INTRODUCTION
With antiretroviral therapy (ART), there has been a significant decline in AIDS-associated morbidity and mortality [1–3]. However, between 5–45% of adults who start ART experience clinical deterioration as the immune system recovers [1,2,4]. This paradox is referred to as the Immune Reconstitution Inflammatory Syndrome (IRIS). Two distinct clinical scenarios of IRIS occur. ‘Paradoxical IRIS’ is the clinical recrudescence of a previous successfully treated opportunistic infection (OI). The symptoms of paradoxical IRIS can be quite similar to the initial infection potentially leading to clinical misclassification as an OI relapse and immunologic ART failure. The second scenario, ‘unmasking IRIS,’ is the immunologic unmasking of subclinical infections after ART initiation and is characterized by the rapid development of new OI’s with accelerated, atypical, or exaggerated symptoms. Distinguishing unmasking IRIS from clinical deterioration due to ongoing immunodeficiency is ill defined and controversial [5].
The incidence of IRIS is highly dependent on the population studied, specifically with the degree of immunosuppression and the OI burden [6]. Unlike adults, little is known about the magnitude of IRIS in children [7,8]. A prospective Thai study reported a pediatric IRIS incidence of 19% but was increasingly becoming common in children [8,9]. Whether these results are generalizable to Sub-Saharan Africa is unknown. In Africa, the majority of HIV-infected children present late to healthcare after they are severely immunosuppressed and infected with OI. These factors likely may increase the risk of IRIS [9]. In South Africa, nearly 10% of children die within 6 months of initiating ART, and in Uganda, 40% of children are hospitalized within the first 6 weeks of ART with 74% of the deaths occurring within 6 months of starting ART [12,13]. With the global ART roll out, there is a need for more information on IRIS in resource-limited settings. We sought to determine the prevalence, clinical epidemiology and risk factors associated with IRIS in HIV-infected children having newly initiated ART in Uganda.
METHODS
Setting and Design
The multi-center, prospective cross-sectional study was conducted between December 2006 and October 2007 at three Joint Clinical Research Centre (JCRC) clinics located in three regions in Uganda. JCRC had 52 satellite clinics and attached 25 outreaches in all regions of Uganda and provided ART to > 40,000 adults and children in 2008. Subjects were recruited consecutively from Kampala, Mbale, and Fort Portal sites located in the Central, Eastern and Western regions of Uganda respectively.
Study population
Consecutive patients aged <18 years presenting to the clinics were screened for enrollment. Inclusion criteria were children receiving ART between 2 weeks to 24 weeks and whose caregivers provided informed consent. Assent was sought for adolescents >14 years of age. Exclusion criteria were those with pre-existing liver or kidney insufficiency (>5x above normal) or ART non-adherence (<95% adherence by self report). The study was approved by the Makerere University Faculty of Medicine and Uganda National Council of Science and Technology.
Study procedures
A physician based at each site consecutively screened and enrolled patients. Enrolled patients were evaluated with a detailed history and physical examination documented using a standardized questionnaire. A history of contact with an adult with chronic cough or with tuberculosis was captured. Laboratory investigations included: 1) complete blood count (CBC), 2) Giemsa stained blood smear for malaria, 3) Liver and renal function tests, 4) T-cell profile, 5) HIV viral load, 6) C-reactive protein (CRP), 7) Erythrocyte sedimentation rate (ESR) and 8) Tuberculin skin test (TST). Past medical records of enrolled patients were reviewed. Data on previous diagnosis of OIs and baseline laboratory values were recorded. As this was a cross-sectional study, evaluation for IRIS events only occurred at time of the study visit.
IRIS case definition
A case of IRIS was defined as a clinical situation where a research participant fulfilled at least 1 major clinical criterion with a decrease of viral load of ≥11og10 or ≥1 major clinical criterion with ≥2 minor criteria according to an adopted IRIS diagnostic criteria from French [14]. In this pediatric population with a frequent inability to produce an expectorated sputum, the diagnosis of TB was primarily made on clinical history (cough >2wks, weight loss, anorexia, fever, contact with an adult with TB), physical examination, chest radiograph, and tuberculin-skin test(TST). All children then received anti-TB therapy, and per the IRIS clinical case definition were required to have a clinical response. Thus, while the strength of the TB diagnoses is considered at best as possible/probable TB, the clinical pattern of improvement with anti-TB therapy followed by clinical deterioration after ART initiation is very compatible with probable ART-associated TB.
Immunological and viral success: Immunological success was defined as an increase from pre-ART in CD4+ T-cell% ≥15% and viral success was achieving a HIV viral load of <50 copies/mL.
Data analysis
Data were entered using EPI-DATA 2.1b (CDC, Atlanta) and analyzed with SPSS 12.0 (SPSS, Chicago). Comparisons were made between cases with IRIS and controls without IRIS from clinical and laboratory data collected at the study visit. Univariate analysis was performed and categorical variables were summarized as frequencies and analyzed using the Chi-square test. Continuous variables were analyzed using means, median and standard deviation, student’s t-test and displayed using tables and figures.
