Abstract
The sodium iodide symporter (NIS) directs the uptake and concentration of iodide in thyroid cells. We have extended the use of NIS-mediated radioiodine therapy to other types of cancer, we transferred and expressed the sodium-iodide symporter (NIS) gene into prostate, colon, and breast cancer cells using adenoviral vectors. To improve vector efficiency we have developed a conditionally replicating adenovirus (CRAd) in which the E1a gene is driven by the prostate specific promoter, Probasin and the cassette RSV promoter-human NIScDNA-bGH polyA replaces the E3 region (CRAd Ad5PB_RSV-NIS). In vitro infection of the prostate cancer cell line LnCaP resulted in virus replication, cytolysis, and release of infective viral particles. Conversely, the prostate cancer cell line PC-3 (androgen receptor negative) and the pancreatic cancer cell line Panc-1 were refractory to the viral cytopathic effect and did not support viral replication. Radioiodine uptake was readily measurable in LnCaP cells infected with Ad5PB_RSV-NIS 24 hours post-infection, confirming NIS expression. In vivo, LnCaP tumor xenografts in nude mice injected intratumorally with Ad5PB_RSV_NIS CRAd expressed NIS actively as evidenced by 99Tc uptake and imaging. Administration of therapeutic 131I after virus injection significantly increased survival probability in mice carrying xenografted LnCaP tumors compared to virotherapy alone. The data indicate that Ad5PB_RSV_NIS replication is stringently restricted to androgen positive prostate cancer cells and results in effective NIS expression and uptake of radioiodine. This construct may allow multimodal therapy, combining cytolytic virotherapy with radioiodine treatment, to be developed as a novel treatment for prostate cancer.
Keywords: prostate cancer, probasin, adenovirus, sodium iodide symporter, virotherapy, gene therapy
INTRODUCTION
Prostate cancer is the second leading cause of cancer death in men 1. To date, no uniformly curative therapy for metastatic prostate cancer has been developed. In some malignancies, for which existing treatment regimens are not completely effective, suicide gene therapy and virotherapy strategies targeting the tumor-associated genetic alterations represent rational directions for the development of novel therapeutics 2–4.
The sodium iodide symporter (NIS) is a transmembrane glycoprotein that mediates uptake of iodide into cells, especially thyroid follicular cells 5,6. The presence of NIS on the basolateral membrane of thyroid cells has been exploited for many years for diagnostic imaging purposes as well as for ablative therapy of differentiated thyroid cancer using radioactive iodide (131I). This non-invasive therapy has proven to be a safe and effective treatment for thyroid cancer, even in advanced, metastatic disease 7,8. In order to extend the use of NIS-mediated radioiodine therapy to other types of cancer, we have successfully transferred and expressed the sodium-iodide symporter (NIS) gene in prostate, colon, and breast cancer cells, both in vivo and in vitro, using adenoviral vectors. Our experience with adenovirus-mediated NIS transfer and radioiodine therapy was confirmed in large animal model and has culminated in the opening of a phase I trial for prostate cancer that is currently accruing patients 9–14.
All gene therapy approaches depend upon the ability to deliver therapeutic genes to target cells. However, the limited ability to efficiently transduce tumors with effective levels of therapeutic transgenes has been identified as the fundamental barrier to effective cancer gene therapy 15, 16. To address this issue, conditionally replicating virus, including adenovirus, have been constructed and their efficacy evaluated 17–19. Two important strategies towards inducing tumor specificity in replicating viral constructs have been pursued: either viral genes essential for viral replication are expressed and controlled by tumor-specific promoters or, alternatively viral genes are mutated in such a way that expression of specific tumor genes are needed for complementation and viral gene induction. Of these two strategies, transcriptional dependent regulation seems to be the most efficient to date 20.
A second conclusion that can be drawn from recent virotherapy clinical trials is that multimodal therapy, combining virotherapy (i.e. viral mediated tumor cytolysis) with chemo- or radiotherapy may be necessary for more complete tumor eradication as opposed to mono-therapy using virotherapy alone 21. Novel mechanisms that allow such multimodal approaches towards metastatic prostate cancer are sorely needed and thereby enhance anti-tumor effectiveness are sorely needed.
Our approach towards the current problems associated with virotherapy/gene therapy has been the development of tumor specific, conditionally replicating adenoviral vectors that also harbor the NIS gene. Transcriptional control of the E1A gene would reduce extratumoral toxicity and by inducing tumor selective replication and tumor lysis. NIS expression will allow for non- invasive imaging and reporting as well as radioiodine mediated therapy. This combination of virus mediated oncolysis and NIS mediated radioiodine therapy has been termed “Radiovirotherapy” 22. We report here the development of a conditionally replicating adenovirus (CRAd) in which the E1a gene is driven by the prostate specific promoter Probasin, thereby targeting prostate cells, and the transcriptional cassette RSV promoter-hNIScDNA-bGH polyA is inserted at the E3 region, inducing NIS expression in permissive prostate cells. Our results suggest that this CRAd may represent a novel approach to prostate cancer gene therapy.
Materials and Methods
Cell Culture
The Androgen-dependent (LnCaP), Androgen-independent (PC-3) prostate cancer cell line, and the pancreatic cancer cell line Panc-1 were cultured as described 10–12,23. Adenovirus infection was carried out for four hours in serum free media. Cells were then washed once with PBS and replenished with fresh culture media.
