Abstract
Objectives
To compare treatment outcomes by starting CD4 counts using data from the CIPRA-South Africa trial.
Design
Observational cohort study.
Methods
Patients presenting to primary care clinics with CD4 cell counts <350 cells/mm3 were randomized to either doctor- or nurse-managed HIV care and followed for at least two years after ART initiation. Clinical and laboratory outcomes were compared by baseline CD4 count.
Results
812 patients were followed for a median of 27.5 months and 36% initiated with a CD4 count >200. While 10% of patients failed virologically (VF), the risk was nearly double among those with a CD4 ≤200 vs. >200 (12.2% vs. 6.8%). 21 deaths occurred, with a five-fold increased risk for the low CD4 group (3.7% vs. 0.7%). After adjustment, those with a CD4 count ≤200 had twice the risk of death/VF (HR 1.9; 95% CI: 1.1–3.3) and twice the risk of incident tuberculosis (HR: 1.90; 95% CI: 0.89–4.04) as those >200. Those with either a CD4 ≤200 (HR 2.1; 1.2–3.8) or a WHO IV condition (HR 2.9; 0.93–8.8) alone had a two to three-fold increased risk of death/VF vs. those with neither, but those with both conditions had a 4-fold increased risk (HR 3.9; 95% CI: 1.9–8.1). We observed some increased loss to follow-up among those initiating <200 (HR 0.79; 95% CI: 0.50–1.25).
Conclusions
Patients initiating ART with higher CD4 counts had reduced mortality, tuberculosis and less virologic failure than those initiated at lower CD4 counts. Our data support increasing CD4 count eligibility criteria for ART initiation.
Keywords: Human Immunodeficiency Virus, Sub-Saharan Africa, Highly Active Antiretroviral Therapy, CD4 count, Mortality, Virologic Failure, Tuberculosis
INTRODUCTION
With the increase in global funding for HIV/AIDS, the developing world has seen unprecedented access to lifesaving antiretroviral therapy (ART) over the past five years. Funds mandated towards rapidly scaling up access to ART have been successfully targeted and nearly four million people are now on ART[1]. When large-scale treatment programs began, in most cases, treatment was limited to patients with advanced disease. The ideal time to initiate ART is currently unknown[2]. While guidelines for resource rich environments currently recommend initiating at CD4 counts < 350[3;4], developing country guidelines recommended initiating ART at CD4 counts ≤200 in the absence of clinical disease until November 2009 when the WHO recommended initiating treatment at CD4 < 350 [5;6]. As new evidence from resource rich environments has accumulated showing that starting ART at higher CD4 counts is associated with better treatment outcomes[7–9], programs in resource limited settings, given limited resources, must make difficult choices about whether or not to raise initiating CD4 count thresholds to higher levels.
The debate about when to initiate treatment is difficult as ART is a lifelong treatment that has significant cost and can have significant side effects. On an individual level decisions about when to initiate therapy must balance the potential medical benefits of initiating at higher CD4 counts[10] and reductions in HIV transmission[11] with the risk of toxicity and the costs associated with longer time on treatment. On a public health level, a decision about when to initiate ART must balance any expected population level benefits of initiating treatment at higher CD4 counts with the cost implications of potentially increased demand for treatment if treatment thresholds are raised, but also any possible cost savings associated with earlier treatment including reduced hospitalization and treatment of opportunistic infections.[12] The first step towards rationalizing decisions on when to initiate ART is to assess, using data from resource limited settings, the likely treatment benefits that can be expected.
Recent evidence from the developed world suggest starting at CD4 counts below 350 improves treatment outcomes and decreases mortality compared to waiting to below 200[13–17], and the benefits may even begin when initiating at CD4 counts above 350,[13] yet limited evidence from the developing world exists to inform policy[18]. To date only one study from a developing country has attempted to randomize patients to initiate ART at CD4 counts below 350 compared to waiting until the CD4 count drops below 200. An interim analysis of the Comprehensive International Program of Research on AIDS (CIPRA)-Haiti trial[19;20] found that initiating at CD4 counts under 200 was associated with a 4-fold increased risk of mortality and a 2-fold increased risk of incident tuberculosis compared to starting at a CD4 count between 200–350.
The recent changes in WHO guidelines have yet to be adopted globally[21]. In order to support decision making around when to initiate ART, we assessed the association between treatment outcomes and starting ART at higher CD4 counts using data collected as part of the CIPRA-SA randomized trial comparing nurse monitored antiretroviral treatment with doctor monitored treatment in South Africa[22].
METHODS
Study Design
The data for this study was collected as part of the CIPRA-SA trial, an unblinded, prospective, randomized controlled trial comparing nurse- vs. doctor-monitored HIV care and demonstrated equivalence of the two monitoring strategies for treatment failure over two years (HR 1.09; 95%CI 0.89–1.33)[22]. The study enrolled 812 HIV-positive ART naïve patients ≥16 years old with a CD4 count ≤350 or prior AIDS defining illness (CDC category B/C) at one of two sites in South Africa (Soweto, Johannesburg and Masiphumelele, Cape Town). All patients were managed under South African National Guidelines for HIV treatment and were given standard ART regimens consisting of lamivudine given with either zidovudine or stavudine and either efavirenz or nevirapine[5]. A protease inhibitor (PI) based regimen was used in a limited number of cases (N=62) for women of childbearing potential with a CD4 count >350. Patients were randomized 1:1 to have their HIV care monitored by either a nurse or a clinical officer and were followed for a minimum of 96 weeks. Details of the CIPRA-SA trial can be found elsewhere.[22]
Patient Follow-up and Data Collection
Patients were seen at baseline and then returned for follow up visits at weeks 0, 2, 4, 9, 12, then every 12 weeks. At each visit patients had a clinical examination, symptom screening for tuberculosis and a blood draw for lab testing including CD4 count, viral load, hematology and biochemistry.
