, bi-directional analyses were averaged and a single mean coupling coefficient was calculated for cell pairs at each phase of the experiment.
Analysis of Neuronal Cell Cultures
The effects of exogenous serotonin on electrical coupling between neurons in cell culture were examined as described above. Cells were penetrated with microelectrodes, hyperpolarizing current pulses were injected, and coupling coefficients were calculated. 50 µM 5HT in DM was perfused over each cell pair for 30 seconds, followed by 5–10 min DM wash.
Calculation of coupling resistance
To determine the effect of serotonin on soma-soma pairs, we calculated the coupling resistance (Rc) as an average of pre- and postjunctional responses to current injection using the equation:
CC = coupling coefficient = V2/V1
Ri = averaged input resistance of both neurons 19 in a soma-soma pair, since neurons are identical
Rc = averaged coupling resistance between neurons 19 in a soma-soma pair.
Model Electrical Synapse
To verify that an increase in the H conductance would explain the observed decrease in the electrical coupling coefficient, a model was developed which included two identical cells with a gap junction between them. Each cell had only leak (Gleak) and H (GH) conductances (Fig. 5A). The parameters for this model (C, capacitance; Ggap, Gleak and GH) were determined using the experimental data (see Figure 2A). To determine Ggap, we measured the steady state voltage drops into the two cells, which we call ΔV1 and ΔV2. For the circuit shown in Figure 5A, Kirchoff's Laws can be used to show that
| (1) |
We therefore set Ggap to this value. Using similar reasoning, we set Gleak to:
| (2) |
The capacitance value was found as follows. Our two cell model is mathematically equivalent to a single cell two compartment model. Thus, we can write the response of this circuit to a current step as the sum of two exponentials (Golowasch et al., 2009).
| (3) |
Adapting the parameters of the Golowasch et al. (2009) two compartment model to our two cell model, we write:
| (4) |
| (5) |
| (6) |
| (7) |
where Rleak = 1/Gleak and Rgap = 1/Ggap.
At time = τ0, the voltage is given by:
| (8) |
where:
Since all variables on the right are known, τ0 can be found by examining the point at which the voltage trace crosses V(τ00). Plugging this value into τ00 = Rleak C gives us our final capacitance value. GH was tuned by trial and error to best match the sag observed in cell 1. Once the model parameters were established for cell 1 in control conditions (saline), the H conductance was adjusted until the sag of cell 1 matched that in the experimental 5HT condition. Electrical coupling coefficients were calculated for both peak voltage change (ECCp) and steady state “sag” conditions (ECCs) for both saline and 5HT conditions. The parameters for this model were found to be: C = 5.38 nF, Ggap = 11.7 nS, Gleak = 20.5 nS and GH = 3.01 nS.
Data Analysis
Comparisons of electrical coupling during and after serotonin perfusion were analyzed for recovery from treatment using Student’s t-tests. Regression analyses were performed using StatView 4.1 (Abacus Concepts, Inc.). Data is presented as mean plus or minus standard error of the mean (s.e.m.) unless otherwise indicated.
Table 2.
Effect of H-current on ECC of model electrical synapses.
| Model H value (uS/nF) | Sag (mV) | ECCp (peak) | ECCs (sag) |
|---|---|---|---|
| 0.000000 | 0 | 0.37 | 0.37 |
| 0.000200 | 0.4 | 0.34 | 0.34 |
| 0.000400 | 1.1 | 0.33 | 0.31 |
| 0.000560* | 1.8 | 0.32 | 0.2 |
| 0.000600 | 1.9 | 0.31 | 0.29 |
| 0.000800 | 2.8 | 0.31 | 0.27 |
| 0.001000 | 3.6 | 0.30 | 0.25 |
| 0.001200 | 4.4 | 0.29 | 0.24 |
| 0.001255** | 4.6 | 0.29 | 0.24 |
| 0.001400 | 5.2 | 0.29 | 0.23 |
indicates H value used to simulate normal saline conditions.
indicates H value used to simulate 5HT-treatment conditions.
