Abstract
Prefrontal cortical (PFC) working memory functions depend on pyramidal cell networks that interconnect on dendritic spines. Recent research has revealed that the strength of PFC network connections can be rapidly and reversibly increased or decreased by molecular signaling events within slender, elongated spines, a process we term Dynamic Network Connectivity (DNC). This newly discovered form of neuroplasticity provides great flexibility in mental state, but also confers vulnerability and limits mental capacity. A remarkable number of genetic and/or environmental insults to DNC signaling cascades are associated with cognitive disorders such as schizophrenia and age-related cognitive decline. These insults may dysregulate network connections and erode higher cognitive abilities, leading to symptoms such as forgetfulness, susceptibility to interference, and disorganized thought and behavior.
Vulnerabilities in prefrontal cortical networks
The cognitive operations of the prefrontal cortex (PFC) are especially vulnerable to physiological, genetic and environmental factors; they are altered by changes in arousal state such as fatigue or stress [1], they decline early in the aging process [2], and are profoundly impaired in most mental illnesses [3–5]. PFC cognitive functions rely on networks of interconnecting pyramidal cells [6]. New research is revealing that PFC network connections are influenced by powerful molecular events that determine whether a network is connected or disconnected at a given moment, thus determining the strength of cognitive abilities [7, 8]. These mechanisms provide great flexibility, but also confer vulnerabilities and limit mental capacity. A remarkable number of genetic and/or environmental insults to these molecular signaling cascades are associated with cognitive disorders such as schizophrenia and age-related cognitive decline. These insults may dysregulate network connections and weaken higher cognitive abilities. In this article, we describe some of the molecular events that can rapidly alter PFC network strength, a process we call Dynamic Network Connectivity (DNC). We propose that DNC may be considered a novel form of very rapid plasticity that coordinates momentary changes in the state of arousal with appropriate cognitive network operations.
PFC networks subserving representational knowledge
The PFC is able to represent information that is not currently in the environment through networks of pyramidal neurons that excite each other to maintain information “in mind”. This process has been referred to as representational knowledge, working memory, or our “mental sketch-pad”, and is thought to be a fundamental component of abstract thought [6]. Information such as a rule or goal is held temporarily in working memory and used to guide behavior, attention or emotions, dependent on the PFC region(s) involved. The circuitry underlying representational knowledge in PFC has been most intensively studied in the visuo-spatial realm. In primates, visuo-spatial information is processed by the parietal association cortices, and fed forward to the dorsolateral PFC, where pyramidal cells excite each other to maintain information briefly in memory (see Box 1 for details). Network activity is “tuned” by inhibitory GABAergic interneurons so that the contents of working memory are specific and informative (Box 1). Pyramidal cell networks interconnect on dendritic spines (Fig. 1A), exciting each other via postsynaptic NMDA receptors that pass both sodium (Na+) and calcium (Ca2+) (Figs. 1C-D, 2A). NMDA currents are particularly evident in PFC during network interactions (Fig 2C, [9]), and seem to be necessary for delay-related firing in monkeys performing a working memory task (Wang, Yang, Gamo and Arnsten, unpublished).
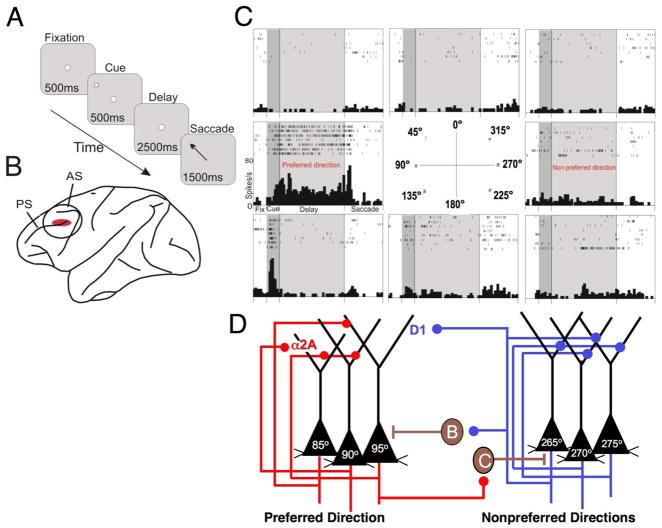
Box 1. PFC microcircuitry underlying spatial working memory.
The PFC microcircuitry subserving spatial working memory was discovered by Patricia Goldman-Rakic and her colleagues [5] using anatomical tracing techniques and physiological recordings from monkeys performing an oculomotor spatial working memory task (Fig. A). The dorsolateral PFC in the principal sulcus is key for spatial working memory (Fig. B), and many neurons in this region exhibit spatially tuned, persistent firing during the delay period in a spatial working memory task (Fig. C). Goldman-Rakic posited that the delay-related firing arises from pyramidal cells with similar spatial characteristics (e.g. 90°) exciting each other to maintain information in working memory (Fig. D). The microcircuits interconnect on dendritic spines, although it is still not known if these occur on the apical and/or basal dendrites; the connections on the apical dendrites in Figure D have been positioned for artistic clarity. These neurons likely reside in deep layer III, which contains the extensive horizontal connections that are characteristic of recurrent connections [5]. Network activity is “tuned” by GABAergic interneurons to provide lateral inhibition, e.g. when 90° pyramidal cells are active they excite GABAergic interneurons that suppress the firing of 270° pyramidal cells, and vice versa (Fig. D). These interneurons are basket cells or chandelier cells, the GABAergic neurons altered in schizophrenia (see text). Thus, GABA inputs are needed to sculpt the network activity so that there is firing to a “preferred” direction, but not to other “nonpreferred” directions. For the sake of simplicity, Figures 2–5 show only one nonpreferred direction, as well as the preferred direction, for each neuron. Optimal working memory occurs when there are high rates of firing throughout the delay period for the preferred direction, but not during the delay period for the nonpreferred directions. PFC networks must maintain firing over the entire delay period (often in the face of distractions) for information to be effective in guiding behavior.
Box 1 Figure 1.
The neural circuitry underlying spatial working memory task as envisioned by Goldman-Rakic and colleagues [6]. A. The oculomotor delayed response (ODR) task, a test of spatial working memory. B. The region of the monkey dorsolateral PFC dedicated to spatial working memory. PS=principal sulcus; AS=arcuate sulcus. C. A representative neuron in PFC with spatially tuned firing during the delay period of the ODR task. For details, see [7]. D. The PFC microcircuits subserving spatially tuned firing during the delay period in a spatial working memory task.
