1. Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is characterized by inattention, hyperactivity and/or impulsivity inappropriate for one’s age that negatively impacts at least two aspects of the individual’s life (e.g., school, work, and/or home life) (DSM-IV). Although early conceptualizations of ADHD suggested the disorder was limited to childhood, recent research indicates that the majority of individuals with ADHD continue to exhibit impaired functioning in adulthood (Faraone & Biederman, 2005; Faraone, Biederman & Mick, 2006; Kessler et al., 2007). Although little is known about brain morphometry in adults with ADHD, neuroimaging studies have documented clear differences in brain morphometry between children and adolescents diagnosed with ADHD as compared to control samples. Using various volumetric and region of interest (ROI) approaches, these studies have typically examined differences at the level of lobes (e.g., parietal) or specific subcortical structures (e.g., the caudate). A recent meta-analysis of such studies (Valera et al., 2007) revealed that anatomical differences are most often noted for total cerebral volume and right cerebral volume, cerebellar regions, the splenium of the corpus callosum, and the right caudate. Although fewer studies have investigated the anatomy of frontal regions specifically, findings of reduced cerebral volume in these areas exist as well (Castellanos et al., 1996; Durston et al., 2004; Filipek et al., 1997; Hill et al. 2003; Kates et al., 2002; Mostofsky et al., 2002).
Despite an abundance of literature using voxel based morphometry (VBM) to examine the brain morphology of children and adolescents with ADHD, only a few studies have examined adults who have been diagnosed with ADHD (Seidman et al., 2006; Makris et al., 2007; Monuteaux et al., 2008; Biederman et al. 2007; Perlov et al., 2008; Hesslinger et al., 2002). Hesslinger and colleagues (2002) found reduced left orbitofrontal cortex (OFC) in individuals with ADHD using a manual segmentation technique. A study by Makris and colleagues (2007) report decreased cortical thickness in a number of regions involved in executive functioning including bilateral DLPFC, orbitofrontal cortex, regions of the cingulate gyrus, and the inferior parietal lobule. A study by Seidman and colleagues (2006), examined the same sample as that of Makris and colleagues, found decreased volume in the left superior frontal gyrus and anterior cingulate cortex of the right hemisphere. Using a priori defined ROIs, Monuteaux and collegues (2008) found reductions in overall mean volume of DLPFC and cerebellum, while Biederman and colleagues (2007) found reduced volume of neocortex grey matter, overall frontal lobe, superior frontal cortex and anterior cingulate regions as well as reduced volume of cerebellar grey matter. Hence, evidence is mounting that the brains of adults with ADHD show morphological differences from control samples.
Given the relative paucity of research on brain morphology in adults with ADHD, further investigation is warranted. The current study utilized a college-aged sample, unlike previous studies examining brain morphology in adults with ADHD (Monuteaux et al., 2008; Biederman et al. 2007; Perlov et al., 2008) in which the mean age was in the early or mid-30s,. Furthermore, because the majority of prior studies examining VBM in adults with ADHD have used manual tracing segmentation techniques or ROI based approaches (Seidman et al., 2006; Makris et al., 2007; Monuteaux et al., 2008; Biederman et al. 2007; Perlov et al., 2008) an investigation using an unbiased approach to VBM (Optimized VBM) is warranted.
Optimized VBM was used in the present study to accomplish two main goals. First, we compared morphometry in a well-characterized sample of young adults who met DSM-IV (American Psychiatric Association, 2000) diagnostic criteria for ADHD but who were not co-morbid for learning disabilities or psychiatric conditions to controls similar in age, gender and IQ. In this manner, any morphometric differences between the groups are likely to be due specifically to ADHD and also to be common across individuals with the disorder. Second, because the causes of ADHD are thought to be multi-factorial, we took an individual differences approach examining whether the degree of disruption in behavioral performance within our ADHD group is associated with morphometric features. We selected our measures of behavioral performance by choosing those that consistently yield the largest effect size across studies when individuals with ADHD are compared to those without: processing speed (PS), response inhibition (RI) and response variability (RV) (Willcutt et al. 2005). Furthermore, these behavioral performance measures assess well identified dysfunction in ADHD, as it relates to attention towards specific stimuli, processing of those stimuli, and the need to inhibit irrelevant stimuli. Although these measures have mainly been identified in studies with children with ADHD, they provide a reasonable starting point for studies with adults. We expected that group differences in brain morphology might exhibit themselves in regions found in prior reports, and that relationships would be observed between behavioral performance variables and brain morphology. To our knowledge, this is one of the few optimized VBM (unbiased, operator free segmentation) study on adults with ADHD to examine how behavioral performance relates to grey matter morphometry.