Bivariate analysis with IRIS as the outcome to determine associations was done. Statistical significance was determined at two-sided P-value <.05. A multivariate analysis using forward stepwise logistic regression was used to control for confounders to determine adjusted odds ratio (OR). All potential correlates were entered into the model and the variables that did not retain significance (P<.05) were eliminated.
RESULTS
Sample characteristics
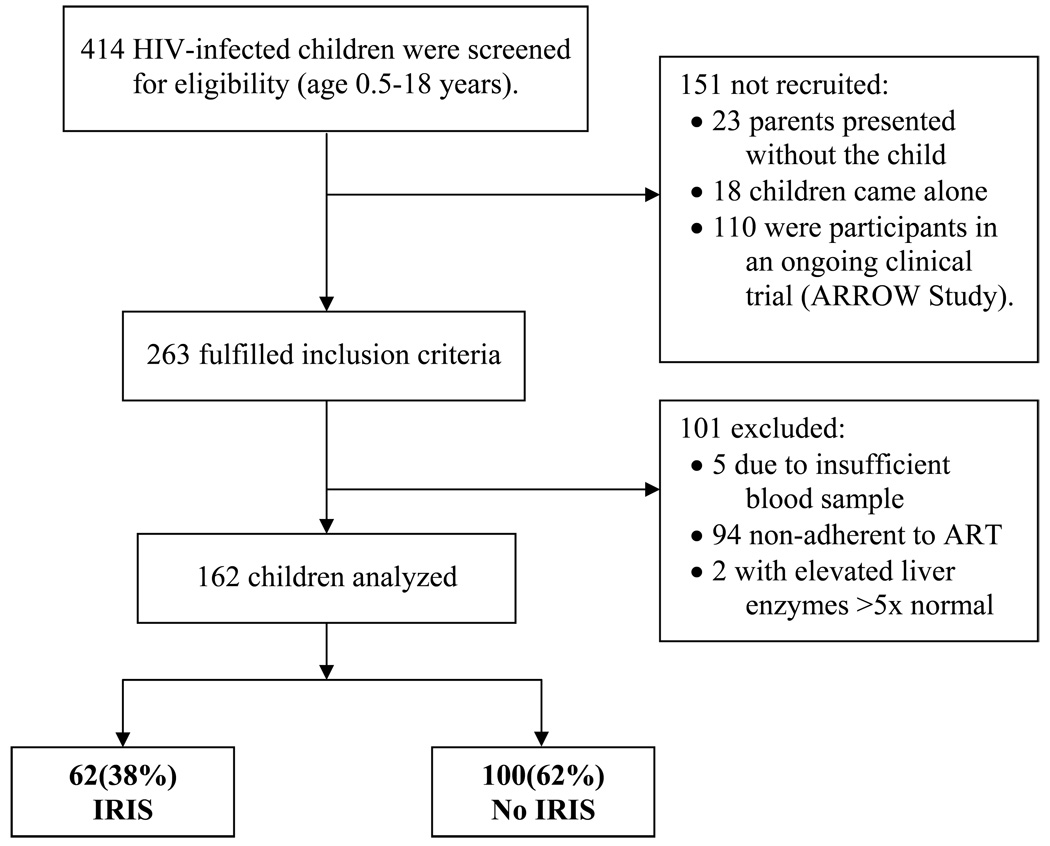
Of 414 children screened, 263 (64 %) fulfilled the inclusion criteria, of whom 162 (62%) were consented and included in the analysis (Figure 1). Of the 162 children studied, 57.4 % (93/162) were female. The age range was 0.5– 18 years with a median age of 6 years (IQR: 2.5–11 yrs). At initiation of ART, using the WHO classification system, 73% of enrolled children were stage III/IV (Table 1). At the time of ART initiation none of the study patients had clinically active OIs, and all patients were receiving trimethoprim-sulfamethoxazole prophylaxis. At time of the study interview, the median time on ART was 11.5 weeks (Interquartile Range: 3.5 to 20 weeks). No ART interruptions occurred according to self-report and clinic based pill counts.
Figure 1.
Study Profile
Table 1.