While current guidelines in South Africa allow for initiation of ART for patients with CD4 counts ≤200 or WHO Stage IV condition[5], and because the CIPRA-SA study enrolled patients with a CD4 count ≤350, we conducted a prospective cohort study assessing differences in treatment failure among those initiated at a CD4 count >200 and those initiated ≤200.
Definition of Study Variables
The primary exposure was CD4 cell count >200 vs. ≤200 at ART initiation. CD4 count was measured at randomization in the main CIPRA-SA study and was assessed using CD4+ flow cytometry (FlowCount Fluorospheres, Beckman Coulter-Immunotech, France).
We assessed the impact of starting treatment at higher CD4 counts on three indicators of program failure: 1) treatment failure (an indicator of death or failure to achieve or maintain viral suppression); 2) incident tuberculosis; and 3) program failures (indicated by patients who leave care). Virologic failure was defined as either: 1) failure to reach a 1.5 log10 drop in viral load by 12 weeks on treatment; or 2) two consecutive viral loads >1,000 copies/ml within one month of each other after 24 weeks on treatment. We defined loss to follow up (LTFU) as missing three or more consecutive study visits (in the main study shown as lost to follow up and defaulting clinic schedule); we did not include patients who voluntarily withdrew from the study as many of these subjects would remain in care just not on the study protocol.
To determine if there were any association between toxicity related outcomes and initiating treatment at higher CD4 counts, we examined the relation between initiating CD4 count and treatment related toxicities. Details of the toxicities that occurred are given elsewhere[22] but included any toxicity which required discontinuation of the study regimen, with a resulting treatment interruption of >6 weeks.
For all analyses, person-time accrued from initiation of treatment through the date of the earliest of: 1) experiencing a treatment outcome (defined above); 2) completion of 48 months of treatment; 3) becoming lost to follow-up (except in analyses where LTFU was the outcome); or 4) date of closing the dataset (January 20, 2009).
All patients in the CIPRA-SA trial signed informed consent forms. The CIPRA-SA trial was approved by the Institutional Review Boards (IRB) of the University of the Witwatersrand and the University of Cape Town. The Boston University IRB gave approval for analysis of the data in a de-identified manner.
Statistical Methods
We compared differences between study groups by stratifying our data by baseline CD4 count group. We looked for crude associations between baseline predictors and treatment outcomes and compared groups using relative risks and 95% confidence intervals. We explored the relation between initiating CD4 count and treatment failure by describing the rate of treatment failure using crude Kaplan-Meier curves. We fit adjusted models of the association between low initiating CD4 count on treatment failure using Cox Proportional Hazards regression. All models were adjusted for age, sex (stratified at baseline into males, pregnant women and non-pregnant women), study site and randomization group. We did not include post-baseline measures as these may be caused by baseline CD4 count. We looked for a dose response between higher CD4 count and treatment outcomes by fitting a model with finer categorizations of baseline CD4 count. Finally we looked for interactions between CD4 count and other markers of immunosuppression at baseline (e.g. viral load, WHO stage, body mass index (BMI)).
RESULTS
Cohort Description
Baseline characteristics of the 812 subjects enrolled in the CIPRA-SA cohort stratified by baseline CD4 count group are shown in Table 1. Patients were followed for a median of 27.5 months (IQR 13.8–33.1) with no differences by study group. As expected based on randomization, roughly half of those in each CD4 count group had nurse-monitored and half had doctor-monitored ART care. Median age was 32 years and more than 2/3 were female with no differences by CD4 count group. Prior ART use (typically for prevention of mother-to-child transmission), was balanced between CD4 groups. Those in the low CD4 group were more likely to have nevirapine in their baseline regimen than efavirenz while those in the higher CD4 count group were more likely to have lopinavir-ritonavir.
Table 1.