Acknowledgments
The authors wish to thank T. Brookings, M. Goeritz, A. Pereda, J. Poyer, J. Richardson and M. Turner for valuable comments. Research conducted in the Department of Biology at Texas A&M University and was supported by a National Science Foundation Grant (IBN-9421372) and National Institutes of Health Grant (P01 NS39546) to M.J.Z. Jonathan S. Caplan was supported by a National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT) Grant (DGE-0549390) at Brandeis University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Achee NL, Zoran MJ. Serotonin-induced modulation of excitability in an identified Helisoma trivolvis neuron. J Exp Biol. 1997;200:1537–1548. doi: 10.1242/jeb.200.10.1537. [DOI] [PubMed] [Google Scholar]
- Antonsen BL, Edwards DH. Mechanisms of serotonergic facilitation of a command neuron. J Neurophysiol. 2007;98:3494–3504. doi: 10.1152/jn.00331.2007. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Pines J, Blumenthal S. Role of motor neuron gap junctional coupling in neuromuscular synapse elimination. Soc Neurosci Abstr. 1995;21:1748. [Google Scholar]
- Baxter DA, Byrne JH. Differential effects of cyclic AMP and serotonin on membrane current, action-potential duration and excitability in somata of pleural sensory neurons of Aplysia. J.Neurophysiol. 1990;64:978–990. doi: 10.1152/jn.1990.64.3.978. [DOI] [PubMed] [Google Scholar]
- Beltz B, Eisen JS, Flamm R, Harris-Warrick RM, Hooper SL, Marder E. Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans (Panulirus interruptus, Homarus americanus and Cancer irroratus) J Exp Biol. 1984;109:35–54. doi: 10.1242/jeb.109.1.35. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Physiology of electrotonic junctions. Ann NY Acad Sci. 1966;137:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Electrical transmission: a functional analysis and comparison to chemical transmission. In: Kandel ER, editor. Handbook of Physiology. Volume 1. Bethesda: Amer Phys Soc; 1977. pp. 357–416. [Google Scholar]
- Bennett MVL. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC. Gap junctions: New tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Berdan RC, Shivers RR, Bulloch AGM. Chemical synapses, particle arrays, pseudo-gap junctions and gap junctions of neurons and glia in the buccal ganglion of Helisoma. Synapse. 1987;1:304–323. doi: 10.1002/syn.890010404. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Spira ME, Kandel ER, Siegelbaum SA. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in Aplysia sensory neurons. Neuron. 1990;5:487–499. doi: 10.1016/0896-6273(90)90088-w. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Verselis V, Levitan IB, Spray DC. Electrotonic synapses between Aplysia neurons in situ and in culture: aspects of regulation and measurements of permeability. J Neurosci. 1988;8:1656–1670. doi: 10.1523/JNEUROSCI.08-05-01656.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Wu C-F, White K. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J Neurosci. 1989;9:2866–2877. doi: 10.1523/JNEUROSCI.09-08-02866.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch AG, Kater SB. Selection of a novel connection by adult molluscan neurons. Science. 1981;212:79–81. doi: 10.1126/science.7209523. [DOI] [PubMed] [Google Scholar]
- Bulloch AG, Kater SB, Murphy AD. Connectivity changes in an isolated molluscan ganglion during in vivo culture. J Neurobiol. 1980;11:531–546. doi: 10.1002/neu.480110604. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Carrow GM, Levitan IB. Selective formation and modulation of electrical synapses between cultured Aplysia neurons. J Neurosci. 1989;9:3657–3664. doi: 10.1523/JNEUROSCI.09-10-03657.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombaioni L, Brunelli M. Neurotransmitter-induced modulation of an electrotonic synapse in the CNS of Hirudo medicinalis. Exp Biol. 1988;47:139–144. [PubMed] [Google Scholar]
- Curti S, Pereda AE. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J Neurosci. 2004;24:3999–4010. doi: 10.1523/JNEUROSCI.0077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach TJ, Sloley BD, Goldberg JI. Neurite branch development of an identified serotonergic neuron from embryonic Helisoma: evidence for autoregulation by serotonin. Dev Biol. 1995;167:282–293. doi: 10.1006/dbio.1995.1023. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Schulz DJ, Marder E. Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci. 2009;12:1424–1430. doi: 10.1038/nn.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JI. Serotonin regulation of neurite outgrowth in identified neurons from mature and embryonic Helisoma trivolvis. Perspect Dev neurobiol. 1998;5:373–387. [PubMed] [Google Scholar]