Demographics of the 162 Children
| Characteristics | Total N = 162 |
Immune Reconstitution Inflammatory Syndrome |
P-value | |
|---|---|---|---|---|
| Yes n = 62 |
No n = 100 |
|||
|
Sex Female Male |
93 (57.4) 69 (42.6) |
29 (31) 33 (48) |
64 (69) 36 (52) |
0.023 |
|
Age 6 – 12 months 13 – 59 months 5 – 12 years 12 – 18 years |
16 (9.9) 57 (35.2) 56 (34.6) 33 (20.4) |
3 (4.8) 21 (37) 26 (46) 12 (36) |
13(81) 36(63) 30(54) 21(64) |
0.24 |
|
Scheduled visit Yes No |
113 (69.8) 49 (30.2) |
40(35) 22(45) |
73(65) 27(55) |
0.17 |
|
Duration on ART 2 weeks – < 1 month 1 month – < 3 months 3 months – 6 months |
51 (31.7) 25 (14.9) 86 (53.4) |
28(55) 10(40) 24(28) |
23(45) 15(60) 62(72) |
0.007 |
|
Weight for age* < −2 SD below normal ≥ −2 SD below normal |
57 (37) 97 (63) |
21(37) 38(39) |
36(63) 59(61) |
0.46 |
|
†Type of ART NNRTI based Regimen PI based Regimen Triple NRTI Regimen |
147 (90.7) 9 (5.6) 6 (3.7) |
53(36) 6(67) 3 (50) |
94(64) 3(33) 3(50) |
0.16 |
|
CD4+%, Pre-ART* ≤ 15% > 15% |
112 (70.4) 47 (29.6) |
49(44) 12(25) |
63(56) 35(75) |
0.033 |
|
WHO Clinical stage, Pre-ART I / II III / IV |
44 (27.2) 118 (72.8) |
15(34) 47(40) |
29(66) 71(60) |
0.31 |
NNRTI = non-nucleoside reverse transcriptase inhibitors, PI = Protease Inhibitor, NRTI = Nucleoside reverse transcriptase inhibitors.
Six children were not assessed for nutritional status, and three were not assessed for the CD4+ levels
Prevalence of IRIS
The overall prevalence of IRIS was 38% at the study visit. The prevalence of IRIS in males was higher than in females 48% vs 31%; OR: 2.02; 95% CI: 1.06–3.86, P=.023. Unmasking IRIS events accounted for 77% of the episodes compared to paradoxical IRIS events (23%). The prevalence of IRIS was found to be highest in the 5–12 year age group with a median age of 6.75 years. The prevalence of IRIS was highest in the first month of ART, followed by the next 3 months period and least in the 3–6 months of ART.
Clinical Pattern of IRIS
The clinical presentation of patients with IRIS is shown in Table 4. TB was the most common IRIS presentation accounting for 25 (29%) of the cases with a prevalence of 15.4% (25/162). Of mycobacterial-IRIS, 20 (80%) had pulmonary TB, 2 had disseminated TB, and 3 had Bacille Calmette Guerin (BCG) lymphadenitis (BCG-IRIS). Fourteen subjects (8.6% prevalence) had no prior TB diagnosis and were considered ART-associated TB (i.e. unmasking TB-IRIS). Paradoxical TB-IRIS occurred in 11 children who had a prior TB diagnosis with an overall prevalence of 6.8%.
Table 4.
Clinical Patterns and Prevalence of IRIS related events in Children
| Clinical Scenario of IRIS |
Overall Prevalence N=162 |
Proportion of IRIS Events |
Unmasking IRIS Proportion N (%) |
Paradoxical IRIS Proportion N (%) |
|---|---|---|---|---|
| Tuberculosis | 25 (15%) | 25 (29%) | 14 (56%) | 11 (44%) |
| Pruritic Papular eruption | 7 (4.3%) | 7 (7.5%) | 6 (86%) | 1 (14%) |
| Candida | 7 (4.3%) | 7 (7.5%) | 4 (67%) | 2 (33%) |
| Bacterial pneumonia | 6 (3.7%) | 6 (6.5%) | 5 (83%) | 1 (17%) |
| Verruca planus | 4 (2.5%) | 4 (4.3%) | 4 (100%) | 0 (0%) |
| Tinea umbilicus | 4 (2.5%) | 4 (4.3%) | 3 (75%) | 1 (25%) |
| Otitis Media | 3 (1.8%) | 3 (3.2%) | 2 (67%) | 1 (33%) |
| Kaposi sarcoma | 3 (1.8%) | 3 (3.2%) | 2 (67%) | 1 (33%) |
| Molluscum contagiosum | 3 (1.8%) | 3 (3.2%) | 2 (67%) | 1 (33%) |
| Abscess | 2 (1.2%) | 2 (2.2%) | 1 (50%) | 1 (50%) |
| Encephalitis | 2 (1.2%) | 2 (2.2%) | 1 (50%) | 1 (50%) |
Additional events include one episode each of cryptococcal meningitis (unmasking), oral hairy leukoplakia, Pneumocystis jiroveci pneumonia, conjunctivitis, varicella zoster, generalized pustular lesions, and acute unmasking of H. influenza bacterial meningitis.
The other presentations were worsening or recurrence pruritic papular eruption and extensive oral candidiasis 7(7.5%), acute bacterial pneumonia 6 (6.5%), verruca planus 4(6.5%), and taenia skin manifestations 4 (4.3%) with extensive involvement of the face, hair and trunk and with patients having reported the flaring up of pre-existing scanty lesions.