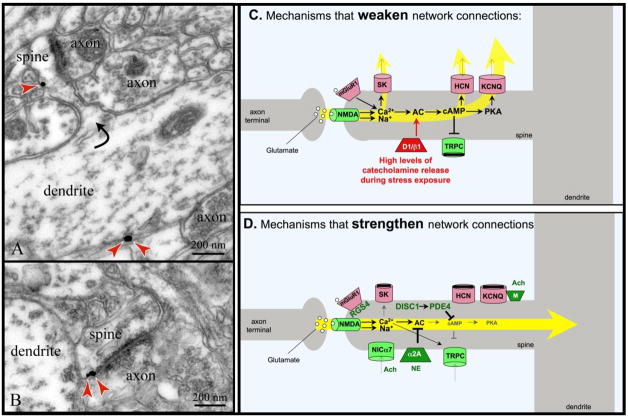
Figure 1.
A working model of DNC mechanisms. DNC signaling proteins are often found in long, thin spines with narrow spine necks in the superficial layers of monkey dorsolateral PFC. A. Immunoelectron micrograph of HCN channels (red arrows) on a spine neck across from an asymmetric (presumed glutamatergic) synapse, and on the parent dendrite. HCN channels in spines appear to gate synaptic inputs onto the spines, while those on dendrites may regulate excitability (adapted from [7]). B. Immunoelectron micrograph of HCN channels (red arrows) on a spine head next to the post-synaptic density of an asymmetric (presumed glutamatergic) synapse (adapted from [7]). C. Working model of molecular mechanisms that weaken PFC network connectivity. D. Working model of molecular mechanisms that strengthen network connectivity. For both C and D, molecules that strengthen connectivity are shown in green; those that weaken connectivity are shown in red.
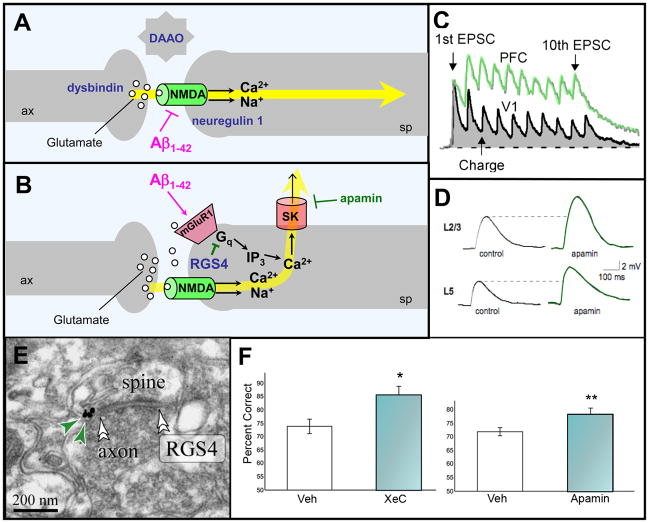
Figure 2.
A working model of glutamate actions at network synapses in monkey dorsolateral PFC. A. Stimulation of NMDA receptors in the synapse mediates network inputs. B. In contrast, stimulation of perisynaptic mGluR1/5 provides negative feedback via Gq signaling. For both A and B, examples of genetic insults in schizophrenia are shown in navy blue; dysbindin regulates glutamate release; DAAO (D-amino acid oxidase) regulates levels of the NMDA modulator, D-serine, while neuregulin 1 regulates NMDA receptor structure and function at the synapse [79]. The actions of soluble Aβ in AD are shown in magenta; Aβ internalizes NMDA receptors in the presence of α7 nicotinic receptors, and stimulates mGluR1. C. Layer V pyramidal cell connections in rat PFC show larger NMDA currents than those in primary visual cortex (V1) in response to a sequence of 10 stimulating pulses and a recovery pulse. Figure courtesy of W.J. Gao with the permission of PNAS USA [9]. D. The NMDA current in rat PFC is increased in the presence of apamin, a selective blocker of SK channels. Figure courtesy of E.S. Faber [16]. E. RGS4 in PFC spines is typically found perisynaptically (double arrowheads point to the synapse) in monkey PFC, where it is strategically positioned to inhibit mGluR1/5-Gq signaling (adapted from [19]). RGS4 is shown in navy blue in B to highlight its reduced expression in PFC in patients with schizophrenia. RGS4 levels are also reduced in AD. F. Blockade of SK channels in rat PFC with apamin, or of IP3-induced Ca2+ release using xestospongin C (XeC), improves working memory performance compared to vehicle (veh) control infusion. Results represent mean ± SEM percent correct. Adapted from [20].
Dynamic Network Connectivity- A new form of rapid plasticity
Recent research has revealed that the physiological strength of PFC network connections can be dramatically altered in a dynamic manner. Molecular signaling events in the spines of PFC pyramidal dendrites can open or close ion channels near synaptic connections to rapidly and reversibly alter the strength of network inputs [7]. For example, Ca2+ entry into the spine appears to play a key role in providing negative feedback, directly or indirectly (via the production of cAMP), opening ion channels that shunt network connections (Fig. 1C). This process can occur in a precise subset of spines to sculpt network inputs to a neuron, or more globally, e.g. to rapidly collapse network activity in response to an acute stressor. In contrast, inhibition of cAMP can close these ion channels [7], and open other depolarizing channels, e.g. canonical transient receptor potential (TRPC) channels [10], to strengthen network connections (Fig. 1D). These actions can occur rapidly, in the timeframe of seconds, and thus allow great flexibility in cognitive ability. Electron microscopy suggests that the spines that mediate DNC have a distinct ultrastructure: they are long, pedunculated spines with a narrow spine neck (Fig. 1; and unpublished data, C. Paspalas). Computational models suggest that narrow width and long length are important properties for effective shunting of network inputs (J.J. Pereira and X.-J. Wang, personal communication). These characteristics contrast with plasticity mediating long-term changes in synapse strength (e.g. LTP), where sessile spines enlarge to become mushroom-type spines, thus preserving new memories through stable architectural changes in neural connections [11]. We propose that under normal conditions the architecture underlying DNC would remain unchanged, but the physiological strength of the connections could rapidly change to accommodate for the state of arousal and cognitive or physiological demands.