Differences in baseline characteristics by baseline CD4 count group among 812 patients enrolled in the CIPRA-SA trial at two sites in South Africa*
| Variable | Baseline CD4 ≤ 200 (N=518) |
Baseline CD4 > 200 (N=294) |
Relative Risk (95% CI)* |
|
|---|---|---|---|---|
| Follow-up (months) (Median (IQR)) | 27.5 (13.4–33.1) | 27.5 (13.8–33) | ||
| Site | Johannesburg | 299 (57.7%) | 150 (51.0%) | Reference |
| Cape Town | 219 (42.3%) | 144 (49.0%) | 0.86 (0.74 – 1.01) | |
| Study group | Primary Health Care Nurse |
260 (50.2%) | 144 (49.0%) | Reference |
| Medical Officer | 258 (49.8%) | 150 (51.0%) | 0.98 (0.85 – 1.12) | |
| Gender | Male | 155 (29.9%) | 84 (28.6%) | Reference |
| Female | 363 (70.1%) | 210 (71.4%) | 0.98 (0.90 – 1.08) | |
| Age at baseline (Median (IQR) | 32 (28.1–37.3) | 32.5 (27.8–36.7) | ||
| Race | Black/African | 515 (99.4%) | 289 (98.3%) | Reference |
| Mixed Race | 3 (0.6%) | 5 (1.7%) | 0.34 (0.080 – 1.41) | |
| Baseline ART* regimen |
d4T-3TC-EFV | 370 (71.4%) | 227 (77.2%) | Reference |
| d4T-3TC-NVP | 119 (23.0%) | 34 (11.6%) | 1.87 (1.32 – 2.65) | |
| d4T-3TC-LPVr | 25 (4.8%) | 30 (10.2%) | 0.54 (0.33 – 0.90) | |
| d4T-3TC-NLF | 4 (0.8%) | 3 (1.0%) | 0.82 (0.19 – 3.63) | |
| Prior ART exposure* |
None | 390 (75.3%) | 219 (74.5%) | Reference |
| sdNVP | 113 (21.8%) | 54 (18.4%) | 1.14 (0.85 – 1.52) | |
| AZT mono | 2 (0.4%) | 4 (1.4%) | 0.28 (0.05 – 1.54) | |
| NVP/AZT | 13 (2.5%) | 16 (5.4%) | 0.47 (0.23 – 0.97) | |
| ART | 0 (0%) | 1 (0.3%) | ||
| WHO stage at baseline |
1 | 71 (13.8%) | 80 (28.0%) | Reference |
| 2 | 154 (29.8%) | 96 (33.6%) | 1.25 (1.07 – 1.47) | |
| 3 | 197 (38.2%) | 84 (29.4%) | 1.44 (1.22 – 1.69) | |
| 4 | 94 (18.2%) | 26 (9.1%) | 2.32 (1.62 – 3.33) | |
| CDC category | A | 177 (34.2%) | 124 (42.2%) | Reference |
| B | 135 (26.1%) | 94 (32.0%) | 1.00 (0.82 – 1.22) | |
| C | 206 (39.8%) | 76 (25.9%) | 1.42 (1.16 – 1.73) | |
| CD4 count at baseline (Median (IQR)) | 123 (77–159) | 254.5 (224–308) | ||
| Baseline hemoglobin (Median (IQR)) | 11.7 (10.3–13.1) | 12.4 (11.4–13.5) | ||
| BMI at baseline (Median (IQR)) | 23.1 (20.5–26.6) | 24.1 (21.3–28.4) | ||
| Baseline viral load |
<100,000 | 174 (33.6%) | 177 (60.2%) | Reference |
| ≥ 100,000 | 344 (66.4%) | 117 (39.8%) | 1.67 (1.43 – 1.95) | |
| Viral load at baseline (× 1000) Median (IQR) |
201.5 (64.8–525) | 66.9 (20.9–188) | ||
CI = confidence interval, CIPRA-SA = Comprehensive International Program of Research on AIDS-South Africa, ART = antiretroviral therapy, sdNVP = Single done Nevirapine, AZT = Zidovudine, NVP = Nevirapine, d4T = Stavudine, 3TC = Lamivudine, EFV = Efavirenz, NLF = Nelfinavir, LPVr = Lopinavir-ritonavir
518 subjects (64%) fell into the low CD4 count group (≤200) while the remaining 294 (36%) were in the high CD4 count group (>200). Those in the low CD4 group were more immunosuppressed at baseline as indicated by viral loads ≥100,000 (RR: 1.67; 95%CI: 1.43 – 1.95), WHO stage IV (RR: 2.32; 95% CI: 1.62–3.33) or CDC C (RR: 1.42; 95% CI: 1.16–1.73). Few subjects had extremely low or high CD4 counts; only 16% (85/518) of those in the low CD4 group had a CD4 count <50 and only 16% (46/294) of those in the high CD4 count group had a CD4 count >350.
Death and Virologic Failure
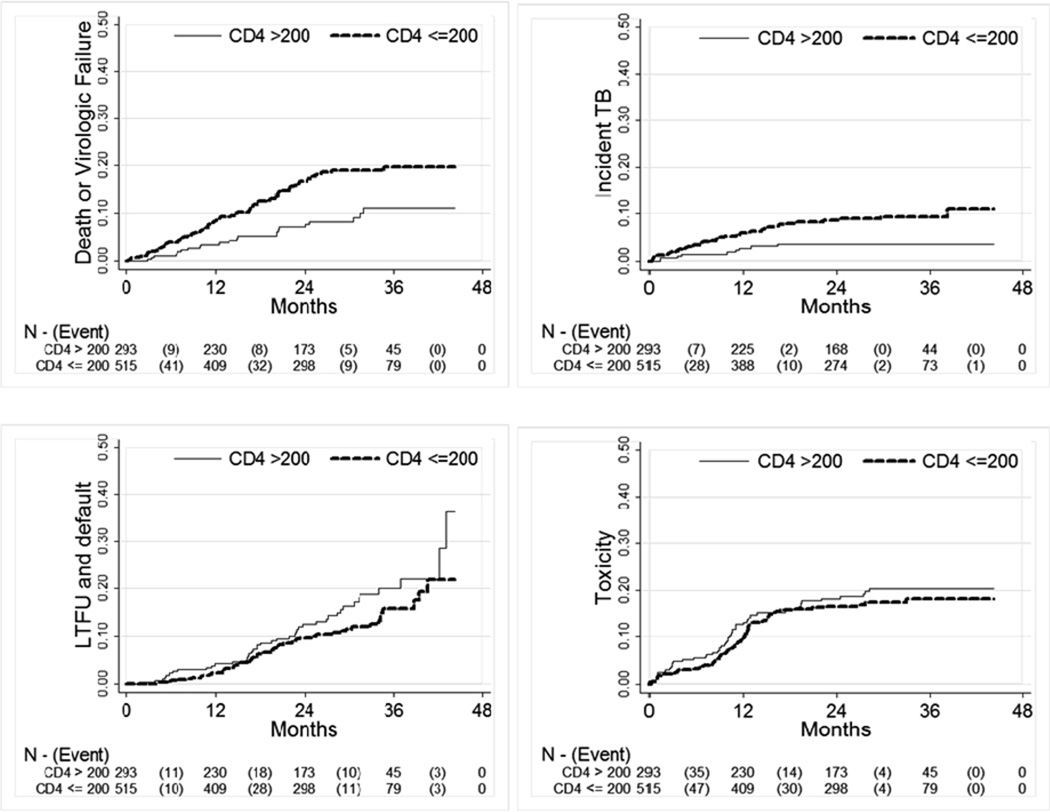