Clinical Findings Pre-ART and at interview
The most common historical feature was previous OI treatment in the past 1 year, which was present in 61.7% (100/162), but this was not predictive of IRIS (OR=1.71, 95% CI: 0.87–3.34, P=.14). Pre-ART clinical findings which were associated with IRIS included: hepatomegaly (OR=1.36, 95% CI: 0.63–2.93, P=.033) or lymphadenopathy (OR=2.76, 95% CI: 1.39–5.46, P=.005) prior to ART initiation (Table 2). At the study visit, Clinical symptoms associated with IRIS included: having cough (OR=3.24, 95% CI: 1.63–6.47, P=.001) or skin lesions (OR=2.00 95% CI: 1.05–3.80, P=.025).
Table 2.
Clinical Signs and Symptoms
| Characteristics | Pre-ART Clinical findings | Clinical findings at Interview | ||||||
|---|---|---|---|---|---|---|---|---|
| IRIS N=62 |
No N=100 |
Odds ratio (95% CI) |
P-value | IRIS N=62 |
No N=100 |
Odds ratio (95% CI) |
P-value | |
| Fever | 56% (35) |
42% (42) |
1.79 (0.94–3.40) |
0.078 | 50% (31) |
36% (36) |
1.78 (0.93–3.39) |
0.056 |
| Cough | 58% (36) |
49% (49) |
1.44 (0.76–2.73) |
0.33 | 74% (46) |
47% (47) |
3.24 (1.63–6.47) |
0.001 |
| Opportunistic infection in previous 1 year |
69% (43) |
57% (57) |
1.71 (0.87–3.34) |
0.14 | 61% (38) |
52% (52) |
1.46 (0.77–2.78) |
0.16 |
| Lymphadenopathy | 45% (28) |
23% (23) |
2.76 (1.39–5.46) |
0.005 | 52% (32) |
41% 41) |
1.54 (0.81–2.91) |
0.12 |
| Oral lesions | 24% (15) |
19% (19) |
1.36 (0.63–2.93) |
0.43 |
21% (13) |
21% (21) |
1.00 (0.46–2.17) |
0.58 |
| Hepatomegaly | 53% (33) |
35% (35) |
2.11 (1.12–4.03) |
0.03 | 44% (27) |
48% 48) |
0.84 (0.44–1.58) |
0.63 |
| Splenomegaly | 32% (20) |
32% (32) |
1.01 (0.51–2.00) |
1.0 | 27% (17) |
38% 38) |
0.62 (0.31–1.23) |
0.18 |
| Skin lesions | 44% (27) |
43% (43) |
1.02 (054–1.94) |
1.0 | 58% (36) |
41% 41) |
2.00 (1.05–3.80) |
0.025 |
Data are % (n). Opportunistic infections were verified by pharmacy prescription records.
Pre-ART Laboratory Parameters
The majority (70%) of study participants were severely immunosuppressed with a CD4+% ≤15% and these children were more likely to develop IRIS (OR=1.00, 95% CI: 1.07–4.82, P=.033), when compared to those with a CD4+% ≥15% at ART initiation (Table 3). Study participants with ≤1000 CD8+ cells/µL were more likely to develop IRIS (OR= 3.60, 95% CI: 1.70–7.63, P<.001). One hundred and seventeen (74%) of the children were anemic at the time of ART initiation. Of these 90 (77%) developed IRIS. However, anemia at ART initiation (OR=1.39, 95% CI: 0.66–2.92, P= .46) or any other pre-ART hematological indexes were not predictors of development of IRIS in this study population.
Table 3.