The ability to rapidly vary the strength of PFC network connections would provide a number of important advantages: 1) An inherent mechanism to weaken network connections could prevent over-excitability, a particular danger in recurrent excitatory connections. As described below, there appear to be negative feedback mechanisms which may prevent seizures in PFC microcircuits; 2) Recurrent PFC network activity is very energy-intensive, as demonstrated by 2-deoxyglucose studies in monkeys [12], and fluorodeoxyglucose-PET imaging studies in humans [13]. DNC mechanisms that weaken network connections could conserve energy during states of fatigue when energy is scarce; 3) Conversely, during rested waking when energy is available and PFC cognition is needed, DNC mechanisms that strengthen network connections can promote PFC cognitive abilities. Precise DNC regulation appears to allow for flexible network connections, where the breadth of inputs can be rapidly altered according to cognitive demands; 4) DNC mechanisms can rapidly take PFC “off-line”, in response to danger, to switch control of behavior to more primitive brain regions that mediate instinctive reactions. For example, high levels of catecholamine release during stress exposure drives the production of cAMP, which disconnects PFC networks but strengthens the amygdala and related structures [1]. Thus, DNC is particularly important for coordinating higher cortical connectivity with the state of arousal.
Although there are many advantages to rapid changes in cortical connectivity, the same mechanisms may confer vulnerability to cognitive dysfunction. We hypothesize that the negative feedback that prevents seizures- e.g. the rise in cAMP which in turn opens potassium channels- also limits working memory storage [7], and may explain why hippocampal connections are needed to store memories over delays greater than 10–30 sec [14]. The precise regulation of DNC also appears to erode with advancing age or with genetic insults (see below). In particular, the purposeful disconnection of networks during stress exposure likely contributes to a number of stress-induced psychiatric disorders, and may explain why so many cognitive disorders are worsened by exposure to stress [1]. In the following section we put forward a working model of DNC mechanisms.
A working model of Dynamic Network Connectivity
A summary of a working model of DNC mechanisms in dorsolateral monkey PFC is presented in Figure 1. The model illustrates the emerging picture of how molecular signaling mechanisms in dendritic spines weaken or strengthen network connections. In this article, we focus where possible on the superficial layers of dorsolateral PFC in monkeys, as this region has been most intensively studied, and is known to be important for delay-related network firing during spatial working memory. There is accumulating evidence that cAMP alters PFC network strength through the regulation of potassium channels, while more preliminary data point to other signaling pathways that may contribute as well.
It is important to note that DNC modulatory influences may not be visible unless networks are actively engaged in a cognitive operation. Thus, molecular influences on excitability in vitro may not always be relevant to DNC actions. There are also likely differences in DNC mechanisms between species, between PFC subregions (e.g. greater serotoninergic influences in orbital PFC [15]), and even between PFC laminae (although a number of these mechanisms have been observed in rodent medial PFC as well, indicating some degree of generalization). Thus, this early working model seeks to illustrate examples of mechanisms that alter network strength, with the understanding that the details governing operational-, regional- and species-specific mechanisms will be the subject of future research.
Pathways that weaken network connections
A variety of intracellular mechanisms can weaken PFC network connections (Fig. 1C). For example, negative feedback arises from Ca2+ entry through NMDA receptor channels, which in turn opens small conductance Ca2+-activated K+ (SK) potassium channels, thus reducing excitability (Figs. 2B, D [16]). Negative feedback may also involve glutamate “spillover”, engaging perisynaptic metabotropic receptors (mGluR1/5), which have been localized on spines in monkey PFC (Fig 2B; [17]). mGluR5 stimulation can modulate excitability in CA1 hippocampal neurons via SK channels [18]. Our preliminary data indicate that stimulation of mGluR1/5 in monkey PFC reduces PFC network firing (Wang, Yang, Gamo and Arnsten, unpublished data), consistent with a negative feedback mechanism. The second messenger basis for this reduction is currently being examined, but likely involves Gq-protein signaling. Gq signaling is inhibited by RGS4 (regulator of G-protein signaling 4), which is also found next to axospinous synapses (Fig 2E; [19]). Gq signaling initiates IP3-mediated intracellular Ca2+ release, which in turn can open SK channels to suppress PFC firing (Fig. 2B; [10] and impair working memory (Fig 2F; [20]). Calcium can also activate Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC), both of which impair PFC function [21, 22]. PKC signaling is increased during stress exposure [22], and has been related to loss of dendritic spines (see below).
Calcium might also weaken PFC network connections indirectly, through the activation of adenyl cyclases and the generation of cAMP (Fig. 3A; [23]). Cyclic AMP opens HCN (hyperpolarization-activated cyclic nucleotide-gated) channels and KCNQ (voltage-gated K+; Kv7) channels, the latter through activation of protein kinase A (PKA) (Fig. 3A [24]). Increased cAMP in PFC markedly reduces network activity during the delay period and impairs working memory performance [7], Importantly, the very same treatments in PFC can strengthen longer-term memory consolidation [25]. For example, either the phosphodiesterase 4 (PDE4) inhibitor, etazolate, or the PDE4 resistant cAMP analog, Sp-cAMPS, cause rapid network collapse, and network activity is rescued by blocking HCN or KCNQ channels (Fig. 3C [7, 26], and Wang, Yang, Gamo and Arnsten, unpublished data). Cyclic AMP may also weaken connectivity by closing TRPC channels [10, 27].