Treatment outcomes by CD4 group are shown in Table 2. There were 21 deaths in the study (2.6%); however in crude analyses, those with a baseline CD4 count ≤200 had a five-fold increased risk vs. those >200 (RR: 5.4; 95% CI: 1.3–23.0). Virologic failure occurred in 10% of the cohort, with the majority (84%) of these virologic failures based on 2 consecutive viral loads >1000 and not based on failure to achieve a 1.5 log10 drop in viral load from baseline by 12 weeks (16% 13/83). Those with a CD4 ≤200 at baseline had nearly twice the crude risk of virologic failure as those >200 (RR: 1.79; 95% CI: 1.10–2.90). The Kaplan-Meier curve of death or virologic failure presented in Figure 1a shows that the difference between the two groups in death and virologic failure emerges mainly between 6 and 24 months on treatment.
Table 2.
Reasons for ART treatment failure stratified by baseline CD4 count group among 812 patients enrolled in the CIPRA-SA trial at two sites in South Africa*
| Baseline CD4 count group | ||||
|---|---|---|---|---|
| Outcome | ≤ 200 (N=518) |
> 200 (N=294) |
Total (N=812) |
Relative Risk (95% CI)* |
| Death | 19/518 (3.7%) | 2/294 (0.7%) | 21 (2.6%) | 5.39 (1.26 – 22.0) |
| Virologic Failure | 63/518 (12.2%) | 20/294 (6.8%) | 83 (10.2%) | 1.79 (1.10 – 2.90) |
| 1.5 log drop# | 8/518 (1.5%) | 5/294 (1.7%) | 13 (1.6%) | 0.91 (0.30 – 2.75) |
| 2 VL > 1000^ | 55/518 (10.6%) | 15/294 (5.1%) | 70 (8.6%) | 2.08 (1.20 – 3.62) |
| Incident tuberculosis | 41/515 (8.0%) | 9/293 (3.1%) | 50 (6.2%) | 2.59 (1.28 – 5.26) |
| OTHER STUDY OUTCOMES | ||||
| Toxicity failure | 81/518 (15.6%) | 53/294 (18.0%) | 134 (16.5%) | 0.87 (0.63 – 1.19) |
| Loss to follow-up | 52/518 (10.0%) | 42/294 (14.3%) | 94 (11.6%) | 0.70 (0.48 – 1.03) |
| Default clinic schedule | 40/518 (7.7%) | 30/294 (10.2%) | 70 (8.6%) | 0.76 (0.48 – 1.19) |
| Lost to follow-up | 12/518 (2.3%) | 12/294 (4.1%) | 24 (3%) | 0.57 (0.26 – 1.25) |
CI = confidence interval, CIPRA-SA = Comprehensive International Program of Research on AIDS-South Africa
Failure to achieve a 1.5 log10 drop in viral load from baseline at 12 weeks on treatment
VL = viral load, 2 consecutive viral loads > 1,000 copies/ml within one month of each other after 24 weeks on treatment
Figure 1.
Kaplan-Meier analysis of time to a) death or virologic failure, b) tuberculosis c) loss to follow-up and d) toxicity by baseline CD4 count group among 812 patients enrolled in the CIPRA-SA trial at two sites in South Africa*
* CIPRA-SA = Comprehensive International Program of Research on AIDS-South Africa, TB = tuberculosis. Note that y-axis goes from 0 to 0.5.
Table 3 shows three different crude and adjusted models of the relation between baseline CD4 count and virologic failure or death. We present three separate models which are identical except that they use different categorizations of CD4 count (models 1 and 2) or include an interaction between baseline WHO stage and CD4 count (model 3). Model 1, which uses the CD4 categories used previously (≤200 vs. >200) shows that after adjusting for age, sex and pregnancy, site, treatment arm and other indicators of immunosuppression, those with a baseline CD4 count ≤200 had twice the risk of virologic failure or death (HR: 1.94; 95% CI: 1.14–3.30). Having a baseline WHO stage IV (HR: 1.98; 95% CI: 1.18–3.33), a viral load >100,000 vs. <10,000 (HR: 2.05; 95% CI: 0.71–5.89) and having a nelfinavir-based regimen (HR: 4.27; 95% CI: 1.17–15.6) were also predictive of virologic failure or death independent of CD4 count.
Table 3.