Immunologic Associations with IRIS
| Characteristic | Pre-ART Laboratory Values | Current Laboratory Values at Study Visit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory Test | Total | IRIS Yes |
No |
Odds ratio (95% CI) |
P-Value | Total | IRIS Yes |
No |
Odds Ratio |
P-value |
|
CD4+ % < 15% ≥ 15% |
112(70.4) 47(29.6) |
49 12 |
63 35 |
Ref 2.27 (1.07–4.82) |
0.03 |
47(30.3) 108(69.7) |
16 44 |
31 64 |
Ref 1.33 (0.65–2.72) |
0.43 |
|
CD4+:CD8+ ratio < 0.25 ≥ 0.25 |
86(57.0) 65(43.0) |
39 20 |
47 45 |
Ref 1.76 (0.90–3.90) |
0.09 |
61(40.9) 88(59.1) |
32 36 |
38 52 |
Ref 0.87 (0.42–1.79) |
0.74 |
|
CD8+ < 1000 cells/µL ≥ 1000 cells/µL |
57(37.3) 96(62.7) |
30 30 |
27 66 |
2.44 (1.18–5.08) Ref |
0.011 |
54(36) 96(64) |
32 28 |
22 68 |
3.53 (1.71–7.50) Ref |
<0.001 |
|
CD8+ % < 75% ≥ 75% |
66(47.1) 74(52.9) |
29 29 |
37 45 |
1.22 (0.59–2.52) Ref |
0.61 |
81(56.2) 63(43.8) |
39 19 |
42 44 |
2.15 (1.02–4.58) Ref |
0.04 |
|
Viral load ≥ 280,000 < 280,000 copies/mL |
41(50.6) 40(49.4) |
24 20 |
17 20 |
Ref 1.41 (0.54–3.72) |
0.51 |
41(50) 41(50) |
26 20 |
15 21 |
Ref 1.82 (0.69–4.84) |
0.27 |
|
CD4+ Change <25 cells/µL increase ≥25 cells/µL increase |
40(25.8) 115(74.2) |
8 52 |
32 63 |
Ref 3.30 (1.40–7.78) |
0.005 | |||||
|
Viral load Change < 1 log10 decrease ≥ 1 log10 decrease |
23(44.2) 29(55.8) |
10 19 |
13 10 |
Ref 2.47 (0.80–7.61) |
0.16 | |||||
Laboratory and Immunologic Profile at Study Visit
At the study visit 55% (87/162) study subjects were anemic with a hemoglobin level <11.5g/dL. Thrombocytopenia was found in five subjects. Anemia (OR=2.17, 95% CI: 1.12–4.19, P=.023) at interview on ART was associated with IRIS on univariate but not multivariate analysis. All patients had a malaria blood smear performed with only 2% (3/162) having detectable parasitemia.
Children with an increase of ≥25 CD4+ T-cells/µL at time of the study visit from the pre-ART baseline were more likely to have IRIS (OR=3.30, 95% CI: 1.40–7.78, P=.005) (Table 3). HIV-1 viral loads were decreased from baseline with a median of 501 HIV RNA copies/mL (Range: <40 – 7,800,000 copies/mL) at the study visit. Of the 82 children who had viral loads measured only 4 achieved undetectable plasma viral suppression (<40 copies/mL) at a median time on ART of 11.5 weeks.
Risk Factors associated with IRIS
On multivariate analysis, the factors independently associated with IRIS were male sex, Pre-ART CD4+% of <15%, study visit CD8+ absolute count <1000/µL, and cough at study visit (Table 5).
Table 5.
Independent Factors associated with IRIS.
| Variable | Univariate Odds Ratio (95% CI) |
Multivariate Odds Ratio (95% CI) |
P-value |
|---|---|---|---|
| Male sex | 2.32 (1.06–3.86) | 2.96 (1.30–6.74) | 0.010 |
| Pre-ART CD4+% <15% | 2.27 (1.07–4.82) | 4.39 (1.62–11.08) | 0.004 |
| CD8+ <1000 cells/µL (current) | 3.53 (1.76–7.11) | 4.56 (2.01–10.34) | 0.001 |
| Cough (current) | 3.24 (1.62–6.47) | 4.30 (1.84–10.08) | 0.001 |
DISCUSSION
Despite numerous descriptions of the infectious and non infectious causes of IRIS, the overall incidence remains largely unknown, particularly in many resource-limited areas and pediatric populations. There is no consensus on case definitions for IRIS in children, yet we continue to recognize probable IRIS-like presentations. We report a cross-sectional prevalence of IRIS of 38% during the first 6 months of ART in Uganda. The majority of these events (77%) would be considered unmasking IRIS manifestations. The vast majority of these events were atypical in having exaggerated clinical presentations, though the contribution of ongoing immunosuppression cannot be discounted. The overall, very important implication is that pediatric HIV care consists much more than simply delivering antiretroviral medications to children and that in the first few months of ART, subclinical infections are frequently unmasked. This should be an expectation for patients, their caregivers, healthcare workers, and policy makers.
The ability of comparison of this cross-sectional study with other cohorts is limited, as the majority of studies have been retrospective in design and/or focused on adults, with a few exceptions [9]. The prevalence of IRIS in this Sub-Saharan African cohort appeared to be higher than the 23.4% IRIS prevalence in hospitalized Thai children [9]. Another retrospective pediatric cohort study reported an IRIS incidence of 20% among 91 children in Lima, Peru [16]. Retrospective design has some limitations with incomplete documentation leading to misclassification of such a relatively new entity as IRIS. Our study does match up similarly to the 41% incidence of probable and possible IRIS prospectively observed in a large adult cohort in Durban, South Africa [17].
The high prevalence of IRIS in this study population is possibly attributed to late presentation and a high background burden of subclinical infections. Diagnosis of HIV-infection in infants is complicated, and in resource-limited areas often delayed, due to lack of diagnostic resources and lack of parental awareness of their own HIV-status and thereby the infant’s risk. The result is continued late presentation of HIV-infected children seeking HIV care only after developing advanced immunosuppression and presenting with one or more OIs. This is reflected in this study population as the majority (70%) started ART with baseline CD4+% <15% indicative of severe immunosuppression and at risk of OIs. Second, in this resource-limited environment, children are frequently exposed to high background of infectious disease from OIs, environmental, and childhood vaccine preventable diseases. In the absence of comprehensive pre-ART screening, sub-clinical infections are likely frequently present when starting ART.