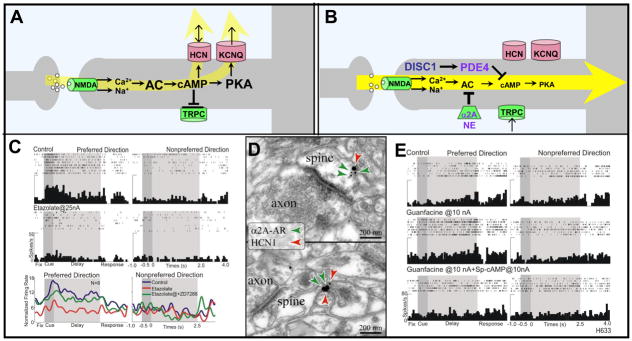
Figure 3.
cAMP signaling weakens PFC network connectivity. A. A working model of cAMP signaling mechanisms that weaken network connectivity. cAMP directly increases the open probability of HCN channels, while indirectly affecting KCNQ2/3 channels via PKA. High levels of cAMP also can reduce the depolarizing TRPC current. B. A working model showing mechanisms that inhibit cAMP signaling and strengthen network connections. NE stimulation of α2A-ARs inhibits cAMP production, while DISC1 activates PDE4s to catabolize cAMP. DISC1 is shown in navy blue to highlight its genetic perturbation in some families with schizophrenia. PDE4, NE and α2A-ARs are shown in purple to emphasize their decline with normal aging. C. High levels of cAMP arising from PDE4 inhibition markedly reduce PFC network firing, which can be reversed by blocking HCN channels with ZD7288 (shown) or blocking KCNQ2/3 channels with XE991 or linopirdine (not shown). Reduced PFC network firing also has been seen following increased cAMP signaling with Sp-cAMPS or yohimbine (not shown). Adapted from [7]. D. Dual immunoelectron microscopy showing α2A-AR/HCN1 channel colocalization in the spine head (top) and the spine neck (bottom) in the superficial layers of the monkey dorsolateral PFC. Adapted from [7]. E. Iontophoretic application of the α2A-AR agonist, guanfacine, increases PFC network firing for the preferred direction, while co-application of the cAMP analog, Sp-cAMPS, reverses this effect. Adapted from [7].
Relevance to epilepsy
Certain genetic insults to DNC signaling pathways can lead to over-excitability and seizures. Genetic reductions in HCN channel expression in animals lower seizure threshold in cortex [28, 29], although HCN channels in hippocampus may promote excitability in epilepsy models e.g. [30]. Importantly, a mutation that prevents PKA from opening KCNQ2/3 channels is associated with childhood epilepsy[31], demonstrating the importance of negative feedback on network excitability.
The key role of DNC during stress
Exposure to even mild, acute stress rapidly impairs PFC working memory function when the subject feels out of control [1]. Stress exposure commandeers DNC pathways that weaken connectivity by markedly increasing cAMP production [1]. While moderate release of dopamine (DA) is helpful to working memory (see below), high levels of release during stress suppresses all PFC firing and impairs working memory via increased DA D1 receptor (D1R) activation of cAMP signaling [8]. Stress-induced impairment also involves excessive norepinephrine (NE) stimulation of α1 adrenergic receptors, which activate Ca2+/PKC signaling [22], and possibly β1 receptors, which increase cAMP signaling [32]. Catecholamine actions appear to be augmented by cortisol [33] that blocks catecholamine reuptake [34]. In contrast, high levels of NE, DA and glucocorticoids enhance amygdala function [33]. In this way, control of behavior can be rapidly and reversibly switched from slow, thoughtful PFC regulation to more reflexive amygdala responses in the presence of an acute danger.
Chronic stress exposure additionally induces architectural changes that may arise from sustained weakening of DNC synapses: dendrites and spines retract in PFC [35], and spine loss can be prevented by inhibiting PKC or cAMP signaling [36]. Dendritic atrophy also occurs in hippocampus with very prolonged stress, while dendrites actually elongate with stress exposure in amygdala [37]. Weakened PFC connectivity has even been seen in humans undergoing a sustained stressor [38]. These architectural changes in animals and humans reverse once the stressor is removed [38]. Importantly, spine loss in PFC is a hallmark of aging [39] and schizophrenia [40] (see below).
Pathways that strengthen network connections
A separate set of DNC signaling pathways strengthen PFC connectivity (Fig. 1D). Under optimal arousal conditions, catecholamines and acetylcholine (ACh) are released in response to interesting, relevant events in the environment (reviewed in [15]). Indeed, the PFC regulates the firing patterns of subcortical NE, DA and ACh cell groups, which in turn determine PFC functional state, thus providing the potential for “vicious vs. delicious cycles” in their regulation; please note that other arousal systems are likely involved as well, e.g. serotonin and the orexins, but these are less understood. The release of optimal levels of NE, DA and ACh strengthens and sculpts network firing through regulation of cAMP signaling, and through direct depolarizing actions on spines.
Optimal levels of NE release in PFC inhibit cAMP production through stimulation of α2A-adrenergic receptors (α2A-ARs) (Fig. 3B; [7]). Alpha2A-ARs have been co-localized with HCN channels in spines near synapses and in the spine neck (Fig. 3D; [7]). Stimulation of these receptors enhances network firing for the preferred direction of the neuron (i.e. increasing “signal”) (Fig. 3E), and improves working memory, especially under distracting conditions. In contrast, α2A-AR blockade suppresses cell firing [7, 41], and impairs working memory and impulse control [42, 43]. The α2A-AR agonist, guanfacine (Intuniv™), is now in use to treat symptoms of poor impulse control and distractibility in patients with Attention Deficit Hyperactivity Disorder (ADHD) and related disorders [44].
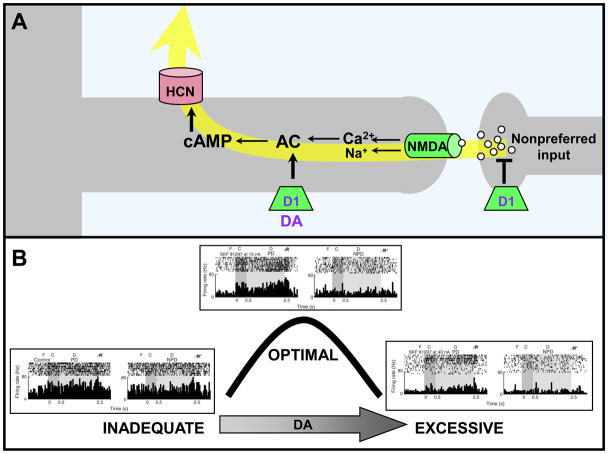
Dopamine D1Rs appear to be on a separate subset of spines from α2A-ARs [45], and moderate stimulation of D1Rs sculpts network inputs by suppressing firing to nonpreferred directions (i.e. reducing “noise”) (Fig. 4; [8]). This process is similar to the tuning induced by GABA (Box 1), but can be adjusted based on the state of arousal. D1R-mediated synaptic shunting is beneficial for cognitive operations requiring a narrow range of network inputs (e.g. spatial working memory for a small location in space), but is harmful if a broader range of inputs is required (e.g. attentional set-shifting [46]). Under optimal conditions, DA release is likely dynamically regulated by the PFC according to momentary cognitive demands (for an extensive review, see [45]). However, when there are high levels of DA release during stress, all firing is suppressed due to excessive cAMP production (Fig 4B; [8]). D1R suppression of neuronal firing additionally may involve presynaptic inhibition of glutamate release [47, 48].