CRAd Construction
The shuttle vector pVQPB is a derivative of the ViraQuest shuttle vector pVQAD5 and was constructed as follows; the E1A gene flanked by a 5′ blunt end and an EcoRI 3′ end was isolated from plasmid pCD512_F2 (a gift from Dr. Richard Vile, Mayo Clinic). The shuttle vector pVQA_PB-NIS 24 was digested with BamHI and blunt ended with T4DNA pol, then digested with EcoRI to release the NIS gene. The resulting vector was then dephosphorylated with CIP and ligated to the E1A gene insert. The human NIS cDNA, under Rous Sarcoma Virus LTR promoter control, was subcloned into an Ad5 genomic vector containing an E3-deleted version (ViraQuest). The pVQPB vector and the NIS containing Ad5 genomic plasmid were recombined to yield the CRAd Ad5PB_RSV-NIS. Cell plaques were grown, purified, and characterized by Vira Quest, North Liberty, IA 24. The final plaque forming unit (pfu) to viral particle (vp) was 1 to 100.
Cytopathic effect assays
1 × 106 cells were infected at MOI 1. Four days post-infection, cells (triplicates) were photographed under a light microscope at 100 × magnification.
MTS Assay
5×105 cells were seeded in 96-well tissue culture plates and incubated at 37°C, 5% CO2 in triplicate. Twenty four hours after plating, cells were infected at the indicated MOI. At time points, 50μl of CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega Corporation) was applied to each well and incubated at 37°C, 5% CO2 for 2 hours. Wells were then read at the 490nm wavelength using a plate spectrophotometer.
Replication Assay of the CRAd vector
4.4 × 106 cells were plated in 150 cm2 culture flasks and infected at MOI 1 or MOI 0.1. Seventy two hours post-infection media and cells were collected separately. The virus was then purified from each source using the ViraBind Adenovirus Purification Kit (Cell Biolabs). Viral titers were quantified by plaque assay on HEK 293 cells.
125I Uptake
Uptake of 125I by virus-infected cells was measured as described 25,26. Briefly, 1 × 106 cells/well were plated on 36-well tissue culture plates and infected at MOI 20. At indicated time points, iodide uptake measurement was performed in triplicate in HBSS supplemented with 10 mM HEPES, pH 7.3, 10 μM NaI, and 1 × 105 cpm of 125I. Control wells were incubated in the same buffer plus 10 μM KClO4. Plates were gently mixed and incubated at 37° C, 5% CO2 for 45 minutes. Following incubation, buffers were aspirated and the cells gently washed once with 4°C 10 mM HEPES, pH 7.3 buffer. Cells were lysed in 1 ml 1N NaOH with shaking for 20 minutes at RT, and lysates were assayed for 125I on a gamma counter.
Animal Experiments
All experimental protocols were approved by the Mayo Foundation Institutional Animal Care and Use Committee.
Subcutaneous Tumor Model
Xenografts derived from the LnCaP and Panc-1 cell lines were established into the right flanks of 4–6-week-old athymic nude Foxn1nu mice (Harlan) by subcutaneous injection of 4 × 106 cells resuspended in 0.125μl media and 0.125μl of BD Matrigel basement membrane matrix (BD Biosciences, Bedford, MA). Mice were maintained on a low iodine diet and T4 supplementation (5mg/l) in their drinking water throughout the duration of the experimentation to maximize radioisotope uptake in the tumor and minimized uptake by the thyroid. The mice were examined daily for tumor development.
Titration of intratumoral virus by QPCR
Mice were xenografted with LNCaP or Panc1 tumors. Once tumor size exceeded 100 mm3, mice were injected intratumorally with the indicated doses of Ad5PB_RSV-NIS. At day ten post-infection, mice were sacrificed and the tumors harvested. Three tumor samples weighing 30 mg were taken from each tumor and DNA was extracted using the DNEasy tissue kit (Qiagen, Valencia, CA, USA) with overnight lysis of tumor tissue in ATL buffer/Proteinase K. Each sample was assayed for Ad5PB_RSV-NIS genome copies using the Applied Biosystems TaqMan Universal PCR System according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA, USA). Briefly, reactions contained 300 nM of a forward primer GACGGCTACGACTGAATG from nt27837 to nt27854 in the Ad5 genome, 300 nM of a reverse primer complementing RSV sequences 118 bp upstream of the RSV promoter, CCGCTTTTCGCCTAAACACAC, and 250 nM of a TaqMan probe binding to the forward strand of the junction between Ad5 E3 sequences and the RSV sequences, 6′ FAM – ATATCTGGCCCGTACATCGCAGAT – Iowa Black FQ, (Integrated DNA Technologies, Coralville, IA, USA). Five μl of template DNA was amplified in 25 μl reactions using ABI Prism 7900HT Real Time PCR System (Applied Biosystems, Foster City, CA, USA). Genome copies per milligram of tissue were quantitated using a standard curve generated by amplification of known quantities of viral genomes.