2. Methods and Materials
2.1 Participants and Recruitment
We recruited the current sample as a part of a comprehensive examination of the internal and external validity of different approaches to the measurement of ADHD in adults (Willcutt et al. submitted).
2.1.1 Screening procedures
Initially, an unselected sample of 3,913 undergraduates completed a battery of self-report rating scales that included a measure of current and childhood symptoms of DSM-IV ADHD (Barkley & Murphy, 1998; American Psychiatric Association, 2000), with 70% of all providing consent to obtain parallel ratings of DSM-IV ADHD symptoms from one of their parents. Participants who scored above the 90th percentile of the total screening sample on either the inattention or hyperactivity-impulsivity dimensions were invited for a more extensive individual testing session. That testing session included a structured interview regarding DSM-IV ADHD (DSM-IV-R), the Matrix Reasoning and Vocabulary subtests from the Wechsler Adult Intelligence Scale, Third Edition (Weschler, 1997), and subtests from the Woodcock-Johnson Tests of Achievement, Third Edition (WJ-III)(Woodcock, McGrew & Mather, 2001), that assess achievement in mathematics (Calculations and Math Fluency) and reading-related domains (Letter-Word Identification, Word Attack, and Spelling)(See Table 1).
Table 1.
Descriptive characteristics of the Control and ADHD samples.
| GROUP | |||
|---|---|---|---|
| Control | ADHD | ||
| M (SD) | M (SD) | t | |
| Descriptive characteristics | |||
| Age | 19.3 (1.1) | 20.0 (1.7) | 1.59 |
| Percent female | 61.1% | 38.9% | 1.78 |
| DSM-IV ADHD symptoms | |||
| Childhood | |||
| Inattention | 0.9 (0.9) | 7.3 (2.1) | 11.96*** |
| Hyperactivity-impulsivity | 1.1 (1.1) | 6.3 (2.0) | 9.64*** |
| Current | |||
| Inattention | 1.3 (1.4) | 6.3 (2.4) | 7.65*** |
| Hyperactivite-impulsivity | 1.6 (1.1) | 4.9 (2.4) | 5.23*** |
| WAIS-III Estimated IQ scores | |||
| Performance | 113.1 (11.0) | 114.1 (9.1) | 0.33 |
| Verbal | 112.2 (11.4) | 114.6 (11.4) | 0.64 |
| Full Scale | 112.6 (9.1) | 114.2 (7.3) | 0.55 |
| WJ-III | |||
| Reading and Spelling | |||
| Letter Word ID | 103.6 (8.1) | 99.2 (9.4) | 1.52 |
| Word Attack | 97.1 (8.4) | 99.1 (8.8) | 0.70 |
| Spelling | 104.5 (6.9) | 102.1 (8.8) | 0.82 |
| Math | |||
| Calculations | 108.3 (15.4) | 105.7 (16.2) | 0.46 |
| Math Fluency | 101.2 (11.3) | 94.9 (9.3) | 1.68 |
=p<0.05
=p<0.01
=p<0.001.
All participants completed multiple measures of functional impairment to ensure significant impairment across setting. The DSM-IV rating scales included several questions regarding the impact of ADHD symptoms on the individual’s social, occupational, educational, and overall daily functioning. Participants also completed a more detailed questionnaire that we developed to specifically measure impairment in academic performance (e.g., GPA, completion of assignments, retention of material), interpersonal relationships, money management (e.g., bounced checks, credit card debt, impulsive purchases), driving performance (e.g., tickets, accidents), and occupational functioning (e.g., retention of jobs, completion of assigned tasks). Finally, Global Assessment of Functioning Scale that corresponds directly to Axis V in DSM-IV was assessed by asking the participant and parent to rate the individual’s lowest overall functioning during the past year. Significant impairment was quantified based on cutoff scores at the 93rd percentile of our overall sample on a composite measure of each domain of impairment (Willcutt et al. submitted).