In this study, the peak time of IRIS occurrence was within the first month of commencing ART. In the Shelburne study most IRIS events presented in the first 60 days after ART initiation and Bakeera study the median time TB-IRIS occurred was at 14 weeks [9,10]. The probable reason why IRIS may occur earlier in children than adults is two-fold. First, children have a more robust improvement of their immune system with ART than adults, specifically children often achieve a 10-fold CD4+ T-cell increase, mainly of naïve T-cells [18]. In our study a rise of ≥25 CD4+ cells/µL from baseline carried a 3-fold risk of having an IRIS event, and the CD4 increase was most marked in the first month on ART when most IRIS events occurred. Our analysis did not adjust for time on ART. Thus, even though IRIS cases had a shorter duration of ART than controls without IRIS, the CD4 response was more robust in IRIS cases.
Demographic factors also were associated with IRIS for both clear and unclear reasons. Increasing age was associated with IRIS, peaking in children who were 5–12 years of age. Age correlated with the degree of immunosuppression, thus on multivariate analysis, age was not an independent risk factor. Increasing age also represents increasing time at risk of TB exposure. Older children also had easier clinical history taking making it easier to diagnose IRIS-related events. Sex was also associated with IRIS. In our study, the prevalence of IRIS in males was higher, and remained so in multivariate analysis. This is similar to Shelburne et al who reported a 2.65-fold risk in males for IRIS [9], yet differs from other prospective adult cohorts in Africa [19,20]. The biological plausibility of sex as a risk factor for IRIS in children is unknown.
IRIS can manifest with a wide variety of clinical symptoms, depending on the target of the inflammatory response thus the resulting clinical manifestation of IRIS depends on the prevailing infections in the locality. In our study multiple and varied presentations of IRIS occurred. There were 86 clinical episodes of IRIS characterized as 18 distinct clinical scenarios which occurred in 62 children. The clinical scenarios involved either unusual manifestations of a previously treated OI or unmasking of a previously subclinical infection [1,17,20–23].
Mycobacterial-IRIS
Considering that Uganda is a high TB burden country, it was not surprising that unmasking TB-IRIS was the most common single clinical scenario of IRIS. The proportion of TB-IRIS events (29%) was similar to the proportion of mycobacterial-related IRIS observed in Thailand (44%) and Peru (35%) [8,16]. In adult studies, paradoxical TB-IRIS manifestations primarily occur within the first 3 months of ART and have been reported as: fever, lymphadenitis, subcutaneous abscesses, pulmonary infiltrates, or inflammatory masses [23]. In our pediatric cohort, the most common symptoms of TB-IRIS were fever, cough, and lymphadenopathy. The 15% overall incidence of ART-associated TB in this study was similar to adult studies[11,24–26], and similar to the 17% incidence previously reported by Bakeera- Kitaka et al from Kampala, Uganda and higher than the 9% incidence reported in Chiang Mai, Thailand and 6.5% in Peru [9,10,16]. The higher prevalence of unmasking ART-associated TB in Uganda than in Thailand or Peru may reflect the increased burden of TB in Sub-Saharan Africa.
Our finding that cough for >7 days at the time of enrolment was associated with IRIS is not surprising since TB was the most common clinical infection. Uganda is in the top 15 TB burden countries globally with a high incidence of HIV-TB co-infection. Children are continually exposed to adults with TB, and those family members are also HIV-infected.
Of the 25 children with mycobacterial-IRIS, 3 (2.1%) had clinical BCG-IRIS with ipsilateral lymphadenopathy with ulceration or abscess formation at the site of the BCG scar at time of the study visit. This has also been described in a South African pediatric cohort of children <24 months with a higher 11.2% prevalence of BCG-IRIS [27]. In restricting our cohort to children <12 months, the prevalence of BCG-IRIS was 19% at time of the study visit. The overall incidence may have been higher.
Dermatologic IRIS
Little information exists on how ART affects the course of dermatologic conditions in children whereas in adults dermatological manifestations of IRIS appear to be the most frequent [17,20,28]. This study has demonstrated that dermatological manifestations were the most common IRIS events. Some of the types of dermatologic events have been reported in adults [7,19], such as paradoxical Kaposi sarcoma, molluscum contagiosum, and varicella zoster.
Other manifestations were considered as unmasking IRIS events due to their unusual, exaggerated presentation. For example, three otitis media episodes presented with exaggerated purulent discharge in conjunction with immunologic success and viral suppression.
This vast clinical manifestation of IRIS depends on the prevailing infections in the locality of HIV-care. Our study was interested in all manifestations of IRIS, thus the diverse presentations observed. In resource-limited areas where screening for OIs is primarily clinical, unmasking of subclinical OIs appears to be common.