Noninvasive imaging of xenografted tumors
Four mice were subcutaneously engrafted with LnCaP and three with Panc-1 cells as described above. When tumors reached 100 mm3, mice received one intratumoral (IT) injection of 1011 vp of the Ad5PB_RSV-NIS CRAd. In vivo micro-SPECT-CT imaging was performed one hour after intraperitoneal injection of 0.5μCi of 99Tc. Animals were anesthetized using a Summit Medical Anesthesia Machine (Summit Medical Equipment Company, Bend, Oregon). Oxygen (2 LPM) was used throughout induction and exam. Induction was performed with 4% Isoflurane using an induction chamber. Mice were kept anesthetized throughout the full measurement by 2% Isoflurane using a nose cone. Images were acquired using a Gamma Medica XSPECT system (Gamma Medica, Inc., Northridge, CA). The SPECT scan was performed with a low energy, high resolution parallel-hole collimator, Field of View of 12.5 cm, with a Reported Resolution of 1 to 2 mm. Sixty four projections, 10 sec/projection, were acquired with a Total Acquisition Time of 13:46 min. The CT Scan was performed with a circular orbit 256. Images were acquired at 80kVp and 0.28 mA with a Slice Thickness of 50μm and a Reported resolution of 43 μm. Images were analyzed using the PMOD Biomedical Image Quantification and Kinetic Modeling Software (PMOD Technologies, Switzerland). The level of 99Tc uptake by the tumor was expressed as tumor activity in mCi.
Efficacy Studies
Mice were subcutaneously engrafted with LnCaP as described above. When tumors were visible, one group of mice was used as control (C), a second group received a single intratumoral dose of Ad5PB_RSV-NIS at 1011 vp (virotherapy V), and the third group received a single intratumoral dose of Ad5PB_RSV-NIS at 1011 vp and 4 days later a single intraperitoneal dose of 3mCi 131I (radiovirotherapy V+I). The volume of the tumors was measured twice weekly.
RESULTS
Virus construction
The conditionally replicating adenovirus (CRAd) Ad5PB_RSV-NIS harbors the transcriptional cassette RSV promoter-human NIScDNA-bGH polyA inserted at the E3 region. In this CRAd, the E1a gene is driven by a small composite ARR2-Probasin androgen-dependent prostate-specific promoter (PB) 27.
Conditional replication, oncolysis, and spread of Ad5PB_RSV-NIS
To test the specificity of virus replication, three CAR positive cell lines were selected; the androgen dependent prostate cancer cell line LnCaP, the androgen receptor negative prostate cancer cell line PC-3, and the pancreatic cell line Panc-1.
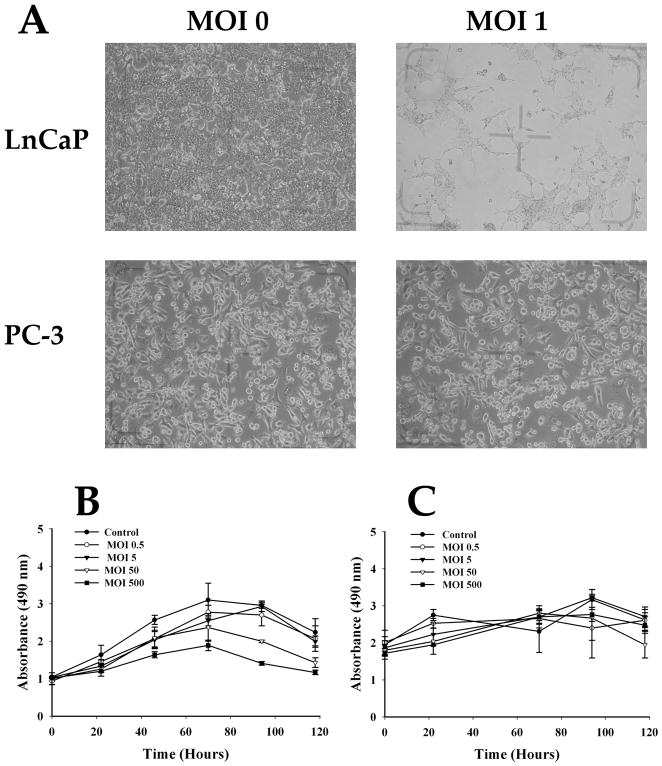
We assayed the specificity of Ad5PB_RSV-NIS replication using several tests. We first examined the specificity of viral protein expression by Western blot for Probasin-driven E1A and Hexon expression (Not Shown). We next measure the specificity of cytopathic effect (CPE) by light microscopy. Cells were infected in triplicates with Ad5PB_RSV-NIS at MOI 1 and examined four days later for signs of CPE. The morphology of the PC-3 cells was unchanged after infection with Ad5PB_RSV-NIS. However, clear signs of CPE in LnCaP were detected under the same conditions (Figure 1A).
FIGURE 1. Virus Mediated Cell Killing.
A) Cytopathic effect. Ad5PB_RSV-NIS-induced cytopathic effect was assessed by light microscopy. B) Virus-mediated cell-specific killing. The prostate cancer cell line LnCaP and the pancreatic cell line Panc-1 were infected with Ad5PB_RSV-NIS at the indicated MOI. At time points cell viability was assayed by MTS.