2.1.2 Identification of groups with and without ADHD
Four criteria were used to operationally define participants with ADHD for the current study: (1) Retrospective ratings by the participant or the parent indicated that he or she met DSM-IV criteria for the combined type in childhood; (2) the participant either currently met criteria for DSM-IV ADHD (N = 28) or scored above the 90th percentile on the ADHD symptom measures while exhibiting marked functional impairment, consistent with the DSM-IV specification of ADHD in partial remission (N = 3); (3) the ADHD symptoms led to significant functional impairment across domains; and (4) the onset of the ADHD symptoms was prior to 12 years of age. Although this last criterion is slightly less stringent than the DSM-IV requirement of symptom onset prior to age 7, it has been used by other studies of adult ADHD due to evidence that it may be more reliable and valid than the threshold specified in DSM-IV (Barkley & Biederman, 1997).
2.1.3 Exclusionary criteria
Potential participants were excluded from both groups if they reported a previous diagnosis of a Learning Disability or scored above a validated screening cutoff for reading disability on the Adult Reading History Questionnaire (Lefly & Pennington, 2000). Individuals with current psychiatric disorders, drug addiction, who were pregnant, left handed, had metal in their body that could not be removed (e.g., cardiac pacemaker), or had a previous history of seizures or a head injury (all self reported), were excluded.
The final groups included 31 participants with ADHD and 21 controls recruited from the same overall sample of participants but who did not meet current or lifetime criteria for any ADHD subtype based on parent or self-report. Initial comparisons of these groups support the validity of our diagnostic procedures. Of the 31 participants in the ADHD group, 28 had received a previous diagnosis of ADHD (mean age when diagnosed = 11.0 years), and 24 had received a prescription for stimulant medication.
2.2 Behavioral Performance
Variables All behavioral performance variable methodology can be found in supplementary materials S1.
2.3 MR Image Acquisition
T1 weighted 3D IR-SPGR anatomical images were also collected along the coronal plane (TR = 9 ms, TE = 2.0 ms, flip angle = 10°, inversion time = 500 ms; 220 mm FOV, 256 × 256 matrix, 0.87 × 0.87 mm2 in-plane resolution, 124 slices, 1.7-mm slice thickness).
2.4 VBM Analysis
2.4.1 Whole Brain Analyses
Structural data was analyzed with FSL-VBM, a voxel-based morphometry style analysis (Ashburner & Friston, 2000; Good et al., 2001) carried out with FSL tools (Smith et al., 2004). First, structural images were brain-extracted using BET (Smith, 2002). Next, tissue-type segmentation was carried out using FAST4 (Zhang, Brady & Smith, 2001). The resulting grey-matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT, followed optionally by nonlinear registration using FNIRT. The resulting images were averaged to create a study-specific template, to which the native grey matter images were then non-linearly re-registered using FNIRT. The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 2, yielding full width half max (FWHM) 2×2.3mm = 4.6mm FWHM. Finally, voxelwise GLM was applied using permutation-based non-parametric testing using Monte Carlo simulations, correcting for multiple voxel comparisons across space.
2.4.2 Correlational Analyses
Behavioral performance variables were taken from a neuropsychological battery conducted on all ADHD and control individuals. Three composite measures of 1) Processing Speed, 2) Response Inhibition, and 3) Response Variability were computed in the following manner. 1) Processing Speed was derived from the WAIS digit symbol and symbol search subtests. 2) Response Inhibition was derived from commission errors from both Conners’ continuous performance task (CPT) and the AX-CPT, as well as standard stop signal reaction time (SSRT) from the Stop Signal task. 3) Response Variability was derived from both the standard deviation of reaction time of total hits from the CPT and AX-CPT, as well as the standard deviation of go reaction time from the Stop-Signal paradigm. To detect group differences, scores for all variables were converted to Z scores computed across all participants. For examining individual differences as they related to brain morphometry within a group, scores were converted to Z scores computed only across the participants within the group. In both cases, the composite measure was computed by averaging across the Z values for the variables within that given composite measure. These Z values were then used as covariates which were entered into an FSL regression model predicting grey matter differences within groups.
2.4.3 ROI Based Analysis
For a more fine-grained analysis of potential group differences in brain morphology, we utilized ROI masks for brain regions that yielded significant associations between behavior and brain morphology determined from the whole brain analysis. These ROI masks, provided by the Montreal Neurological Institute (MNI), were applied to each group’s concatenated warped group image, applied to the study specific template and then were utilized to extract grey matter probability estimates of volume within two regions: the superior parietal lobule (voxel=1570), and the right inferior frontal gyrus (rIFG; voxel=3643). Furthermore, to examine morphometric characteristics of rIFG in more detail, three masks (MNI) differentiating the pars opercularis (voxel=824), pars triangularis (voxel=682) and the pars orbitalis (voxel=1247) regions within the rIFG were used. These individual masks provided probability estimates of grey matter with which behavioral performance variables could be correlated. The r values derived from these correlations were then compared between groups to assess significance via Fisher’s Z. Finally these masks were used to extract the overall grey matter volume within each region for each group separately.