Figure 4.
DA stimulation of D1Rs weakens PFC network connections. A. Working model showing D1Rs on a different subset of spines than those containing α2A-ARs; the spines receive network inputs from neurons with dissimilar tuning characteristics (e.g. a neuron with a preferred visuospatial direction of 90° receiving an input from a neuron with a preferred response to 120°). D1R stimulation leads to cAMP generation and the opening of ion channels that shunt nearby synaptic inputs. DA and D1R are shown in purple as they decline with normal aging. B. Stimulation with the D1R agonist, SKF8129, produces an inverted-U dose-response whereby moderate, optimal doses enhance spatial tuning by reducing firing during the delay period only for the memory of nonpreferred directions. In contrast, high doses of D1R stimulation suppress firing for all directions. Adapted from [8]. F=fixation; C=cue; D=delay; R=eye movement response; PD=preferred direction of the neuron; NPD=an example of a nonpreferred direction of the neuron.
Neuronal cAMP concentrations are also regulated by the brain PDE4 enzymes (e.g. PDE4A, B and D), which hydrolyze cAMP (Fig. 3B). Loss of PDE4 activity leads to cAMP build-up and network collapse (Fig. 3A, C; [7]). Recent data indicate that DISC1 (Disrupted In SChizophrenia) increases PDE4 activity under conditions of high cAMP production, e.g. as occur during stress exposure (Fig. 3B; [49]). In PFC, DISC1 is found in spines [50], where it is colocalized with HCN channels and PDE4s (Paspalas and Arnsten, unpublished data), and thus ideally positioned to regulate network connectivity. Therefore, genetic insults to DISC1 may render DNC mechanisms in PFC particularly vulnerable to stress exposure (see below).
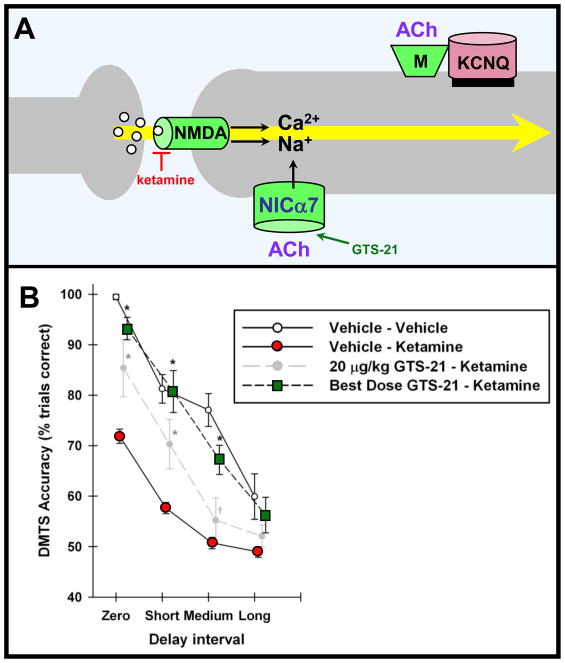
Acetylcholine is important for vigilant attention [51], and for strengthening NMDA-mediated working memory [52]. It increases DA and NE release via α4β2 and α7 nicotinic receptors on catecholamine terminals [53]. In addition, α7 nicotinic receptors are localized directly on spines, where they are positioned to modulate NMDA receptor inputs directly (Fig 5A; [54]). Preliminary data indicate that stimulation of α7 receptors in monkeys performing a working memory task enhances network firing while receptor blockade seems to markedly reduce network firing (Yang, Gamo, Arnsten and Wang, unpublished data). Behavioral data are also consistent with α7 nicotinic modulation of NMDA inputs, as an α7 agonist can partially rescue the cognitive deficits induced by the NMDA blocker, ketamine (Fig 5B; [52]). Acetylcholine may also strengthen network connections by closing KCNQ channels via muscarinic receptors (the so-called “M-current” [24]). Thus, release of ACh as well as of catecholamines in response to relevant environmental events appears to enhance PFC network connectivity.
Figure 5.
ACh strengthens PFC network connectivity. A. A working model showing that ACh may strengthen PFC network connections through direct stimulation of α7 nicotinic receptors on spines [54], and indirectly through stimulation of muscarinic receptors which in turn closes KCNQ2/3 channels. Nicotinic α7 receptors are shown in navy blue due to their genetic alteration in some families with schizophrenia; ACh is shown in purple due to its decline with normal aging. B. Systemic administration of the α7 receptor agonist, GTS-21, to monkeys partially rescues the deficits in working memory induced by the NMDA receptor blocker, ketamine. Figure adapted from [52] with the kind permission of A.V. Terry, Jr..
Insults to Dynamic Network Connectivity in cognitive disorders
The complex, genetic and environmental contributions to cognitive disorders are beginning to be understood. As these data emerge, it is evident that many genetic and environmental insults would impact DNC signaling molecules. Alterations in DNC mechanisms appear to be associated with a variety of cognitive disorders, ranging from mild PFC impairment (e.g. ADHD, normal aging) to severe deficits (schizophrenia, Alzheimer’s Disease (AD)). The following provides a brief oveview; further information on ADHD can be found in [55].
Aging
Decline in PFC cognitive abilities is common with normal aging. Although neuronal death does not occur in healthy aged PFC, there are changes in white matter [56] and loss of dendritic spines, particularly the long, thin spines characteristic of DNC synapses [39]. There are also numerous age-related changes in the arousal systems and second messenger cascades that regulate network connectivity (shown in purple in Figs. 3–5). Unexpectedly, cAMP signaling becomes disinhibited with advancing age in PFC, in contrast to the aged hippocampus where there is reduced cAMP [57]. Dysregulated cAMP signaling may arise from a decline in α2A-ARs [58] and PDE4A (A.A. Simen, personal communication). Disinhibited cAMP signaling weakens PFC network connectivity and impairs working memory abilities, as supported by behavioral data [57, 59], and preliminary physiological recordings from aged monkeys (Wang, Yang, Gamo and Arnsten, unpublished data). Depletion of ACh with age may also contribute to cognitive deficits [60], and DA neurons actually degenerate with normal aging [61]. However, D1R agonists have limited therapeutic value in aged monkeys due to the increased susceptibility to detrimental D1R-mediated actions [62].