Crude and adjusted hazard ratios (HR) of death or virologic failure among 812 patients enrolled in the CIPRA-SA trial at two sites in South Africa*
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Predictor of Death or VL Failure | Crude HR (95% CI*) |
Adjusted HR (95% CI*) |
Crude HR (95% CI*) |
Adjusted HR (95% CI*) |
Crude HR (95% CI*) |
Adjusted HR (95% CI*) |
| Age at baseline (continuous) | 1.00 (0.97–1.03) | 1.01 (0.98–1.04) | 1.00 (0.97–1.03) | 1.01 (0.98–1.04) | 1.00 (0.97–1.03) | 1.01 (0.98–1.04) |
| BMI at baseline (continuous) | 0.99 (0.95–1.03) | 1.00 (0.96–1.04) | 0.99 (0.95–1.03) | 1.00 (0.96–1.04) | 0.99 (0.95–1.03) | 1.00 (0.96–1.04) |
| Pregnant vs. not pregnant female | 1.60 (0.99–2.58) | 1.53 (0.80–2.91) | 1.60 (0.99–2.58) | 1.51 (0.79–2.87) | 1.60 (0.99–2.58) | 1.52 (0.80–2.89) |
| Male vs. not pregnant female | 0.94 (0.62–1.43) | 1.03 (0.62–1.71) | 0.94 (0.62–1.43) | 1.02 (0.62–1.69) | 0.94 (0.62–1.43) | 1.02 (0.62–1.69) |
| Johannesburg vs Cape Town | 1.22 (0.82–1.81) | 1.23 (0.79–1.92) | 1.22 (0.82–1.81) | 1.20 (0.77–1.88) | 1.22 (0.82–1.81) | 1.22 (0.78–1.90) |
| Nurse vs. Doctor | 1.10 (0.75–1.62) | 1.13 (0.75–1.69) | 1.10 (0.75–1.62) | 1.14 (0.76–1.71) | 1.10 (0.75–1.62) | 1.10 (0.74–1.64) |
| Regimen with LPVr vs NNRTI* | 1.41 (0.71–2.79) | 1.57 (0.69–3.58) | 1.41 (0.71–2.79) | 1.54 (0.67–3.52) | 1.41 (0.71–2.79) | 1.51 (0.66–3.44) |
| Regimen with NLF vs NNRTI* | 3.92 (1.24–12.37) | 4.27 (1.17–15) | 3.92 (1.24–12.37) | 4.20 (1.14–15.42) | 3.92 (1.24–12.37) | 3.56 (0.98–12.96) |
| Viral load >100,000 vs <10,000 | 1.77 (0.69–4.55) | 2.41 (0.86–6.78) | 1.77 (0.69–4.55) | 2.40 (0.85–6.76) | 1.77 (0.69–4.55) | 1.89 (0.74–4.82) |
| Viral load 10,000 – 100,000 vs <10,000 | 2.43 (0.98–6.03) | 2.05 (0.71–5.89) | 2.43 (0.98–6.03) | 2.05 (0.71–5.89) | 2.43 (0.98–6.03) | 1.58 (0.61–4.12) |
| CD4 ≤ 200 vs > 200 | 2.11 (1.32–3.38) | 1.94 (1.14–3.30) | ||||
| CD4 0–99 vs. ≥300 | 3.29 (1.16–9.34) | 3.08 (0.92–10.4) | ||||
| CD4 100–199 vs. ≥300 | 3.17 (1.15–8.76) | 3.23 (0.99–10.6) | ||||
| CD4 200–299 vs. ≥300 | 1.72 (0.58–5.08) | 1.86 (0.54–6.45) | ||||
| WHO Stage IV vs. III/II/I | 1.81 (1.13–2.90) | 1.98 (1.18–3.33) | 1.81 (1.13–2.90) | 2.00 (1.19–3.37) | ||
| WHO IV, CD4 >200 vs. No WHO IV, CD4 > 200 | 2.64 (0.89–7.80) | 2.87 (0.93–8.83) | ||||
| No WHO IV, CD4 ≤200 vs. No WHO IV, CD4 > 200 | 2.24 (1.33–3.78) | 2.14 (1.20–3.81) | ||||
| WHO IV, CD4 ≤200 vs. No WHO IV, CD4 > 200 | 3.36 (1.75–6.46) | 3.91 (1.88–8.14) | ||||
CI = confidence interval, HR = hazard ratio, NNRTI = non-nucleoside reverse transcriptase inhibitor, NLF = Nelifinavir, LPVr = Lopinavir-ritonavir, CIPRA-SA = Comprehensive International Program of Research on AIDS-South Africa. Crude models are adjusted for each predictor by itself while adjusted models are adjusted for all other variables in the model. All adjusted models included regimen age, sex, site, randomization group, baseline viral load and BMI. Model 1 included dichotomous predictors for baseline CD4 count (≤ 200 vs > 200) and WHO stage (IV vs. III/II/I) with no interaction terms. Model 2 included a dichotomous predictor for baseline WHO stage (IV vs. III/II/I) and four categories for baseline CD4 count (0–99, 100–199, 200–299 vs > 300) with no interaction terms. Model 3 included dichotomous predictors for CD4 count (≤ 200 vs > 200) and WHO stage (IV vs. III/II/I) as well as an interaction term between baseline CD4 count and WHO stage.
In model 2 we looked for a CD4 count dose response between decreasing baseline CD4 count and risk of death or virologic failure by stratifying baseline CD4 count into finer categories (0–99, 100–199, 200–299, ≥300). After adjustment we found those with a CD4 count between 100–199 and those with a CD4 count <100 had a roughly 3-fold increased risk of death or virologic failure as those ≥300 (CD4 <100 vs. >300, HR 3.08; 95% CI: 0.92–10.4 and CD4 100–199 vs. >300, HR: 3.23; 95% CI: 0.99–10.6) while those with a CD4 200–299 had twice the risk (HR: 2.00; 95% CI: 1.19–3.37). Thus higher baseline CD4 count does appear to be associated with lower risk of virologic failure and death.