- Goldberg JI, Kater SB. Expression and function of the neurotransmitter serotonin during development of the Helisoma nervous system. Dev Biol. 1989;131:483–495. doi: 10.1016/s0012-1606(89)80019-3. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Thomas G, Taylor AL, Patel A, Pineda A, Khalil C, Nadim F. Membrane capacitance measurements revisited: dependence of capacitance value on measurement method in nonisopotential neurons. J Neurophysiol. 2009;102:2161–2175. doi: 10.1152/jn.00160.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. Reliable neuromodulation from circuits with variable underlying structure. Proc Natl Acad Sci U S A. 2009;106:11742–11746. doi: 10.1073/pnas.0905614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow B, Kater SB. Identified higher-order neurons controlling the feeding motor program of Helisoma. Neurosci. 1977;2:1049–1063. [Google Scholar]
- Guan X, Cravatt BF, Ehring GR, Hall JE, Boger DL, Lerner RA, Gilula NB. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J Cell Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie PB, Lee RE, Rehder V, Schmidt MF, Kater SB. Self-recognition: a constraint on the formation of electrical coupling in neurons. J Neurosci. 1994;14:1477–1485. doi: 10.1523/JNEUROSCI.14-03-01477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RD, Bodnar DA, Kater SB. Formation of electrical synapses between isolated, cultured Helisoma neurons requires mutual neurite elongation. J Neurosci. 1985;5:3145–3153. doi: 10.1523/JNEUROSCI.05-12-03145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Activation of a D2 receptor increases electrical coupling between retinal horizontal cells by inhibiting dopamine release. Proc Natl Acad Sci USA. 1992;89:9220–9224. doi: 10.1073/pnas.89.19.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. The formation of chemical synapses between cell-cultured neuronal somata. J Neurosci. 1988;8:1032–1038. doi: 10.1523/JNEUROSCI.08-03-01032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Kater SB. The differential regulation of formation of chemical and electrical connections in Helisoma. J Neurobiol. 1988;19:636–655. doi: 10.1002/neu.480190706. [DOI] [PubMed] [Google Scholar]
- Haydon PG, McCobb DP, Kater SB. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984;226:561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- Haydon PG, McCobb DP, Kater SB. The regulation of neurite outgrowth, growth cone motility, and electrical synaptogenesis by serotonin. J Neurobiol. 1987;18:197–215. doi: 10.1002/neu.480180206. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Zoran MJ. Chemical synapses in cell culture. In: Chad H, Wheal H, editors. Cellular neurobiology: a practical approach. New York: Oxford University Press; 1991. pp. 57–71. [Google Scholar]
- Hughes SW, Crunelli V. Hardwiring goes soft: long-term modulation of electrical synapses in the mammalian brain. Cellscience. 2006;2:1–9. [PMC free article] [PubMed] [Google Scholar]
- Johnson BE, Peck JH, Harris-Warrick RM. Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A. 1993;172:715–732. doi: 10.1007/BF00195397. [DOI] [PubMed] [Google Scholar]
- Johnson BE, Peck JH, Harris-Warrick RM. Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J Comp Physiol A. 1994;175:233–249. doi: 10.1007/BF00215119. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Levitan IB. Neuromodulation: the biochemical control of neuronal excitability. New York: Oxford University Press; 1987. [Google Scholar]
- Kandler K, Katz LC. Neuronal coupling and uncoupling in the developing nervous system. Curr Op Neurobiol. 1995;5:98–105. doi: 10.1016/0959-4388(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater SB. Feeding in Helisoma trivolvis: the morphological and physiological bases of a fixed action pattern. Am Zool. 1974;14:1017–1036. [Google Scholar]