In contrast to normal aging, AD is associated with neuronal death. However, cognitive deficits precede cell death, and new research is focusing on soluble amyloid-beta peptide (Aβ) impairment of synaptic transmission (illustrated in magenta in Figs. 2A-B). Soluble Aβ internalizes NMDA receptors in the presence of α7 nicotinic receptors, suggesting that DNC synapses may be especially vulnerable (Fig. 2A [63]). In addition, Aβ directly stimulates mGluR1 (Fig 2B; [64]), which would reduce PFC network activity. These actions may contribute to PFC cognitive deficits prior to pyramidal cell death.
Schizophrenia
Schizophrenia is associated with profound deterioration in PFC function [65]. Even “positive” symptoms of delusions and hallucinations have been related to PFC dysfunction [66, 67], and hypofrontality during a working memory task correlates with thought disorder [68]. Thus, disordered PFC function contributes substantially to schizophrenia symptoms. Schizophrenia is associated with altered PFC circuits, arising from both developmental insults in utero, and continuing in the mature brain, e.g. with waves of gray matter loss in late adolescence and adulthood [69]. We propose that impaired DNC regulation contributes to the progressive worsening of symptoms and neuropil loss, especially following stress exposure.
A remarkable number of genetic insults in schizophrenia involve proteins found at DNC synapses (shown in navy blue in Figs 2–5). There are well-established genetic changes associated with NMDA receptor signaling (Fig. 2A; reviewed in [70, 71]), and α7 nicotinic receptors [72]. It is possible that the high prevalence of smoking in patients with schizophrenia arises from their need to increase nicotine stimulation of α7 receptors to strengthen PFC network connections. More recently, a translocation in the gene encoding for DISC1 has been associated with high rates of mental illness in a large Scottish pedigree [49]. Genetic insults to DISC1 likely contribute to altered cortical development [73], and should also lead to weakened network connectivity similar to that shown in Figure 3. Interestingly, significant associations have been observed between reduced PFC activity and single nucleotide polymorphisms in genes encoding for DISC1 and for NMDA, α7 nicotinic and α2A adrenergic receptors in an fMRI study of patients with schizophrenia performing an oddball task [74]. Thus, this nonbiased study uncovered a set of DNC-associated proteins needed for PFC function. It is possible that similar modulatory insults in ventromedial and/or orbital PFC contribute to symptoms of mood disorders, as depression and bipolar disorder have also been linked to genetic insults in DISC1 [75]. Finally, large decreases in RGS4 protein and mRNA have been measured in the PFC of patients with schizophrenia [76, 77]. Loss of RGS4 would disinhibit mGluR1/Gq signaling in the spine and reduce PFC network activity (Figs. 2B, D). Loss of RGS4 would also disinhibit PKC signaling, which could aggravate spine loss [36].
Lewis and Gonzalez-Burgos [78] have described a cascade of primary genetic insults to PFC circuits, followed by compensatory events that may combine to produce the symptoms of schizophrenia. Genetic insults to DNC may play a key role in this sequence of events that lead to schizophrenia symptoms: 1) Neurodevelopmental errors caused by genetic and/or environmental insults as the cortex develops in utero, leading to the formation of abnormal PFC circuitry, followed by 2) progressive PFC spine loss in adolescence leading to greatly reduced pyramidal cell network excitation. The current article provides evidence that genetic alterations in DNC mechanisms may play an essential role in this process. The weakening of pyramidal cell network activity would then lead to a number of compensatory changes: 3) weakening of specific GABA synapses from loss of excitatory drive. This would reduce network tuning (i.e. make information noisier), 4) reduced cortical drive on DA neurons projecting to PFC (further eroding tuning), and 5) increased striatal DA release, which would magnify cortical inputs and PFC network errors. Thus, correcting for genetic errors in DNC, especially at key time periods in adolescence, may slow the progression of PFC network demise and the manifestation of schizophrenia symptoms.
Concluding remarks
Research on neuronal plasticity has generally focused on long-term changes in synaptic connections. However, as we come to appreciate the intricate roles of cortical networks in cognition, it is now increasingly important to understand the rapid and dynamic modulatory influences on cortical network strength. This is a challenging task, as these modulatory effects are best observed in a cognitively-engaged circuit, and this type of research is tedious and technically challenging. This emerging discipline calls on us to fuse the extraordinary details of molecular biology and electron microscopy with the remarkable insights from higher cortical physiology, fields that generally do not intersect. As DNC mechanisms are just beginning to be revealed, there are many more questions than answers (see Box 2). However, we are beginning to glimpse those signaling events that temporarily strengthen the network connections that form our “mental sketch pad”, as well as the molecular limits on our cognitive abilities.
Box 2. Outstanding questions.
It is unlikely that all DNC synapses contain all mechanisms. If they do not, how are they segregated? Are there common molecular partners? Quantitative analyses will be needed for rigorous comparisons of molecular interactions in different cortical areas.
Are DNC mechanisms specific to the superficial PFC microcircuits that are thought to generate persistent network firing during working memory, or are they more generalized throughout layers and across regions? Are some mechanisms shared with all cortical association areas and/or with hippocampus? (e.g. mGluR1 negative feedback via SK channels).
It is likely that a single PFC neuron engages in both long-term (e.g. LTP) and DNC types of plasticity. Might this arise from differing signaling partners in different sets of spines? For example, might cAMP have traditional, long-term strengthening effects predominantly in mushroom-shaped spines without HCN channels, while in other, long thin spines with HCN channels, cAMP would rapidly weaken connectivity?
Are differing PFC cognitive operations regulated in a similar or different manner, and are there molecular consistencies in networks mediating a cognitive operation? For example, are α7 nicotinic receptors on spines of both PFC and parietal networks mediating sustained attention?
DNC mechanisms influence the strength of working memory abilities, but do they also shape the contents of working memory? Studies of D1R actions suggest they do. This will be an exciting area for future research.