We also looked for interactions between baseline CD4 count group and other markers of immunosuppression. Model 3 shows the results of the only significant interaction we identified, that between baseline CD4 count (≤200 vs. >200) and baseline WHO Stage IV. Compared to the reference group of those with a baseline CD4 count >200 and no WHO Stage IV condition, those with either a WHO Stage IV condition alone or a CD4 count ≤200 alone had a 2 to 3-fold increased risk of death or virologic failure (HR: 2.87; 95% CI: 0.93–8.83 and HR: 2.14; 95% CI: 1.20–3.81 respectively). However, those with both a WHO Stage IV condition and a CD4 count ≤200 at baseline had a 4-fold increased risk of death or virologic failure vs. those with neither condition (HR: 3.91; 95% CI: 1.88–8.14).
Tuberculosis
Overall about 6% of all subjects in the study developed incident tuberculosis over the course of follow up with substantially more of it occurring among those initiated on ART at CD4 counts ≤200 than among those initiated >200 (8.0% vs. 3.1%, RR 2.6; 95% CI: 1.3–5.3) (Table 2). In a Kaplan-Meier analysis (Figure 1c) we note that the majority of the difference between the two groups emerged within the first 24 months on treatment. In table 4, we present two separate models of incident TB which are identical except that they use different categorizations of CD4 count (models 1 uses ≤200 vs. > 200 while Model 2 uses <50, 50–199, ≥ 200). After adjusting for site, treatment group, age, sex, use of a PI based regimen, baseline viral load, WHO stage, and BMI (data not shown) using proportional hazards regression we found that those initiated on ART at CD4 counts ≤200 cells/ul were twice as likely to develop TB compared to those initiated >200 cells/ul (HR: 1.9; 95% CI: 0.89–4.0) (Table 4, model 1). When we further stratified the lowest CD4 count group into those above and below 50 (model 2) we found that those with a CD4 count <50 vs. >200 were at strongly increased risk of incident TB (HR 3.4; 95% CI: 1.4–8.5) while those 50–200 vs. >200 were at lower increased risk (HR 1.60; 95% CI: 0.73–3.5) (Table 4).
Table 4.
Crude and adjusted hazard ratios (HR) of incident tuberculosis among 812 patients enrolled in the CIPRA-SA trial at two sites in South Africa*
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Predictor of Death or VL Failure | Crude HR (95% CI*) |
Adjusted HR (95% CI*) |
Crude HR (95% CI*) |
Adjusted HR (95% CI*) |
| Age at baseline (continuous) | 1.02 (0.98–1.06) | 1.02 (0.98–1.06) | 1.02 (0.98–1.06) | 1.02 (0.98–1.06) |
| BMI at baseline (continuous) | 0.90 (0.84–0.97) | 0.91 (0.84–0.99) | 0.90 (0.84–0.97) | 0.91 (0.84–0.99) |
| Female | 0.71 (0.40–1.26) | 1.30 (0.66–2.55) | 0.71 (0.40–1.26) | 1.29 (0.65–2.54) |
| Johannesburg vs Cape Town | 0.58 (0.33–1.02) | 0.60 (0.32–1.12) | 0.58 (0.33–1.02) | 0.69 (0.36–1.32) |
| Nurse vs. Doctor | 0.92 (0.53–1.60) | 1.06 (0.59–1.89) | 0.92 (0.53–1.60) | 1.03 (0.58–1.84) |
| Regimen with a PI* | 0.54 (0.13–2.22) | 0.93 (0.21–4.04) | 0.54 (0.13–2.22) | 0.98 (0.22–4.27) |
| WHO Stage IV vs. III/II/I | 2.09 (1.09–4.00) | 1.27 (0.63–2.56) | 2.09 (1.09–4.00) | 1.12 (0.55–2.29) |
| Viral load >100,000 vs <10,000 | 2.37 (0.73–7.66) | 1.23 (0.36–4.22) | 2.37 (0.73–7.66) | 1.18 (0.34–4.07) |
| Viral load 10,000 – 100,000 vs <10,000 | 0.65 (0.17–2.51) | 0.53 (0.13–2.08) | 0.65 (0.17–2.51) | 0.50 (0.13–1.97) |
| CD4 ≤ 200 vs > 200 | 2.64 (1.28–5.43) | 1.90 (0.89–4.04) | ||
| CD4 0–49 vs. ≥200 | 5.95 (2.57–13.8) | 3.39 (1.35–8.51) | ||
| CD4 50–199 vs. ≥200 | 2.05 (0.96–4.36) | 1.60 (0.73–3.51) | ||
Crude models are adjusted for each predictor by itself while adjusted models are adjusted for all other variables in the model. CI = confidence interval, HR = hazard ratio, PI = protease inhibitor, CIPRA-SA = Comprehensive International Program of Research on AIDS-South Africa. Crude models are adjusted for each predictor by itself while adjusted models are adjusted for all other variables in the model. All adjusted models included regimen, age, sex, site, randomization group, baseline viral load, WHO stage and BMI. Model 1 included a dichotomous predictor for baseline CD4 count (≤ 200 vs > 200). Model 2 included three categories for baseline CD4 count 0–49, 150–199, ≥200).