- Kepler TB, Marder E, Abbott LF. Effect of electrical coupling on the frequency of model neuronal oscillators. Science. 1990;248:83–85. doi: 10.1126/science.2321028. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM. 5-HT modulatin of hyperpolarization-activated inward current and calcium-dependent outward current in a crustacean motor neuron. J Neurophys. 1992;68:496–508. doi: 10.1152/jn.1992.68.2.496. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties ot the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- Kirk MD, Taussig R, Scheller RH. Egg-laying hormone, serotonin and cyclic nucleotide modulation of ionic currents in the identified motoneuron B16 of Aplysia. J. Neurosci. 1988;8:1181–1193. doi: 10.1523/JNEUROSCI.08-04-01181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Camardo J, Kander ER. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc. Natl. Acad. Sci.U.S.A. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Lent CM, Dickinson MH, Marshall CG. Serotonin and leech feeding behavior: obligatory neuromodulation. Amer Zool. 1989;29:1241–1254. [Google Scholar]
- Locke D, Harris AL. Connexin channels and phospholipids: association and modulation. BMC Biol. 2009;7:52. doi: 10.1186/1741-7007-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BJ, McGinley MJ, Westbrook GL. Experience-dependent maturation of the glomerular microcircuit. Proc Natl Acad Sci USA. 2009;106:16865–16870. doi: 10.1073/pnas.0808946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapara S, Parries S, Quarrington C, Ahn KC, Gallin WJ, Goldberg JI. Identification, molecular structure and expression of two clones serotonin receptors from the pond snail, Helisoma trivolvis. J Exptl Biol. 2008;211:900–910. doi: 10.1242/jeb.013953. [DOI] [PubMed] [Google Scholar]
- McMahon DG. Modulation of electrical synaptic transmission in zebrafish retinal horizontal cells. J Neurosci. 1994;14:1722–1734. doi: 10.1523/JNEUROSCI.14-03-01722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Brown DR. Modulation of gap-junction channel gating at zebrafish retinal electrical synapses. J Neurophysiol. 1994;72:2257–2268. doi: 10.1152/jn.1994.72.5.2257. [DOI] [PubMed] [Google Scholar]
- Mears D, Sheppard NF, Jr, Atwater I, Rojas E. Magnitude and modulation of pancreatic beta-cell gap junction electrical conductance in situ. J Membr Biol. 1995;146:163–176. doi: 10.1007/BF00238006. [DOI] [PubMed] [Google Scholar]
- Moss BL, Fuller AD, Sahley CL, Burrell BD. Serotonin modulates axo-axonal coupling between neurons critical for learning in the leech. J Neurophysiol. 2005;94:2575–2589. doi: 10.1152/jn.00322.2005. [DOI] [PubMed] [Google Scholar]
- Murrain M, Murphy AD, Mills LR, Kater SB. Neuron-specific modulation by serotonin of regenerative outgrowth and intracellular calcium within the CNS of Helisoma trivolvis. J Neurobiol. 1990;21:611–618. doi: 10.1002/neu.480210408. [DOI] [PubMed] [Google Scholar]
- Murphy AD, Hadley RD, Kater SB. Axotomy-induced parallel increases in electrical and dye coupling between identified neurons of Helisoma. J Neurosci. 1983;3:1422–1429. doi: 10.1523/JNEUROSCI.03-07-01422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AD, Lukowiak K, Stell WK. Peptidergic modulation of patterned motor activity in identified neurons of Helisoma. Proc Natl Acad Sci USA. 1985;82:7140–7144. doi: 10.1073/pnas.82.20.7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AD, Quinlan EM, Arnett BC. Food extracts stimulate the feeding motor pattern in Helisoma, in part by activating dopaminergic interneuron N1A. Soc Neurosci Abstr. 1996;22:1376. [Google Scholar]
- Nadarajah B, Jones AM, Evans WH, Parnavelas JG. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oland LA, Kirchenbaum SR, Pott WM, Mercer AR, Tolbert LP. Development of an identified serotonergic neuron in the antennal lobe of the moth and effects of reduction in serotonin during construction of olfactory glomeruli. J Neurobiol. 1995;28:248–267. doi: 10.1002/neu.480280210. [DOI] [PubMed] [Google Scholar]