Factors associated with IRIS
We did not find that the type of ART regimen, pre-ART Hgb level, CD4+: CD8+ ratio, previous OI, WHO stage III and IV, a shorter interval between initiating treatment for OI and starting ART, nor a rapid viral load decline to be associated with IRIS, as has been described in other adult cohorts [6,28].
The absolute CD8+ cells at interview <1000 cells/µL was associated with a 4-fold risk of IRIS development. In a U.S. study of 120 adolescents, decreased CD8+ was associated with a temporary clinical deterioration while on ART [29]. In our population having a low CD8+ T-count correlated with older age.
Limitations
There is no validated pediatric IRIS case definition to date, and this creates a challenge in diagnosing IRIS. This study used a prospective case definition in conjunction with expert opinion, similar to other recent studies [17,30]. Additionally, what is and is not “IRIS” remains controversial in the absence of a gold standard; however the adverse clinical events, whether labeled as IRIS or not, appear to be common in this multi-centered prospective study. We believe these results are generalizable to other Sub-Saharan pediatric populations presenting with advanced AIDS. The clinical implication is that pediatric HIV providers need to be aware of the frequent unmasking of subclinical and previously latent OIs within the first few months of initiating ART.
The design of the study was cross-sectional, thus IRIS events occurring prior to or after the study visit were not included. As such, the total incidence of IRIS cannot be estimated. A future prospective pediatric cohort study is needed in Sub-Saharan Africa to better inform pediatric HIV care. Extrapolating solely from adult cohort studies on the incidence, risk factors, and pathogenesis of IRIS is unwise and may lead to incorrect assumptions regarding pediatric HIV-infected populations.
Conclusions
First, the prevalence of pediatric unmasking IRIS in this study was high and is likely to continue for the foreseeable future in the resource-limited countries as long as HIV maternal-to-child-transmission (MTCT) continues unabated coupled with children presenting late for HIV-care with unknown, advanced immunosuppression. Second, there is need for rapid and early pediatric HIV diagnosis so as to facilitate early ART initiation in order to minimize the HIV-related morbidity and mortality [31], to which in part IRIS contributes. Third, there is need to reemphasis vigorous TB screening to minimize unmasking of ART-associated TB as this was the most common unmasked clinical infection. Fourth, providers should be aware of the frequent unmasking of subclinical infections in the first few months of ART and counsel caregivers. Finally, improved implementation of MTCT services is needed as a public health strategy to prevent IRIS through eradication of pediatric HIV.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the incalculable contribution of the statistician Mr Yusuf Mulumba and Dr Okello Ayen; departments of Pediatrics and JCRC Laboratory, with special thanks to: Drs. Hilda Kizito, Abbas Lugemwa, William Kizito and Francis Kiweewa; Department of Pediatrics and Child Health Makerere Medical School, with special thanks to Professor JK Tumwine, Dr Gerald Ojambo and Professor Martyn French without whose assistance this study would not have been possible.
Financial Disclosures: JCRC receives support from the President's Emergency Plan For AIDS Relief (PEPFAR). Dr. Boulware is supported by the National Institutes of Health, NAID K23AI073192-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicts of interest exist.
- Pediatric HIV conference in Uganda as an abstract November 2008
- Uganda Society of Health Scientists Conference as an oral presentation February 2009
- 1st Pediatric HIV Conference in Cape Town, South Africa as an Oral presentation July 2009
- 5th IAS Conference in Cape Town, South Africa as a poster presentation July 2009
Author Contributions: Dr. Orikiiriza has full access to all the data in the study and takes responsibility for the integrity and accuracy of the data and data analysis.