3. Results
3.1 Behavioral Results
3.1.1 Group Classification
As would be expected based on the way the sample was defined, unpaired t-tests indicated that participants with ADHD exhibited significantly elevated symptoms of ADHD in childhood and as young adults (see Table 1). In contrast, the two groups did not differ significantly on measures of Full Scale, Verbal, or Performance IQ estimated from the Matrix Reasoning, Vocabulary subtests, reading or mathematics achievement. Overall, the analysis of our samples indicates they are well matched with regards to overall intellectual ability, although the ADHD group shows clear evidence of attentional dysfunction and significant functional impairment.
3.1.2 Behavioral Performance Variables
T-tests assuming unequal variance and N within each group were performed on the three composite variables to examine group differences. The composite score for processing speed yielded a significant group difference [t(1,36)=1.98, p=0.055], suggesting that ADHD individuals exhibit slower processing speed, as compared to controls. The composite score for response inhibition also yielded a significant group difference [t(1,36)=−2.17, p=0.036], suggesting that ADHD individuals exhibit increased commission errors and stop signal reaction time, which indicates a deficiency in response inhibition as compared to controls. The composite score for response variability yielded no significant group difference [t(1,36)=−.34, p=0.73]. Differences between group Z scores can be found in Figure 1.
Figure 1.
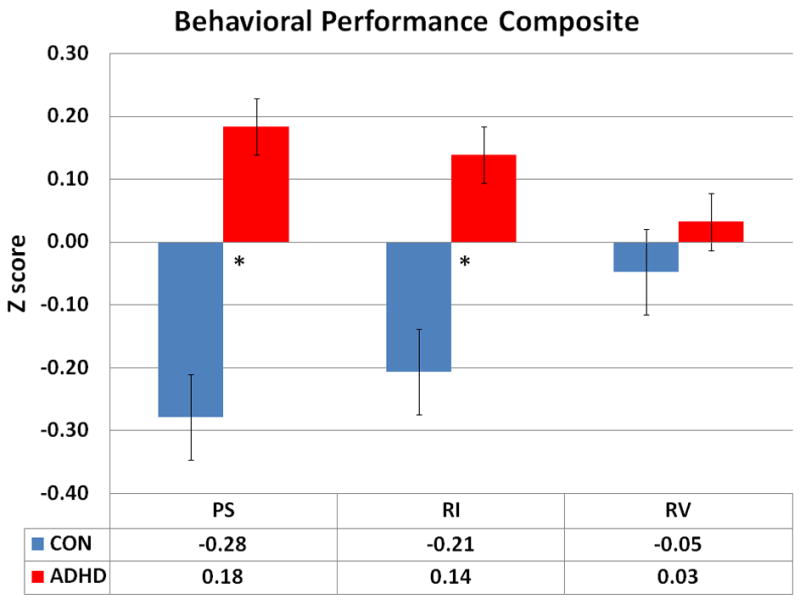
Z scores for all three behavioral performance composite measures by group: PS = Processing Speed, RI = Response Inhibition, RV = Response Variability. * indicates group level significance at p≥0.05 (df = 20, 30; Control, ADHD individuals, respectively).
3.2 Anatomical results
3.2.1 Whole Brain Analyses
We conducted a whole brain optimized VBM analysis to detect grey matter differences between ADHD and control individuals. We found no significant differences between the two groups in overall grey matter volume (p>0.05) nor any significant regional differences (p>0.05). Additionally, a whole brain analysis with overall grey matter volume as a covariate produced no significant group difference (p>.05).
3.2.2 VBM Correlated with Behavioral Measures
The next set of analyses examined whether the three composite measures: Processing Speed (PS), Response Inhibition (RI), and Response Variability (RV) are related to brain morphometry. Each group (ADHD, controls) was tested separately to identify significant correlations between grey matter volume and the composite measures in a whole-brain analysis, using total grey matter volume as a covariate. For the ADHD group, there was a significant positive relationship for PS, as slower PS predicted less grey matter volume in the anterior insula spreading anteriorly to the right inferior frontal gyrus (rIFG; Fig. 2a).