- Parker PR, Cruikshank SJ, Connors BW. Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology. J Neurosci. 2009;29:9761–9770. doi: 10.1523/JNEUROSCI.4568-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993a;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Pereda AE, Rash JE, Nagy JI, Bennett MV. Dynamics of electrical transmission at club endings on the Mauthner cell. Brain Res Brain Res Rev. 2004;47:227–244. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Phelan P, Goulding LA, Tam JLY, Allen MJ, Dawber RJ, Davies JA, Bacon JP. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fibre system. Curr. Biol. 2008;18:1955–1960. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Goldberg JI. Serotonin activation of a cyclic AMP-dependent sodium current in an identified neuron from Helisoma trivolvis. J Neurosci. 1993;13:4979–4987. doi: 10.1523/JNEUROSCI.13-11-04979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Malchow RP, Ripps H. Gap-junctional properties of electrically coupled skate horizontal cells in culture. Visual Neurosci. 1993;10:287–295. doi: 10.1017/s0952523800003680. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Gregory K, Murphy AD. An identified glutamatergic interneuron patterns feeding motor activity via both excitation and inhibition. J Neurophysiol. 1995;73:945–956. doi: 10.1152/jn.1995.73.3.945. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Murphy AD. Plasticity in the multifunctional buccal central pattern generator of Helisoma illuminated by the identification of phase 3 interneurons. J Neurophysiol. 1996;75:561–574. doi: 10.1152/jn.1996.75.2.561. [DOI] [PubMed] [Google Scholar]
- Radu A, Dahl G, Loewenstein WR. Hormonal regulation of cell permeability: upregulation by catecholamine and prostaglandin E. J Membr Biol. 1982;70:239–251. doi: 10.1007/BF01870566. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Rorig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur J Neurosci. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Rosen SC, Kupfermann I, Goldstein RS, Weiss KR. Lesion of a serotonergic modulatory neuron in Aplysia produces a specific defect in feeding behavior. Brain Res. 1983;260:151–155. doi: 10.1016/0006-8993(83)90778-3. [DOI] [PubMed] [Google Scholar]
- Spira ME, Spray DC, Bennett MV. Synaptic organization of expansion motoneurons of Navanax inermis. Brain Res. 1980;195:241–269. doi: 10.1016/0006-8993(80)90063-3. [DOI] [PubMed] [Google Scholar]
- Szabo TM, Faber DS, Zoran MJ. Transient electrical coupling delays the onset of chemical neurotransmission at developing synapses. J Neurosci. 2004;7:112–120. doi: 10.1523/JNEUROSCI.4336-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo TM, Zoran MJ. Transient electrical coupling regulates formation of neuronal networks. Brain Res. 2007;1129:63–71. doi: 10.1016/j.brainres.2006.09.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R, Sweet-Cordero A, Scheller RH. Modulation of ionic currents in Aplysia motor neuron B15 by serotonin, neuropeptides and second messengers. J Neurosci. 1989;9:3218–3229. doi: 10.1523/JNEUROSCI.09-09-03218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble DL, Barker DL. Activation by dopamine of patterned motor output from the buccal ganglia of Helisoma trivolvis. J Neurobiol. 1984;15:37–48. doi: 10.1002/neu.480150105. [DOI] [PubMed] [Google Scholar]
- Trimble DL, Barker DL, Bullard BJ. Dopamine in a molluscan nervous system: synthesis and fluorescence histochemistry. J Neurobiol. 1984;15:27–36. doi: 10.1002/neu.480150104. [DOI] [PubMed] [Google Scholar]
- Wolinsky EJ, Patterson PH, Willard AL. Insulin promotes electrical coupling between cultured sympathetic neurons. J Neurosci. 1985;5:1675–1679. doi: 10.1523/JNEUROSCI.05-07-01675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolszon LR, Gao W, Passani MB, Macagno ER. Growth cone “collapse” in vivo: are inhibitory interactions mediated by gap junctions? J Neurosci. 1994;14:999–1010. doi: 10.1523/JNEUROSCI.14-03-00999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RG, Hadley RD, Kater SB, Hauser GC. Neurite outgrowth in molluscan organ and cell cultures: the role of conditioning factor(s) J Neurosci. 1981;1:1008–1021. doi: 10.1523/JNEUROSCI.01-09-01008.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kaczmarek LK, Kelley DB. Functional specialization of male and female vocal motoneurons. J Neurosci. 2003;23:11568–11576. doi: 10.1523/JNEUROSCI.23-37-11568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran MJ, Haydon PG, Matthews PJ. Aminergic and peptidergic modulation of motor function at an identified neuromuscular junction in Helisoma. J Exp Biol. 1989;142:225–243. doi: 10.1242/jeb.142.1.225. [DOI] [PubMed] [Google Scholar]