Loss to Follow Up
Nearly 12% of the cohort were lost to follow-up and LTFU was more common among those with a baseline CD4 count >200 than ≤ 200 (14.3% vs. 10.2%)(Table 2). After adjusting for age, sex, baseline WHO stage, viral load, use of a PI, treatment arm and site, we found a small decreased risk of being LTFU among those initiated on ART with lower CD4 counts (≤200) vs. those initiated at higher CD4 counts (>200) (HR: 0.79; 95% CI 0.50–1.25). Figure 1b shows that the difference between the groups emerges only after 24 months, the minimum potential follow-up time for the cohort. Those with a baseline WHO stage IV condition had an increased risk of LTFU (HR 1.61; 0.93–2.79), as did those with (data not shown).
Toxicities
Toxicity endpoints were experienced by about 17% of the entire cohort (Table 2). We observed small differences in rates of toxicities between CD4 count groups slightly favoring those with baseline CD4 counts ≤200 vs. >200 (15.6% vs. 18.0%) (Figure 1d). After adjusting for age, sex, baseline WHO stage, viral load, treatment regimen, treatment arm and site, females (HR: 1.95; 95% CI: 1.17–3.24), those who with a BMI >30 vs. 18.5–30 (HR 2.17; 95% CI: 1.41–3.34) or a baseline WHO stage IV condition (HR 1.91; 1.15–3.18) were at increased risk of toxicity failure, but we found little differences between CD4 count groups (HR 0.78; 95% CI: 0.53–1.16) (data not shown). Use of lopinavir-ritonavir vs. efavirenz based regimens decreased the risk of toxicity (HR: 0.40; 95% CI: 0.16–1.02). Nevirapine use was associated with increased risk of toxicity vs. efavirenz based regimens in unadjusted analyses (HR: 1.46; 95% CI: 0.99–2.16), but the association disappeared after adjustment (HR: 1.01; 95% CI: 0.63–1.61).
DISCUSSION
The results of analyses of the CIPRA-SA trial data show a clearly increased risk of death or virologic failure associated with initiating ART at lower CD4 counts. We found that those who started ART at CD4 counts ≤200 had roughly twice the risk of death or virologic failure as those initiated at CD4 counts >200 (HR: 1.94; 95% CI: 1.14–3.30) and twice the risk of developing incident tuberculosis (HR:1.90; 95% CI 0.89–4.04). These findings are in line with numerous observational studies from resource-limited settings showing low baseline CD4 count is a major predictor of death and loss to follow-up[23–27]. Recently an interim analysis of the CIPRA-HT001 trial in Haiti showed a nearly four-fold increased risk of death among those started with a CD4 count ≤200 vs. 200–350,[19] very similar to our five-fold increased risk (RR: 5.4; 95% CI: 1.3–23.0)[19]. Thus a body of evidence is beginning to emerge showing the benefits of earlier treatment initiation in resource-limited settings. This, along with a recent analysis by Lawn and colleagues[28] showing that the longer a patient maintains a CD4 count <100, the higher the risk of death suggest that starting patients at higher CD4 counts may allow them to maintain their CD4 count above the point at which they are at increased risk of death.
Our findings are also consistent with data from resource rich environments. Observational data has shown that higher CD4 counts are associated with lower risk of death[7;9]. More recently, Kitahata and colleagues have shown[13] that patients initiating ART at CD4 counts >500 had substantially reduced risk of mortality vs. those below. While our data cannot be used to comment on CD4 counts >350, our finding of decreased mortality and virologic failure risk associated with having a starting CD4 count >300 is suggestive of a dose-response with increasing baseline CD4 count associated with better outcomes.
While we found a substantial benefit to earlier ART initiation, we also found a slightly increased risk of being lost to follow-up among those with higher baseline CD4 counts which could potentially offset some of the benefits of initiating treatment earlier. However, we urge caution in interpreting these results. Under ideal conditions, assessing the effectiveness of initiating ART at higher CD4 counts would come from randomizing patients to either: 1) immediate initiation of ART when the CD4 count falls <350; and 2) follow patients and delay ART until the CD4 count falls <200[14;29]. In both arms patients would be followed from the time of their first CD4 count <350. In our study patients were initiated onto ART at enrollment as long as their CD4 count was <350 so we do not have any follow-up time to approximate what happens to patients in the time their CD4 count is between 200–350; however, we anticipate some death and loss to follow-up occurs in this time. While methods exist to adjust for this lead time bias,[30] they require pre-ART data which we did not have.
The current analysis has several strengths. The data were from a large prospective randomized trial with excellent follow-up data at standardized intervals which allowed the assessment of the impact of starting treatment at higher CD4 counts. Although the data was from a randomized trial of another intervention, because the trial showed no differences between randomization groups (i.e. nurse- vs. doctor-monitored care) and because adjustment for randomization group had no impact on our current results, there is little evidence that the primary intervention had any impact on our findings.
Still the current analysis should be considered in light of several limitations. First, as noted above, we did not have the ideal comparison group to assess death and virologic failure (i.e. a group followed from a CD4 count of 350 until 200 and then initiated on ART). Thus, any deaths occurring between 350-200 would not be included in our analysis. Since we are missing deaths in the high CD4 count group, this analysis likely underestimates the treatment benefits of starting at higher CD4 counts. Thus our estimates cannot be considered the true causal effect of starting treatment at higher CD4 counts. Second, as the data came from a randomized trial with conservative definitions of toxicity, many subjects who were treatment failures for toxicity might have otherwise continued on treatment under usual practice conditions. This could have biased our toxicity results towards the null and prevented us from observing a true difference between the groups if one existed. Third, in our analysis of loss to follow-up, we were not able to determine the final outcomes of patients lost and therefore we cannot say if patients left care because they were feeling well nor could we determine how many of them have since died. Finally, as the data were from a trial, the study population may have been healthier than the general clinic population.