Figure 2.
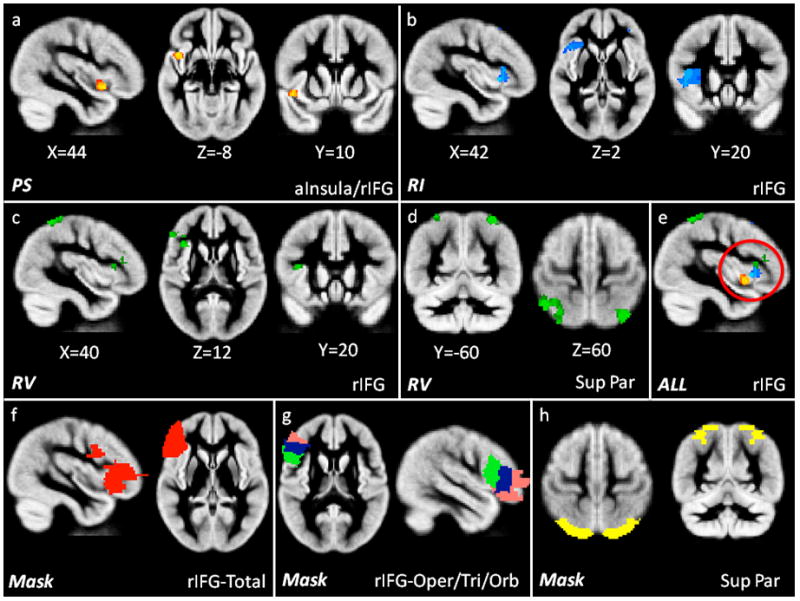
Significant results (p<0.05, df 30) of grey matter morphometry correlated with the composite measures of: 2a) PS (Processing Speed; yellow/red) in the anterior insula and rIFG, 2b) RI (Response Inhibition; blue) in the rIFG, 2c) RV (Response Variability, green) in the rIFG and 2d) superior parietal lobule and 2e) an overlay of all three significant findings, highlighting the localization within rIFG (red circle) in ADHD individuals. Figure 2f–h shows the masks used to extract grey matter volume in the 2f) rIFG-Total (red), 2g) rIFG-Opercularis (green), rIFG-Triangularis (blue), rIFG-Orbitalis (pink) and 2h) the superior parietal lobule (yellow). All images are shown in radiological convention (right on left side of image).
The composite measure of RI yielded a significant negative correlation, such that increases in commission errors and increased stop signal reaction time (SSRT) predicted significantly less grey matter volume in the rIFG (Fig. 2b). The composite measure of RV also yielded a significant negative correlation, indicating increased response variability predicted significantly less grey matter volume in the rIFG (Fig. 2c) and the superior parietal lobule (Fig. 2d). An overlay of all three results within rIFG to localize the anatomical relationship is show in Fig. 2e. Results for the control group produced no significance correlations in a similar whole brain analyses.
3.2.3 ROI Based Analysis on the Right Inferior Frontal Gyrus and Superior Parietal Lobule
Although a whole brain analysis produced no significant differences between ADHD and control individuals in grey matter volume, the finding of a significant correlation between behavioral measures and rIFG and superior parietal lobule grey matter volume within the ADHD group led us to perform a more fine-grained comparison of group differences in grey matter volume in these regions. Anatomical masks (provided by Montreal Neurological Institute) comprising regions that showed correlations in the ADHD group were used to extract the overall grey matter volume. The masks used, localized the rIFG region in general (Fig. 2f) and in an attempt to increase the sensitivity to detect localized differences within the rIFG, the pars opercularis, pars triangularis and pars orbitalis regions (Fig. 2g), as well as a mask for the superior parietal lobule (Fig. 2h). Total grey matter volume was extracted from each ROI and compared via t-tests assuming unequal N to assess any group differences within each ROI. This analysis revealed significantly decreased grey matter volume in ADHD as compared to control individuals in the total rIFG region [t(49)=2.91, p=0.005], the pars triangularis of the rIFG [t(49)=3.77, p=0.0005], and a trend towards significance for the pars opercularis and pars orbitalis of the rIFG [t(49)=1.69, p=0.10; t(49)=1.68, p=0.10, respectively]. The volume analysis of the superior parietal ROI yielded no significant group differences.