Acknowledgments
This work was funded by Public Health Service grants PO1 AG030004, MERIT Award AG06036, and 1RL1AA017536 within U54RR024350, and a NARSAD Distinguished Investigator Award to AFTA.
Glossary
- α2A-AR
The α2A subtype of the epinephrine/norepinephrine receptor is a Gi-protein coupled, seven-transmembrane domain receptor. Its intracellular signaling pathway involves deactivation of adenylyl cyclase, which lowers the production of cAMP. Some of these receptors are found pre-synaptically on noradrenergic axons and cell bodies, but their ability to strengthen PFC network connectivity arises from post-synaptic receptors
- Aβ peptide
Amyloid beta peptide is the main constituent of the amyloid plaques in the brain of patients with Alzheimer’s disease. New data suggest that the soluble form of this peptide (before it aggregates in plaques) may actually mediate its toxic properties
- ACh
Acetylcholine is a neurotransmitter in the central and peripheral nervous system. In the central nervous system, ACh neurons form the cholinergic system that has neuromodulatory functions. ACh exerts its actions via ionotropic (nicotinic) and metabotropic (muscarinic) cholinergic receptors
- Antagonist
-
A receptor antagonist is a chemical substance that binds to a receptor and attenuates or blocks receptor responses to the naturally occurring ligand or an agonist.
Methyllycacontine: antagonist of the α7 nicotinic receptor.
Ro25-6981: antagonist of the NMDA receptor containing the NR2B subunit.
Yohimbine: antagonist of the α2-AR
- Agonist
-
A receptor agonist is a chemical substance that binds to a receptor and triggers a response similar to that triggered by the naturally occurring ligand.
DHPG: agonist of group I mGluRs (mGluR1/5).
Guanfacine: agonist of the α2A-AR.
PHA543613: agonist of the α7 nicotinic receptor.
SKF81297: agonist of the D1R
- CaMKII
Ca2+/calmoduline-dependent protein kinase II is a serine/threonine kinase
- cAMP
Cyclic adenosine monophosphate is a key intracellular second messenger for an array of biological processes. cAMP is a diffusible molecule that is synthesized on the inner aspect of the plasma membrane from ATP by the action of adenylyl cyclase. Its actions are terminated by the phosphodiesterase enzymes that hydrolyze cAMP. Certain G protein coupled receptors (like NE and DA receptors) can regulate cAMP signaling via inhibiting or stimulating adenylyl cyclase
- D1R
The D1 type of the family of dopamine receptors is a Gs protein-coupled, seven-transmembrane domain receptor. Its signaling pathway mainly involves activation of adenylyl cyclase, thus increasing intracellular cAMP levels
- Dendritic spine
The dendrites of cortical pyramidal neurons typically present numerous minute protrusions, the dendritic spines. The majority of the cortical excitatory drive (including the connections between pyramidal cells in cortical networks) terminates onto the spines as axospinous synapses. Dendritic spines sequester a small cytosolic volume, which is continuous with the cytosol of the parent dendrite via a narrow spine neck. Thus the spine can subserve electrical and/or biochemical compartmentalization
- DISC1
The Disrupted in Schizophrenia genetic locus is recognized as a key susceptibility factor for severe psychiatric illness, as a DISC1 loss-of-function translocation is found in families with high rates of schizophrenia, bipolar disorder and major depression. One important emerging role of DISC1 is in neuroplasticity and neurodevelopment. DISC1 is also positioned to have a critical involvement in the cAMP signal transduction pathway via interaction with PDE4
- HCN channels
Hyperpolarization-activated cyclic nucleotide-gated channels form nonselective cation-contacting pores on neuronal plasma membranes, mediating the h-current that affects key aspects of brain physiology. HCN channels open when the cell is hyperpolarized, and their open-state probability is increased in the presence of cAMP
- IP3
Inositol trisphosphate is a second messenger in the phosphoinositide signal transduction system. IP3 is generated in the plasma membrane and diffuses to reach IP3 receptors on endomembranes of the smooth endoplasmic reticulum. Binding of IP3 to the IP3 receptor triggers Ca2+ release from the reticular lumen (i.e. internal Ca2+ release)
- KCNQ channels
KCNQ are voltage-gated K+ channels mediating the M-current in neurons, which is key for controlling membrane excitability. Opening of these channels on spines would hyperpolarize the spine and weaken connections onto the spine. These channels are also found on other parts of the neuron where they decrease cell firing
- mGluR1/5
Metabotropic glutamate receptors 1 and 5 comprise the group I of the G protein-coupled GluRs. Receptor activation triggers the phosphoinositide signaling pathway (please see IP3, above) and IP3-gated Ca2+ release from internal stores
- Muscarinic receptor
Muscarinic acetylcholine receptors are cholinergic ligand-gated G protein-coupled (metabotropic) receptors. Ligand binding to the receptors (including muscarine) can signal via the phosphoinositide or the cAMP signaling pathways. Muscarinic receptors coupled to Gq can close KCNQ channels when they are in the same lipid raft (i.e. immediately next to these channels)
- Nicotinic receptor
Nicotinic acetylcholine receptors are cholinergic ligand-gated ionotropic receptors. Ligand binding to the receptors (including nicotine) opens a non-selective cation channel on the cell surface. Many of these are localized pre-synaptically on cholinergic, catecholamine and glutamate axons, but some are localized directly on PFC spines
- NMDA receptor
N-methyl D-aspartate receptors are ionotropic glutamate receptors. Ligand binding opens a non-selective cation channel, while receptor activation is voltage-depended. NMDA receptors play a central role in synaptic plasticity
- PDE4
Regulation of cAMP actions as well as cellular compartmentalization of cAMP signals is achieved by tight control of its degradation by the cyclic nucleotide phosphodiesterase (PDE) enzymes. The cAMP-specific PDE4 subfamily hydrolyzes and deactivates virtually 100% of the cAMP produced in response to a G protein-coupled receptor activation in cerebral cortex
- PKA
Protein kinase A is a family of enzymes that their activity depends on the levels of cAMP (cAMP-dependent protein kinase). PKA phosphorylates a variety of protein substrates. It also activates PDE4s that catabolize cAMP, providing a feedback regulation of cAMP levels
- PKC
Protein kinase C is a family of enzymes regulated by Ca2+ or diacylglycerol. PKC phosphorylates and controls a variety of other proteins in the downstream signal transduction pathway, thus having important role in intracellular signaling
- Receptor
A protein molecule embedded in the cell membrane or found intracellularly that binds a specific ligand or set of ligands. Ligand binding can trigger a complex array of intracellular signaling cascades and likely initiate a cellular response
- RGS4
Regulators of G protein Signaling (RGS) negatively regulate intracellular signaling via G proteins. RGS4 is perhaps the most widely distributed and highly expressed RGS in the brain. It attenuates the intensity and duration of signal transduction via Gαi and Gαq proteins, for example, diminishing the effects of mGluR1/5 stimulation. RGS4 is markedly reduced in the PFC of patients with schizophrenia
- SK channels
Small conductance Ca2+-activated K+ channels are potassium channels gated by intracellular Ca2+ that underlie afterhyperpolarizing currents in neurons. SK channels contribute to shaping the neuronal firing pattern, i.e. opening these channels leads to a reduction in neuronal firing
Footnotes