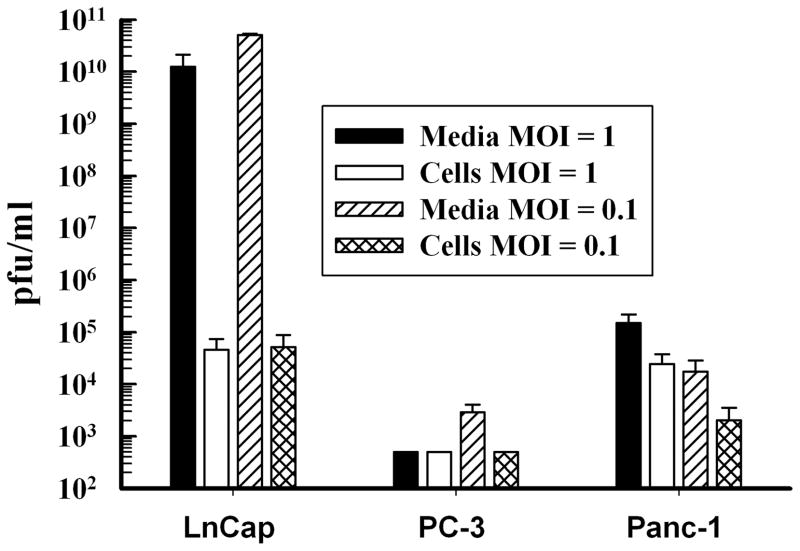
To further test for virus-mediated cell-specific killing, the permissive cell line LnCaP was infected with the Ad5PB_RSV-NIS CRAd at increasing MOIs and then assayed daily by MTS. The results were compared to those obtained with the non-permissive pancreatic cell line Panc-1. The LnCaP cells showed a decreased in viability that was both time and MOI doses dependent (Figure 1B). On the other hand, Panc-1 viability was not affected at all (Figure 1C). Similar negative results were obtained using the androgen-independent prostate cell lime PC-3 (Not Shown). Virus progeny production was measured with the Ad5PB_RSV-NIS CRAd in LnCaP, PC-3, and Panc-1 cells three days post-infection at MOI 1 and 0.1 (Figure 2). The amount of virus produced by the LnCaP cells was 7 orders of magnitude higher than the amount produced by the PC-3 cells and 5 orders of magnitude larger than Panc-1. Moreover, the bulk of viral progeny produced by LnCaP was found in the media indicating that viral infection of LnCaP results in cell lysis with subsequent release of infective viral particles.
FIGURE 2. Cell Specific Replication of Ad5PB_RSV-NIS.
LnCaP, PC-3, and Panc-1 cells were infected with Ad5PB_RSV-NIS at MOI 1 and 0.1. Viral progeny was isolated from media and cells and titrated by plaque assay.
Taken together, these data show that replication of the Ad5PB_RSV-NIS virus is stringently restricted to androgen receptor positive prostate cancer cells confirming that the virus is a conditionally replicating adenovirus or CRAd.
NIS expression and radioiodine uptake
The next task was to investigate NIS expression induced by the construct, which is driven by the ubiquitous RSV promoter. CRAd-mediated NIS expression, in prostate and pancreatic cell lines which do not naturally supports NIS expression, was examined by Western Blot (Not shown) and by NIS-mediated radioiodine uptake (Figure 3). Ad5PB_RSV-NIS infected LnCaP cells kinetics of iodine uptake at MOI 20 showed a maximum at 24 hours post-infection followed by a sharp decrease (Figure 3A). Radioiodine uptake was inhibited by KClO4, a well known inhibitor of NIS activity indicting that it was specific for NIS expression 5. These data indicate that an optimal window in time for NIS expression in permissive cells exists, after which NIS expression declines due to virus replication and induction of CPE. In support of this interpretation, cells infected with the non-replicating virus Ad5PB-RSV, in which the NIS gene under the control of the PB promoter is inserted at the E1A region, maintained radioiodine uptake for three days (Figure 3B). Additionally, Panc-1 cells, which are refractory to Ad5PB_RSV-NIS killing, also supported radioiodine uptake mediated by RSV-driven NIS for three days (Figure 3C) but, as expected, did not express PB-driven NIS (Figure 3D).
FIGURE 3. Kinetics of NIS-mediated Radioiodine Uptake.
LnCaP and Panc-1 cells were infected at MOI 20 with the CRAd or the non replicating virus Ad5PB-NIS in which the E1A region is replaced by the transcription unit PB-NIS-SV40 PolyA. 125I uptake was measured daily.
Our results demonstrate that Ad5PB_RSV-NIS directs NIS expression in permissive cells before the onset of cytopathic effect but, NIS expression is limited in time by cell death. Conversely, in non-permissive cells NIS is expressed with kinetics and dose response characteristics consistent with transient expression such as that seen after non-replicating virus infection.
Noninvasive imaging of xenografted tumors
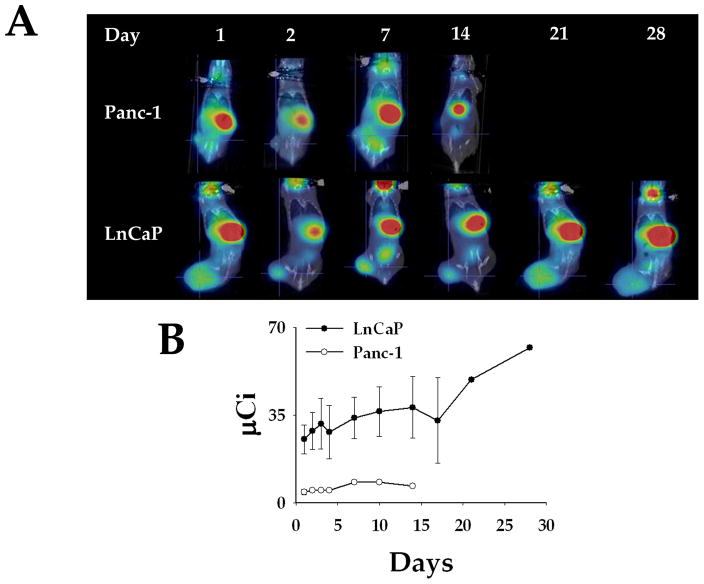
Next, we tested Ad5PB_RSV-NIS ability to direct NIS expression in vivo by imaging. When tumors reached 200 mm3 a single Ad5PB_RSV-NIS dose of 1011 vp of was administered intratumorally. Twenty-four hours later, an intraperitoneal dose of 0.5μCi of 99Tc was administered. NIS-mediated 99Tc uptake was detected with a noninvasive micro SPECT-CT imaging system. Figure 5A shows a classical time lapse uptake in LnCaP and Panc-1 xenografted tumors. One hour after delivery of the radiotracer, uptake can be detected in all animals receiving a 99Tc dose over the stomach due to native expression of NIS in the gastric mucosa, even in the absence of viral injection 5,28. The thyroid also concentrates 99Tc representing the major NIS expressing organ. Finally the bladder, is also imaged as a result of clearance of the radioisotope in the urine. However, due to their position in the plane of the image, these organs are not seen in all images. Very minimal 99Tc uptake was detected in the Panc-1 tumor at any time point after viral administration (Figure 4A). On the other hand, figure 4A reveals strong 99Tc uptake by the LnCaP tumor that has been injected with the Ad5PB_RSV-NIS CRAd 24 hours post-infection. Quantitation of the image yielded 99Tc uptake in infected tumors of more than 12% of the injected dose. These data indicate specific NIS-mediated 99Tc uptake in the permissive tumor.