Overall grey matter volume in each of these regions was then correlated with the three composite behavioral measures within each group and Fisher’s Z-tests were used to assess the difference in magnitude of the correlations between the groups (df = 30 ADHD, 20 Controls, p<0.02 Bonferroni corrected). These results indicate that the ADHD showed a significantly greater correlation than the control group in the magnitude of the correlations of grey matter volume in rIFG and the three distinct regions within, for all three composite measures of behavioral performance (see Table 2).
Table 2.
Correlation coefficients of grey matter volume and behavioral performance composite measures
| rIFG-Total | rIFG-Oper | rIFG-Tri | rIFG-Orb | Sup Par | PS | RI | |
|---|---|---|---|---|---|---|---|
| rIFG-Oper | 0.42 | 1 | |||||
| 0.69 | 1 | ||||||
| rIFG-Tri | 0.79 | 0.24 | 1 | ||||
| 0.68 | 0.56 | 1 | |||||
| rIFG-Orb | 0.90 | 0.15 | 0.57 | 1 | |||
| 0.90 | 0.51 | 0.34 | 1 | ||||
| Sup Par | 0.59 | 0.35 | 0.52 | 0.65 | 1 | ||
| .69 | 0.55 | 0.59 | 0.57 | 1 | |||
| PS | *−0.40 | −0.28 | −0.21 | *−0.40 | −0.26 | 1 | |
| −0.01 | .02 | 0.05 | −0.02 | 0.12 | 1 | ||
| RI | −0.31 | *−0.50 | −0.35 | −0.36 | −0.19 | −0.03 | 1 |
| −0.08 | −0.03 | −0.04 | −0.09 | −0.01 | *−0.58 | 1 | |
| RV | −0.36 | −0.36 | *−0.40 | −0.30 | −0.36 | −0.09 | 0.54 |
| −0.04 | −0.06 | 0.02 | 0.11 | 0.07 | −0.37 | 0.33 |
rIFG-Total = total rIFG volume, rIFG-Oper = rIFG pars opercularis, rIFG Tri = rIFG pars triangularis, rIFG-Orb = rIFG pars orbitalis, Sup Par = superior parietal lobule; PS = processing speed, RI = response inhibition, RV = response variability.
Bold indicates significant correlations within group
indicates between group differences, Bonferroni corrected.
Red indicates ADHD individuals, green indicates control individuals.
4. Discussion
Our results provide evidence that three composite measures of behavioral performance are linked to brain morphometry in young adults with combined-type ADHD. This sample of young adults, who were carefully selected as exhibiting ADHD but not other psychiatric disorders and/or learning disabilities, exhibited poorer behavioral performance as indexed by slower Processing Speed (PS), reduced Response Inhibition (RI) and increased Responvbse Variability (RV). These different manifestations of behavioral performance correlated significantly with reduced grey matter volume in the rIFG and superior parietal lobule. In contrast, no such relationships were observed within the control group, and the difference in the magnitude of the correlations across groups proved significant. Although no differences were found in a whole-brain optimized VBM analysis comparing the ADHD and control group, a more focused analysis provided evidence of reduced grey matter in the ADHD group compared to controls for the the rIFG (in total), the pars triangularis and additional trends towards significance for the pars-opercularis and pars-orbitalis. These findings indicate a possible morphometric anomaly of the rIFG of young adults with ADHD.
While other studies examining brain morphometry in adults with ADHD have used whole brain and ROI approaches, the current study is one in a few examining how behavioral performance predicts unbiased estimates of grey matter differences between ADHD and control individuals. The current results suggest that morphometric differences in young adults with ADHD may indeed underlie and affect how these individuals perform on tasks that assess object based stimulus-response behavioral performance characteristics that have often been found effective in providing evidence for behavioral impairment in ADHD.
It is interesting to consider our results in reference to others who, using ROI parcellation approaches, examined anatomical differences in a somewhat older sample of adults (mid-30s) with ADHD (Seidman et al., 2006; Makris et al., 2007). Whereas we observed grey matter reductions in ADHD individuals specifically in the rIFG, they have tended to observe reduced grey matter in prefrontal, cingulate and lateral inferior parietal cortex. The fact that we did not observe grey matter reduction in these other regions may have occurred due to the highly selected nature of our sample. Our results are consistent with Seidman and colleagues (2006) in that we too found no significant group difference in the caudate or cerebellum, although morphometric differences were found in other regions. The lack of difference in caudate volume is consistent with the report of Castellanos and colleagues (2002) that these differences are observed in ADHD children but not adolescents, suggesting perhaps that the morphological manifestations of ADHD may change with age. The current results are consistent with others, who have found decreased grey matter volume in lateral prefrontal cortex. However, while our study implicated rIFG, others have found reductions in grey matter in DLPFC Monuteaux and colleagues (2008).