Study Concept and Design: Orikiiriza, Bakeera-Kitaka, Mugyenyi, Mworozi
Acquisition of Data: Orikiiriza, Musiime
Statistical Analysis: Orikiiriza
Interpretation of data: Orikiiriza, Bakeera-Kitaka, Boulware
Drafting the manuscript: Orikiiriza, Boulware
Critical revisions for intellectual content: Orikiiriza, Bakeera-Kitaka, Boulware
Obtaining funding: Mugyenyi
Administrative, technical, or material support: Bakeera-Kitaka, Mugyenyi, Mworozi,
REFERENCES
- 1.Hirsch HH, Kaufmann G, Sendi P, Battegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159–1166. doi: 10.1086/383034. [DOI] [PubMed] [Google Scholar]
- 2.Resino S, Resino R, Maria Bellón J, Micheloud D, Gutiérrez MD, de José MI, et al. Long-term effects of highly active antiretroviral therapy in pretreated vertically HIV type 1 Infected children: 6yrs of follow up. Clin Infect Dis. 2006;42:862–869. doi: 10.1086/500412. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Michelet C, Arvieux C, François C, Besnier JM, Rogez JP, Breux JP, et al. Opportunistic infections occurring during highly active antiretroviral treatment. AIDS. 1998;12:1815–1822. doi: 10.1097/00002030-199814000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS. 2008;3:461–467. doi: 10.1097/COH.0b013e3282fe9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune Reconstitution Syndrome (IRIS): review article of common infectious manifestations and treatment options. AIDS Res Therapy. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 8.Puthanakit T, Aurpibul L, Oberdorfer P, Akarathum N, Kanjananit S, Wannarit P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puthanakit T, Oberdorfer P, Akarathum N, Wannarit P, Sirisanthana T, Sirisanthana V. Immune reconstitution syndrome after highly active antiretroviral therapy in human immunodeficiency virus-infected Thai children. Pediatr Infect Dis J. 2006;25:53–58. doi: 10.1097/01.inf.0000195618.55453.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakeera-Kitaka S, Kekitiinwa A, Dhabangi A, Namulema E, Maganda A, Boulware DR. Tuberculosis Immune Reconstitution Inflammatory Syndrome among Ugandan Children. Int J of Inf Dis. 2008 [Google Scholar]
- 11.John L, Baalwa J, Kalimugogo P, Nabankema E, Castelnuovo B, Muhindo G, et al. Response to 'Does immune reconstitution promote active tuberculosis in patients receiving highly active antiretroviral therapy?'. AIDS. 2005;19:2049–2050. doi: 10.1097/01.aids.0000191922.08938.12. [DOI] [PubMed] [Google Scholar]
- 12.Reddi A, Leeper SC, Grobler AC, Geddes R, France KH, Dorse GL, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achan J, Ruel T, Kateera F, Kalyango J, Akello C, Gasasira A, et al. Immune reconstitution inflammatory syndrome in the first 6 months of antiretroviral therapy in HIV-infected Ugandan children. Abstract: MOPDB105. 17th Intl AIDS Conf; August 8, 2009; Mexico City, Mexico. [Google Scholar]
- 14.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 15.Goebel FD. Immune reconstitution inflammatory syndrome (IRIS)--another new disease entity following treatment initiation of HIV infection. Infection. 2005;33:43–45. doi: 10.1007/s15010-005-6105-x. [DOI] [PubMed] [Google Scholar]
- 16.Wang ME, Castillo ME, Montano SM, Zunt JR. Immune Reconstitution Inflammatory Syndrome in Human Immunodeficiency Virus-Infected Children in Peru. Pediatr Infect Dis J. 2009;28:900–903. doi: 10.1097/INF.0b013e3181a4b7fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddow LJ, Easterbrook PJ, Mosam A, Khanyile NG, Parboosing R, Moodley P, Moosa MY. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49:1424–1432. doi: 10.1086/630208. [DOI] [PubMed] [Google Scholar]
- 18.Sharland M, Watkins AM, Dalgleish AG, Cammack N, Westby M. Immune reconstitution in HAART-treated children with AIDS. highly active anti-retroviral therapy. Lancet. 1998;352:577–578. doi: 10.1016/s0140-6736(05)79289-8. [DOI] [PubMed] [Google Scholar]
- 19.Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald AR, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 21.Kumarasamy N, Chaguturu S, Mayer KH, Solomon S, Yepthomi HT, Balakrishnan P, et al. Incidence of immune reconstitution syndrome in HIV/TB-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–1576. doi: 10.1097/00126334-200412150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immune restitution Disease Involving the Innate and Adaptive response. Clin Infect Dis. 2000;30:882–892. doi: 10.1086/313809. [DOI] [PubMed] [Google Scholar]
- 23.Stone SF, Price P, French MA. Immune restoration disease: a consequence of dysregulated immune responses after HAART. Curr HIV Res. 2004;2:235–242. doi: 10.2174/1570162043351345. [DOI] [PubMed] [Google Scholar]
- 24.Manosuthi W, Van Tieu H, Mankatitham W, Lueangniyomkul A, Ananworanich J, Avihingsanon A, et al. Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2009;23:2467–2471. doi: 10.1097/QAD.0b013e32832f7b59. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 26.Baalwa J, Mayanja-Kizza H, Kamya MR, John L, Kambugu A, Colebunders R. Worsening and unmasking of tuberculosis in HIV-1 infected patients after initiating highly active antiretroviral therapy in Uganda. Afr Health Sci. 2008;8:190–195. [PMC free article] [PubMed] [Google Scholar]
- 27.Smith K, Kuhn L, Coovadia A, Meyers T, Hu CC, Reitz C, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 29.Flynn PM, Rudy BJ, Douglas SD, Lathey J, Spector SA, Martinez J, et al. Virological and immunological outcomes after 24 weeks in HIV type-1 infected adolescents receiving HAART. J Infect Dis. 2004;190:271–279. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 30.Robertson J, Meier M, Wall J, Ying J, Fichtenbaum CJ. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis. 2006;42:1639–1646. doi: 10.1086/503903. [DOI] [PubMed] [Google Scholar]
- 31.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]