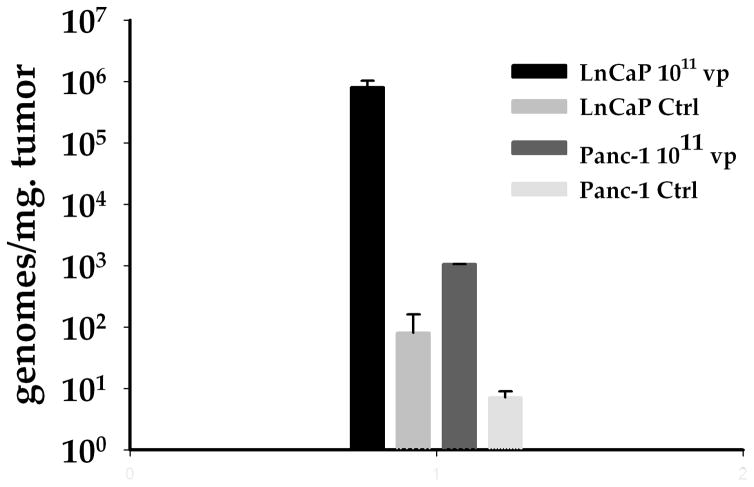
FIGURE 5. Titration of viral genome copies in xenografted tumors.
Two LnCaP and two Panc-1 xenografted tumors were infected with Ad5PB_RSV-NIS at 1011 vp. Non-infected tumors were used as controls. On day ten the animals were sacrificed and the tumors removed. Viral genome copies were titred by QPCR using three different longitudinal sections (left, center, and right) for each tumor
FIGURE 4. Radioiodine Imaging of Xenograft Tumors.
A) LnCaP and Panc-1 xenografted tumors were infected with Ad5PB_RSV-NIS at 1011 vp. At time points post-infection an injection of 0.5 mCi 99Tc was given IP to all mice. Images were captured using a noninvasive micro SPECT-CT imaging system one hour after radioisotope administration. B) Kinetics of imaging were established by quantifying 99Tc tumor uptake using the PMOD Biomedical Image Quantification and Kinetic Modeling Software (PMOD Technologies, Switzerland). The level of 99Tc uptake by the tumor was expressed as tumor activity in μCi.
A kinetic curve of 99Tc uptake was constructed using xenografted LnCaP and Panc-1 tumors to determine viral persistence. An intratumoral injection of 1011 vp was administered at time 0 to all mice in each group. At indicated time points, 0.5 μCi of 99Tc was administered IP and radiotracer uptake was visualized and quantified by micro SPECT-CT imaging. Strong 99Tc NIS-mediated uptake was sustained for up to one month post-infection in the Ad5PB_RSV-NIS infected LnCaP tumors. After this point, mice were sacrificed due to increasing tumor size. On the other hand, only minimal traces of radioisotope accumulation were observed in the infected Panc-1 tumors (Figure 4B). These data indicate that the Ad5PB_RSV-NIS CRAd directs long term expression of functional NIS in LnCaP tumor in vivo and, giving the kinetics of NIS expression, suggest that the Ad5PB_RSV-NIS replicates in vivo.
Further prove of in vivo CRAd replication was obtained by assaying viral genome copy number by QPCR. LnCaP and Panc-1 xenograft tumors established in nude mice were infected with a single dose of Ad5PB_RSV-NIS at 1011 vp. Ten days later, the animals were sacrificed and the tumors were tested for viral genome copy contents. We found that the number of viral genome copies per gram of tumor was 3 log orders higher in the permissive LnCaP tumors than in the non-permissive Panc-1 tumors (Figure 5). Taken together, the data presented in Figures 4 and 5 strongly supports the notion that the Ad5PB_RSV-NIS CRAd replicates intratumorally in permissive tumors.