Our results are also consistent with the broader findings on individuals with ADHD, in which dysfunction of rIFG is frequently noted. Studies show both reductions in rIFG activation (Rubia et al., 2005; Smith et al., 2006) as well as, reduced volume of the inferior rPFC (Filipek et al., 1997; Semrud-Clikeman et al., 2000). The rIFG is thought to be crucial in response inhibition (Aron & Polldrack, 2006), as it sends important signals for interruption of motor commands via the basal ganglia and motor cortex. Not surprisingly, reduced response inhibition has been implicated and considered a hallmark of ADHD dysfunction since research began on these individuals (Barkley, 1997).
Although it is not surprising that our behavioral performance composite measure of RI indicated reduced grey matter volume within the rIFG, explanations as to why the other two composite measures of PS and RV also yielded a significant relationship are less clear. One possibility is that all three composite measures tap into the function of rIFG. Whereas, RI is linked to the rIFG through the execution of inhibiting motor actions, PS and RV are linked to object based stimulus detection and timing of responses. The rIFG is anatomically connected to the posterior cortex and more specifically the ventral visual pathway (Corbetta, 1998; Corbetta, Kincade & Shulman, 2002), which processes and represents object based stimuli. Clearly this processing and representation of object based stimuli is likely to influence both PS and RV. One caveat that is worth mentioning is that RI and RV were found to be highly correlated (r=.54) in ADHD individuals in the current study. This high correlation may result because both composite measures use different components of the same three tasks (CPT, AXCPT, Stop-signal; see methods section for full description), thus they likely share within subject variance. However, the two composite measures showed correlations with reduced grey matter volume in two different regions of the rIFG: RI correlated with grey matter volume in the pars opercularis, whereas RV correlated with grey matter volume in the pars triangularis. Although both regions have been implicated in the inhibition over motor responses (Aron & Polldrack, 2006; Chambers et al., 2007) it is unclear why the two different regions correlated more highly with different composite variables. Finally, perhaps our posterior to anterior dissociation of rIFG-opercularis -triangularis -orbitalis, indicates the relative strength of object based stimulus representation certain tasks elicit. Speculatively, RI and RV are possibly more tightly bound to concrete object based stimulus representation, while PS is tied to more abstract task goals. This idea follows the posterior-anterior gradient to abstraction of stimulus representation, that both dorsal and ventral regions of PFC exhibit (Badre & D’Esposito, 2009).
Although interesting, there are certain limitations of our study. First, our sample size is small compared to some other studies examining brain morphometry in children and adolescents with ADHD (Castellanos et al., 2002). Second, our sample is functioning at a relatively high level, successfully matriculating into a 4-year college degree program. Thus, it is unclear whether any null results (whole brain analysis) are due to: 1) low power, 2) a sample that is not as impaired as prior samples, 3) because of variability in the manifestation of ADHD in adulthood, or 4) changes in the aspects of brain morphology associated with ADHD in young adulthood as compared to childhood, adolescence or later periods of adulthood. Third, the majority of our sample was currently prescribed medication. Although the long term effects of stimulant medication on neural circuitry is largely unknown, it perhaps affects brain regions, in which, we found null results. Further research will have to address this issue. Lastly, our behavioral performance composite measures used to correlate with grey matter volume are highly biased towards measuring response-related aspects of processing. All three measures assessed object based stimulus-response aspects of performance. These aspects of the tasks may explain why we show grey matter reduction specific to the rIFG, which has been implicated in object based stimulus-response processing. It is possible that correlating behavioral performance in tasks that tap other cognitive processes, such as those that place high demands on working memory, would yield different results.
Yet some of these limitations are the exact reasons why we believe our approach is illuminating. First, very few studies have used an unbiased whole-brain study of brain morphometry in individuals with ADHD. Such bias-free and automated methods may be highly appropriate in situations, as is the case with ADHD, in which theory and existing empirical findings do not always mesh cleanly. Second, due to the likely multi-factorial influences leading to ADHD, we also performed correlational analyses to detect individual differences, in addition to examining group differences. Lastly, our ADHD sample was very carefully selected. To our knowledge, no other study has provided anatomical data from an ADHD population that has been so carefully characterized with regards to ADHD symptomology, screened to exclude cognitive or psychiatric comorbidities, and matched for general intelligence. Because of this careful selection, however, our sample will be, by necessity, relatively small. Moreover, because it is difficult or impossible to truly “control” for such variables statistically (Miller & Chapman, 2001), our selected sample allows for a more straightforward interpretation of the results. We are able to attribute any differences as being directly related to attention-deficit/hyperactivity disorder and not other psychiatric disorders or general intelligence.