Disclosure statement- AFTA and Yale University receive royalties from Shire Pharmaceuticals for the sale of Intuniv™ based on a license agreement for the development of guanfacine for the treatment of ADHD and related disorders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnsten AFT. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;32:267–287. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore TL, et al. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR, et al. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Archives General Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 4.Rubia K, et al. Hypofrontality in Attention Deficit Hyperactivity Disorder during higher-order motor control: A study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg HP, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 6.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, et al. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, et al. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagenston AM, et al. mGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cerebral Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swartz BE, et al. Studies of working memory using 18FDG-positron emission tomography in normal controls and subjects with epilepsy. Life Sci. 1996;58:2057–2064. doi: 10.1016/0024-3205(96)00198-1. [DOI] [PubMed] [Google Scholar]
- 14.Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behavioral Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- 15.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faber ES. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J Physiol. 2010;588:1281–1292. doi: 10.1113/jphysiol.2009.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muly EC, et al. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- 18.Mannaioni G, et al. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paspalas CD, et al. Mapping the Regulator of G Protein Signaling 4 (RGS4): Presynaptic and postsynaptic substrates for neuroregulation in prefrontal cortex. Cereb Cortex. 2009;19:2145–2155. doi: 10.1093/cercor/bhn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan AR, et al. Blockade of IP3-mediated SK channel signaling in the rat medial prefrontal cortex improves spatial working memory. Learn Mem. 2008;15:93–96. doi: 10.1101/lm.767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runyan JD, et al. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birnbaum SB, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology. 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- 24.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nature Reviews Neuroscience. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 25.Runyan JD, Dash PK. Distinct prefrontal molecular mechanisms for information storage lasting seconds versus minutes. Learn Mem. 2005;12:232–238. doi: 10.1101/lm.92405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor JR, et al. Activation of protein kinase A in prefrontal cortex impairs working memory performance. J Neuroscience (Online) 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge LD, et al. Modulation of calcium-activated non-specific cation currents by cyclic AMP-dependent phosphorylation in neurons of Helix. J Physiology. 1990;429:131–145. doi: 10.1113/jphysiol.1990.sp018248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kole MH, et al. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578(Pt2):507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Z, et al. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. 2009;29:10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poolos NP, et al. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nature Neuroscience. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder BC, et al. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- 32.Ramos B, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biological Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Roozendaal B, et al. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundemann D, et al. Molecular identification of the cortisone-sensitive extraneuronal catecholamine transporter. Nature Neuroscience. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 35.Radley JJ, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hains AB, et al. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vyas A, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liston C, et al. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Nat Acad Sci USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao J, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 41.Li BM, et al. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacol. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 42.Li BM, Mei ZT. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 43.Ma CL, et al. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 44.Biederman J, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 45.Arnsten AFT, et al. Dopamine’s influence on prefrontal cortical cognition: Actions and circuits in behaving primates. In: Bjorklund A, et al., editors. Dopamine Handbook. Oxford University Press; 2009. pp. 230–249. [Google Scholar]
- 46.Crofts HS, et al. Differential effects of 6-OHDA lesions of the prefrontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 47.Gao WJ, et al. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 50.Kirkpatrick B, et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 51.Gill TM, et al. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. Journal of Neuroscience. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buccafusco JJ, Terry AVJ. A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochem Pharmacol. 2009;78:852–862. doi: 10.1016/j.bcp.2009.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 54.Duffy AM, et al. Spatial and intracellular relationships between the alpha7 nicotinic acetylcholine receptor and the vesicular acetylcholine transporter in the prefrontal cortex of rat and mouse. Neuroscience. 2009;161:1091–1103. doi: 10.1016/j.neuroscience.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters A, et al. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Ramos B, et al. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 58.Moore TL, et al. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Ramos B, et al. Alpha-2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learning and Memory. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wenk GL, et al. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobio Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- 61.Siddiqi Z, et al. Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. Journal of Neuropathology & Experimental Neurology. 1999;58:959–971. doi: 10.1097/00005072-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Cai JX, Arnsten AFT. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283:183–189. [PubMed] [Google Scholar]
- 63.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 64.Blanchard BJ, et al. Mechanism of membrane depolarization caused by the Alzheimer Abeta1–42 peptide. Biochem Biophys Res Commun. 2002;293:1197–1203. doi: 10.1016/S0006-291X(02)00346-7. [DOI] [PubMed] [Google Scholar]
- 65.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 66.Ford JM, et al. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 67.Corlett PR, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130(Pt 9):2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perlstein WM, et al. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 69.Vidal CN, et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 70.Ross CA, et al. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Krystal JH, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 72.Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 73.Ishizuka K, et al. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, et al. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp. 2009;30:241–255. doi: 10.1002/hbm.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blackwood DH, Muir WJ. Clinical phenotypes associated with DISC1, a candidate gene for schizophrenia. Neurotox Res. 2004;6:35–41. doi: 10.1007/BF03033294. [DOI] [PubMed] [Google Scholar]
- 76.Mirnics K, et al. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- 77.Erdely HA, et al. Regional alterations in RGS4 protein in schizophrenia. Synapse. 2006;59:472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- 78.Lewis DA, Gonzalez-Burgos GR. Pathophysiologically based treatment interventions in schizophrenia. Nature Medicine. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 79.Hahn CG, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]