Radioiodine therapy study in vivo
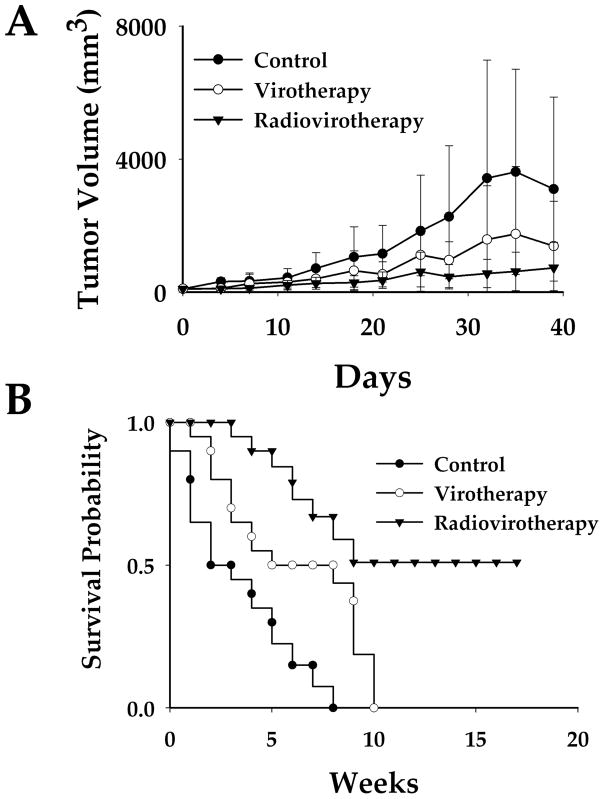
LnCap xenografts were established in three groups of mice. One group (n=10) of mice was used as control (C), a second group (n=10) received a single intratumoral dose of Ad5PB_RSV-NIS at 1011 vp (virotherapy V), and the third group (n=10) received a single intratumoral injection of Ad5PB_RSV-NIS at 1011 vp and 4 days later a single intraperitoneal dose of 3 mCi 131I (radiovirotherapy V+I). Tumor measurements started the day of virus administration and twice weekly thereafter. Mice were followed for a total of 40 days or until tumor burden was such that animals had to be sacrificed (Figure 6A). In the control group, tumors grew steadily. The high values in the standard errors reflect large variations in tumor growth. Virotherapy treatment slowed tumor growth considerably. As for the control group, high values in the standard errors were observed as a consequence of irregular, albeit greatly decreased, tumor growth. When animal received radiovirotherapy tumor growth was completely arrested. This effect was less variated as attested by the small values of the standard errors. Linear mixed effect models with random intercept and slope were fitted for each group. Pairwise comparisons of the slopes using the likelihood ratio test indicated significant differences among all treatment groups after Bonferroni’s adjustment (control vs virus p=0.001, control vs radiotherapy p<0.001, and virus vs radiotherapy p<0.001).
FIGURE 6. Efficacy Studies.
Mice were subcutaneously engrafted with LnCaP, one group of mice was used as control (C), a second group received a unique intratumoral dose of Ad5PB_RSV-NIS at 1011 vp (virotherapy V), and the third group received a unique intratumoral dose of Ad5PB_RSV-NIS at 1011 vp and 4 days later a single intraperitoneal dose of 3mCi 131I (radiovirotherapy V+I). For each group n=10. A) Tumor growth kinetics. B) Survival was analyzed according to Kaplan-Meier (t test p=0.001)
We investigated whether treatment with Ad5PB_RSV-NIS CRAd combined with 131I radiotherapy could increase survival. LnCap xenografts were established in three groups of mice. One group (n=10) of mice was used as control (C), a second group (n=10) received a single intratumoral dose of Ad5PB_RSV-NIS at 1011 vp (virotherapy V), and the third group (n=10) received an intratumoral dose of Ad5PB_RSV-NIS at 1011 vp and 4 days later a single intraperitoneal dose of 3 mCi 131I (radiovirotherapy V+I). Mice were followed and the survival times of each group were displayed using the Kaplan-Meier curve (Figure 6B). The end point event was set at tumor burden ≥ 1,000 mm3, while death before end point was considered as censoring. By week 8 all animals in the control group reached tumor sizes ≥ 1,000 mm3. Virotherapy resulted in a net improvement in probability survival over the control group. Thus, while all control animals have died by week 8, the virotherapy group had a probability of survival of 0.5. However, after week 8, survival in this group plunged precipitously. On the other hand, the radiovirotherapy group achieved a 0.5 probability of survival up to 18 weeks, at which point the experiment was ended. The survival times of the three treatment groups were significantly different as indicated by the log-rank test (p=0.001). Assuming proportional hazard, the hazard ratio of virotherapy vs control was 0.360 (95% CI: 0.125---1.040) and the hazard ratio of radiovirotherapy vs control was 0.115 (95% CI: 0.031---0.423). Taken together, these results indicate that the combination of radiotherapy and cytolytic virotherapy was superior to virotherapy (p=0.001) which was a moderate improvement over control.
DISCUSSION
We have reported the use of the probasin promoter to target expression of NIS to prostate cancer cells in vitro 29. Our initial in vitro studies with the non-replicating virus As5PB-NIS, in which the PB promoter drives the expression of the NIS, yielded high levels of membrane-targeted NIS expression in androgen-positive LnCaP prostate cancer cells. We have extended this study here to an in vivo mouse model of prostate cancer. Radioiodide imaging of tumor bearing mice revealed targeted expression of NIS following adenoviral infection with substantial 99Tc concentration at the tumor site. Moreover, virotherapy combine with therapeutic 131I treatment significantly increased survival probability in mice carrying xenografted LnCaP tumors compared to virotherapy alone.