In conclusion, we found that patients initiated on standard first-line South African ART regimens were at increased risk of death and virologic failure if initiated at CD4 counts <200 compared to those initiated >200. This is consistent with findings from developed areas which have shown that the benefits of starting at earlier CD4 counts outweigh the risks of toxicity and long-term adherence. While the cost implications are unknown, national and international guidelines on the topic of when to initiate ART should consider our findings when deciding on whether to increase initiating CD4 count thresholds. If thresholds are increased, substantial efforts will need to be made to move patients into care earlier in their disease progression in order to obtain the maximum benefit from ART.
Acknowledgements
Support for this study was provided by the US National Institute of Allergy and Infectious Diseases (NIAID) through the Comprehensive International Program of Research on AIDS (CIPRA) network, Grant U19 AI53217. The project described was also supported by K01AI083097 and P30-AI50410 from the National Institute of Allergy And Infectious Diseases and 5U2RTW007373-03 from the Fogarty International Center and was also provided by the United States Agency for International Development (USAID) under the terms of agreement 674-A-00-08-00007-00 with Right to Care (RTC). The content of this publication does not necessarily reflect the views or policies of NIAID, the Fogarty Center, USAID or Right to Care nor does mention of trade names, commercial projects, or organizations imply endorsement by the US Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the Fogarty Center, the National Institutes of Health, USAID, or other parties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
MF performed the primary statistical analyses and drafted the manuscript. IS, FC JZ, CO RI MR MD CvdH JM and RW contributed to the design of the study and data interpretation and revising the article. All authors approved the final manuscript.
Reference List
- 1.Souteyrand Y, Akwara P, Warner Smith M, Beusenberg M, Jashi M, Hayashi C, et al. Scaling up access to antiretroviral therapy (ART) in low- and middle-income countries: global and regional progress in 2008. International AIDS Society Conference WELBD105.2009. [Google Scholar]
- 2.Hughes MD, Ribaudo HR. The search for data on when to start treatment for HIV infection. J Infect Dis. 2008;197:1084–1086. doi: 10.1086/586712. [DOI] [PubMed] [Google Scholar]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed 12/1/2009];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 11-3-2008. [Google Scholar]
- 4.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 5.National Department of Health South Africa. National Antiretroviral Treatment Guidelines. First Edition. Jacana: 2004. [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach (2006 revision) World Health Organization; 2006 [PubMed]
- 7.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study [In Process Citation] BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 9.May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiebaut R, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin CA, Phillips AN. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV-1-infected patients? Curr Opin Infect Dis. 2009;22:191–197. doi: 10.1097/qco.0b013e328326cd34. [DOI] [PubMed] [Google Scholar]
- 11.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 12.Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, Martinson NA, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips AN, Gazzard BG, Clumeck N, Losso MH, Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334:76–78. doi: 10.1136/bmj.39064.406389.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernan MA, Cole SR, Philippakis A, Robins JM. Methods for estimating the optimal time of HAART initiation. International Conference on AIDS; 2002. abstract no. TuPeC4844. [Google Scholar]
- 16.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O'Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan JE, Hanson DL, Cohn DL, Karon J, Buskin S, Thompson M, et al. When to begin highly active antiretroviral therapy? Evidence supporting initiation of therapy at CD4+ lymphocyte counts <350 cells/microL. Clin Infect Dis. 2003;37:951–958. doi: 10.1086/377606. [DOI] [PubMed] [Google Scholar]
- 18.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 19.Severe P, Fitzgerald DW The Haiti CIPRA Team. A randomized clinical trial of early versus standard antiretroviral therapy for HIV-infected patients with a CD4 T Cell Count of 200 – 350 cells/ml (CIPRAHT001). 49th Interscience Conference on Antimicrobial Agentsand Chemotherapy; 9-14-2009; San Francisco. Abstract H-1230c. [Google Scholar]
- 20.Questions and Answers: The CIPRA HT 001 Clinical Trial. National Institute of Allergy and Infectious Diseases, National Institutes of Health; 2009 Jun 8;
- 21.World Health Organization. Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents. Switzerland: 2009 [PubMed]
- 22.Wood R, Fox M, Conradie F, Cornell M, Dehlinger M, Heilberg C, et al. Nurse management is not inferior to doctor management of ARV patients: the CIPRA South Africa randomized trial. IAS 2009 Cape Town, South Africa[Abstract LBPED03] 2009 [Google Scholar]
- 23.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 26.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20:1163–1169. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–342. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane HC, Neaton JD. When to start therapy for HIV infection: a swinging pendulum in search of data. Ann Intern Med. 2003;138:680–681. doi: 10.7326/0003-4819-138-8-200304150-00018. [DOI] [PubMed] [Google Scholar]
- 30.Cole SR, Li R, Anastos K, Detels R, Young M, Chmiel JS, et al. Accounting for leadtime in cohort studies: evaluating when to initiate HIV therapies. Stat Med. 2004;23:3351–3363. doi: 10.1002/sim.1579. [DOI] [PubMed] [Google Scholar]