In summary, our study suggests that the brains of high functioning young adults carefully screened to ensure that they exhibit the symptoms of ADHD and not other psychiatric disorders appear to exhibit less grey matter in the rIFG. Moreover, the amount of grey matter volume in this region predicted behavioral performance on three measures on which ADHD individuals usually show poorer performance than control individuals (which was also the case in our sample). Although our findings will need replication, they are highly suggestive of an underlying neural dysfunction in right inferior regions of prefrontal cortex in young adults with ADHD.
Supplementary Material
Acknowledgments
This research was supported by NIH grant R01MH070037 to the first author. LCB is supported by NIMH F31 MH 78514, EGW was supported by NIH grant R01 MH 63941 during the preparation of this manuscript. Appreciation is given to Bruce Pennington for insightful discussion of our results and also given to Deb Singel and Yiping Du for MR technical issues. We thank Kirsten Orcutt and Paula Villar for help in running the participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Aron AR, Poldrack RA. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Voxel-based morphometry - The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nature Reviews Neursocience. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin and Review. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1204–1210. doi: 10.1097/00004583-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy K. Attention-deficit hyperactivity disorder: A clinical workbook. 2. New York: Guilford Press; 1998. [Google Scholar]
- Biederman J, Makris N, Valera EM, Monuteaux JM, et al. Towards further understanding of the co-morbidity between attention deficit disorder and bipolar disorder: a MRI study of brain volumes. Psychological Medicine. 2007;38:1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences, USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, et al. Dissociable Mechanisms of cognitive control in the prefrontal and premotor cortex. Journal of Neurophysiology. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. What is the prevalence of adult attention deficit/hyperactivity disorder? Results of a population screen of 1019 adults. Journal of Attention Disorders. 2005;9:384–391. doi: 10.1177/1087054705281478. [DOI] [PubMed] [Google Scholar]
- Faraone S, Biederman J, Mick E. The age dependent decline of attention-deficit/hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingrad R, Kennedy D, Biederman J. Volumetric MRI analysis: Comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, et al. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Research. 2002;116:63–81. doi: 10.1016/s0925-4927(02)00066-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley RA, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2007;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefly DL, Pennington BF. Reliability and validity of the Adult Reading History Questionnaire. Journal of Learning Disabilities. 2000;33:286–296. doi: 10.1177/002221940003300306. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical Thinning of the Attention and Executive Function Networks in Adults with Attention-Deficit/Hyperactivity Disorder. Cerebral Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Monuteaux MC, Seidman LJ, Faraone SV, Makris N, et al. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. American Journal of Medical Genetics. 2008;147B:1436–1441. doi: 10.1002/ajmg.b.30870. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Copper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorders. Biol Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: Evidence for selective effects on ADHD symptom dimensions. Journal of Abnormal Psychology. 2005;114:706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, van Elst LT, Ebert D, et al. Hippocampus and amygdala morphology in adults with attention-deficit hyperactivity disorder. Journal of Psychiatric Medicine. 2008;33:509–515. [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Tonne B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;16:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Steingard RJ, Filipek PA, Biederman J, et al. Using MRI to examine brain-behavior relationships in males with attention deficit disorder with hyperactivity. Journal of American Child and Adolescent Psychiatry. 2000;39:477–484. doi: 10.1097/00004583-200004000-00017. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich MW, Beckmann CF, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-Specific Hypoactivation in Prefrontal and Temporoparietal Brain Regions During Motor Inhibition and Task Switching in Medication-Naive Children and Adolescents With Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-Analysis of Structural Imaging Findings in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. A meta-analytic review of the executive function theory of ADHD. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Bidwell LC, Hitt-Laustsen S, Banich MT. Internal and external validity of ADHD in young adults. Manuscript submitted for publication. [Google Scholar]
- Woodcock R, McGrew K, Mather N, Woodcock-Johnson . Itasca, IL: Riverside Publishing Company; 2001. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