Although a number of gene therapy approaches have been developed for many cancers, clinical trials completed to date have fallen short of expectations 30–34 largely due to limited tumor transduction, and this limitation has been defined as the most important fundamental barrier to effective cancer gene therapy 35. This has been addressed by escalating the viral dose, but this tactic has also been limited in effect when viral vectors, including adenoviral vectors, are used because of increasing toxicity with higher viral doses. Finding ways to surmount these obstacles is critical for the development of clinically useful cancer gene therapy and virotherapy approaches. Our approach to address the problem of low tumor transduction was to permit local vector replication after delivery using a conditionally replicating adenovirus. We have utilized a transcriptional regulation method placing the E1A gene under control of the prostate specific promoter probasin and the NIS gene under control of the powerful, but non-specific RSV promoter. The rationale for this approach was that viral replication, and consequently extended NIS expression, will be limited to permissive cell lines and tumors. In the present study we show that PB restricts viral protein synthesis, cytopathic effect, cell lysis, and infectious viral progeny production to probasin positive prostate cancer cells. All negative control cells used in this study expressed the CAR receptor, yet androgen negative prostate and non-prostate cells did not support oncolysis and/or viral replication. Moreover, the finding that these cell lines supported transient expression of NIS shows that they were indeed infected by the Ad5PB_RSV-NIS thus reinforcing the notion that the PB promoter targeted viral replication solely to probasin positive cells. In vivo, QPCR assay revealed that the number of viral genome was 3 log orders of magnitude larger in the permissive LnCaP tumors than in the non-permissive Panc-1 ten days post-infection. In addition, robust 99Tc uptake, and thus correct NIS expression, was maintained for a month in the established LnCaP tumors while only very limited uptake was observed in the non-permissive Panc-1 pancreatic tumors. Taken together, and since Ad5 does not replicate in mouse tissues, we interpret these results as proof that the Ad5PB_RSV-NIS CRAd is also capable of in vivo replication in permissive androgen positive prostate tumors.
The NIS gene must be properly targeted to the cell membrane to be functional 36. Under the control of the RSV promoter, NIS was correctly targeted to the cell membrane thereby promoting high levels of iodine uptake both in cell tissue culture and xenografted androgen positive prostate tumors. NIS expression provides a major advantage of this construct; its expression can be directly and non-invasively monitored using diagnostic doses of radioiodine or other similar radionuclides.
The limited efficacy observed in clinical trials of cancer gene therapies to date have suggested that combinatorial therapies to treat cancer will likely be more effective and possibly required for complete tumor eradication. The hypothesis developed from this observation is that attacking tumor cells through different mechanisms of actions may prevent tumor cells from developing resistance to the treatment combination and may more effectively induce immunity against the tumor 21. Our results show that Ad5PB_RSV-NIS mediated NIS expression improves the therapeutic value of this CRAd by allowing multimodal therapy in a single agent – viral mediated tumor lysis as well as radioiodine mediated therapy. In addition, it has been shown that E1A represses HER-2/neu resulting in induction of apoptosis 37. In prostate cancer, the androgen receptor interacts with the HER-2/neu signaling pathways and this interaction contributes to the development of metastatic disease 38. Therefore, PB restricted expression of E1A to prostate tumors may result in HER-2/neu signaling pathway inhibition thus increasing the efficacy of the Ad5PB_RSV-NIS CRAd.
In conclusion, Ad5PB_RSV-NIS CRAd addresses favorably the major hurdles identified in gene therapy clinical trials for anti-cancer treatments. Radiovirotherapy that combines virotherapy with NIS mediated 131I therapy can be further developed based on this novel CRAd which has the potential to impact prostate cancer treatment.
Acknowledgments
The authors wish to thank Azhal Ahmadi, Robert Parrish, Rachel E. Carlson, Tracy Decklever, and Dr. Jian Qiao for technical help. This work was supported by Prostate SPORE grant P50 CA 091956, Donald J. Tindall, P.I., John C. Morris, Project Director.
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist
References
- 1.Ruijter E, et al. Molecular genetics and epidemiology of prostate carcinoma. Endocrine Reviews. 1999;20:22–45. doi: 10.1210/edrv.20.1.0356. [DOI] [PubMed] [Google Scholar]
- 2.Chiocca EA, Broaddus WC, Gillies GT, Visted T, Lamfers ML. Neurosurgical delivery of chemotherapeutics, targeted toxins, genetic and viral therapies in neuro-oncology. Journal of Neuro-Oncology. 2004;69:101–117. doi: 10.1023/b:neon.0000041874.02554.b3. [DOI] [PubMed] [Google Scholar]
- 3.McCormick F. Cancer gene therapy: fringe or cutting edge? Nature Reviews Cancer. 2001;1:130–141. doi: 10.1038/35101008. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Curiel DT. Cancer gene therapy. Technology in Cancer Research & Treatment. 2005;4:315–330. doi: 10.1177/153303460500400402. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco N. Iodide transport in the thyroid gland. Biochimica et Biophysica Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 6.Jhiang SM, Cho JY, Ryu KY, DeYoung BR, Smanik PA, McGaughy VR, et al. An immunohistochemical study of Na+/I- symporter in human thyroid tissues and salivary gland tissues. Endocrinology. 1998;139:4416–4419. doi: 10.1210/endo.139.10.6329. [DOI] [PubMed] [Google Scholar]
- 7.Van Nostrand D, Wartofsky L. Radioiodine in the treatment of thyroid cancer. Endocrinology & Metabolism Clinics of North America. 2007;36:807–822. doi: 10.1016/j.ecl.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.ELM . Carcinoma of follicular epithelium: Radioiodine and other treatments and outcomes. In: Braverman LE, Utiger RD, editors. The Thyroid: A Fundamental and Clinical Text. 7. Lippincott - Raven; Philadelphia: 1996. pp. 922–945. [Google Scholar]
- 9.Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Therapy. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer RM, Bergert ER, O’Connor MK, Gendler SJ, Morris JC. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clinical Cancer Research. 2005;11:1483–1489. doi: 10.1158/1078-0432.CCR-04-1636. [DOI] [PubMed] [Google Scholar]
- 11.Dwyer RM, Bergert ER, O’Connor MK, Gendler SJ, Morris JC. Sodium Iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Therapy. 2006;13:60–66. doi: 10.1038/sj.gt.3302599. [DOI] [PubMed] [Google Scholar]
- 12.Spitzweg C, Dietz AB, O’Connor MK, Bergert ER, Tindall DJ, Young CY, et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Therapy. 2001;8:1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer RM, Schatz SM, Bergert ER, Myers RM, Harvey ME, Classic KL, et al. A preclinical large animal model of adenovirus-mediated expression of the sodium-iodide symporter for radioiodide imaging and therapy of locally recurrent prostate cancer. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2005;12:835–841. doi: 10.1016/j.ymthe.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. 2009. http://clinicaltrials.gov/ct/show/NCT00788307.
- 15.Herrmann F. Cancer gene therapy: principles, problems, and perspectives. Journal of Molecular Medicine. 1995;73:157–163. doi: 10.1007/BF00188136. [DOI] [PubMed] [Google Scholar]
- 16.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nature Reviews Genetics. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 18.Markert JM, Malick A, Coen DM, Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Therapy. 2002;9:961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- 20.Bazan-Peregrino M, Carlisle RC, Hernandez-Alcoceba R, Iggo R, Homicsko K, Fisher KD, et al. Comparison of molecular strategies for breast cancer virotherapy using oncolytic adenovirus. Human Gene Therapy. 2008;19:873–886. doi: 10.1089/hum.2008.047. [DOI] [PubMed] [Google Scholar]
- 21.Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clinical Cancer Research. 2004;10:5299–5312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- 22.Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 23.Qu CF, Li Y, Song YJ, Rizvi SM, Raja C, Zhang D, et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. British Journal of Cancer. 2004;91:2086–2093. doi: 10.1038/sj.bjc.6602232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Therapy. 2000;7:1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 25.Weiss SJ, Philp NJ, Grollman EF. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology. 1984;114:1090–1098. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- 26.Spitzweg C, Dietz AB, O’Connor MK, Bergert ER, Tindall DJ, Young CY, et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Therapy. 2001;8:1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141:4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 28.Zuckier LS, Dohan O, Li Y, Chang CJ, Carrasco N, Dadachova E. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. Journal of Nuclear Medicine. 2004;45:500–507. [PubMed] [Google Scholar]
- 29.Kakinuma H, Bergert ER, Spitzweg C, Cheville JC, Lieber MM, Morris JC. Probasin promoter (ARR(2)PB)-driven, prostate-specific expression of the human sodium iodide symporter (h-NIS) for targeted radioiodine therapy of prostate cancer. Cancer Research. 2003;63:7840–7844. [PubMed] [Google Scholar]
- 30.Cascallo M, Capella G, Mazo A, Alemany R. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Research. 2003;63:5544–5550. [PubMed] [Google Scholar]
- 31.Humphreys MJ, Greenhalf W, Neoptolemos JP, Ghaneh P. The potential for gene therapy in pancreatic cancer. International Journal of Pancreatology. 1999;26:5–21. doi: 10.1385/IJGC:26:1:5. [DOI] [PubMed] [Google Scholar]
- 32.Hung MC, Hortobagyi GN, Ueno NT. Development of clinical trial of E1A gene therapy targeting HER-2/neu-overexpressing breast and ovarian cancer. Advances in Experimental Medicine & Biology. 2000;465:171–180. doi: 10.1007/0-306-46817-4_16. [DOI] [PubMed] [Google Scholar]
- 33.Miura Y, Ohnami S, Yoshida K, Ohashi M, Nakano M, Fukuhara M, et al. Intraperitoneal injection of adenovirus expressing antisense K-ras RNA suppresses peritoneal dissemination of hamster syngeneic pancreatic cancer without systemic toxicity. Cancer Letters. 2005;218:53–62. doi: 10.1016/j.canlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. Journal of Pathology. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Wolf G, Schmidt-Wolf IG. Human cancer and gene therapy. Annals of Hematology. 1994;69:273–279. doi: 10.1007/BF01696555. [DOI] [PubMed] [Google Scholar]
- 36.Kaminsky SM, Levy O, Salvador C, Dai G, Carrasco N. The Na+/I-symporter of the thyroid gland. Society of General Physiologists Series. 1993;48:251–262. [PubMed] [Google Scholar]
- 37.Hortobagyi GN, Ueno NT, Xia W, Zhang S, Wolf JK, Putnam JB, et al. Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biologic effects: a phase I clinical trial. Journal of Clinical Oncology. 2001;19:3422–3433. doi: 10.1200/JCO.2001.19.14.3422. [DOI] [PubMed] [Google Scholar]
- 38.Ricciardelli C, Jackson MW, Choong CS, Stahl J, Marshall VR, Horsfall DJ, et al. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. 2008;68:830–838. doi: 10.1002/pros.20747. [DOI] [PubMed] [Google Scholar]