Abstract
Vitamin C (ascorbic acid, AA) depletion during pre-natal and post-natal development can lead to oxidative stress in the developing brains and other organs. Such damage may lead to irreversible effects on later brain function. We studied the relationship between AA deficiency and oxidative stress during development in gulonolactone oxidase (gulo) knockout mice that are unable to synthesize their own ascorbic acid. Heterozygous gulo(+/−) mice can synthesize AA and typically have similar tissue levels to wild-type mice. Gulo(+/−) dams were mated with gulo(+/−) males to provide offspring of each possible genotype. Overall, embryonic day 20 (E20) and post-natal day 1 (P1) pups were protected against oxidative stress by sufficient AA transfer during pregnancy. On post-natal day 10 (P10) AA levels were dramatically lower in liver and cerebellum in gulo (−/−) mice and malondialdehyde (MDA) levels were significantly increased. In post-natal day 18 pups (P18) AA levels decreased further in gulo(−/−) mice and oxidative stress was observed in the accompanying elevations in MDA in liver, and F2-isoprostanes in cortex. Further, total glutathione levels were higher in gulo(−/−) mice in cortex, cerebellum and liver, indicating that a compensatory antioxidant system was activated. These data show a direct relationship between AA level and oxidative stress in the gulo(−/−) mice. They reinforce the critical role of ascorbic acid in preventing oxidative stress in the developing brain in animals that, like humans, cannot synthesize their own AA.
Keywords: Vitamin C, Oxidative stress, Gulo, Brain, Development
1. Introduction
Vitamin C (ascorbic acid; AA) is elevated during fetal- and post-natal-development in humans and rodents (Kratzing and Kelly, 1982; Kratzing et al., 1985). AA is important during pregnancy for the production of collagen to form strong membranes and low AA is linked to increases in premature rupture of the membranes (PROM) and preterm labor (Rumbold and Crowther, 2005; Woods et al., 2001). However, preferential sequestration of AA in the brain implies a further role for AA in brain development that has been suggested (Rice, 2000) but is not yet not fully understood. The importance of AA deficiency in the brain is highlighted by the finding that mice lacking the Sodium Vitamin C Transporter (SVCT2) responsible for transferring AA from the blood to the brain die at birth following respiratory failure. They show cerebral hemorrhage, although the exact cause of death is not known (Sotiriou et al., 2002).
Low AA intake may be especially dangerous in neonates due to rapid growth and development and low tissue reserves (Lykkesfeldt et al., 2007). Although it is often assumed that poor nutrition is rare in developed societies, hypovitaminosis for vitamin C may be more prevalent than is currently believed. In 95 pregnant women (in Arizona, USA) in a generally healthy outpatient sample of 350 women, 5.3% had deficient- (<11.4 μM) and 26.3% had depleted-AA levels (<28.4 μM) (Johnston and Thompson, 1998). Salmenpera et al. concluded that marginal intake of AA was more common than expected in a Finnish sample of 200 lactating women and that 6% of maternal plasma AA levels in their study were at deficient levels (<14.1 μM) despite no symptoms of clinical deficiency in the mothers (Salmenpera, 1984). The likelihood of consuming insufficient AA, especially during pregnancy and nursing, is greater in those from low socioeconomic groups (Madruga de Oliveira et al., 2008) and undeveloped countries where diet is further influenced by harvests and rainy seasons (Bates et al., 1983). Furthermore, AA levels tend to decrease as pregnancy progresses and during lactation (Casanueva et al., 2005; McGanity et al., 1958; Poston et al., 2006), highlighting an even greater need for adequate intake to avoid deprivation to the mother and fetus. Given these data, it is important to establish the consequences of low AA on the developing brain.
Most mammals synthesize AA from glucose. Humans and primates, along with guinea pigs and some other mammals and birds, do not possess the functioning gene for the enzyme (gulonolactone oxidase) responsible for the final step in this process and are thus reliant on dietary AA in order to prevent scurvy. A mouse line in which the gulo gene is inactive has recently been created (Maeda et al., 2000) permitting study of the effects of varying maternal AA level on fetal development in mice. In normal rodents, vitamin C synthesis begins in the fetus around day 16 of a 21-day gestation period (Kratzing and Kelly, 1982). In the case of gulo(−/−) mice, fetuses are dependent on maternal supply of AA via the placenta and umbilical cord and later via the milk, until weaning at 21 days. We recently reported that gulo(−/−) mice from gulo(−/−) mothers have sensorimotor deficits and elevated cortical oxidative stress as adults despite sufficient AA intake after weaning (Harrison et al., 2008). Although these changes could be partially attributed to some effect of the inactivation of gulo we have no evidence for this and we hypothesized that irreversible damage occurred in these mice due to inadequate levels of AA during development and that the damage may be due to elevated oxidative stress that results from diminished antioxidant capacity. In this work we show that during nursing, when the brain continues to develop, gulo(−/−) mice have increased oxidative stress that reflects significant decreases in tissue AA levels.
2. Results
2.1 Litter demographics
Demographic data were determined from 4 to 8 litters per age group as follows (−/−):(+/−):(+/+): E20, 4 litters - 15:18:6; P1, 5 litters - 10:22:7; P10, 4 litters - 5:16:6; P18, 8 litters - 8:23:14. Genotype ratios among the pups did not differ from the expected 1:2:1 Mendelian ratios at any age (Ps >0.05).
2.2 AA levels in E20 mice and gulo(+/−) dam
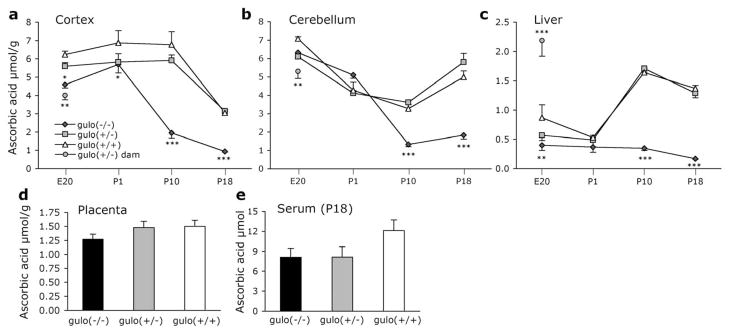
AA levels in the dam were lower than those seen in the fetuses, in both cortex and cerebellum (P<0.002; Fig. 1a,b). Liver AA levels did not differ among the fetuses, but were significantly elevated in dams (P<0.001; Fig. 1c). AA levels did not differ in the placenta according to genotype (P=0.256; Fig. 1d).
Figure 1. Ascorbic acid measurements in gulo(+/−) dams and offspring.
AA in (a) cortex, (b) cerebellum, and (c) liver of gulo(+/−) dams and offspring at each age measured, (d) placenta from E20 mice, and (e) serum from P18 mice. AA decreased with age in each genotype in (a) cortex, P<0.001, and in (b) cerebellum, P<0.05. AA increased with age in gulo(+/+) and gulo(+/−) in liver P<0.01. *P<0.05 different from gulo(+/+) mice only; **P<0.01, and ***P<0.001 from all other groups at particular age.
2.3 AA levels and age
A 4 (age) X 3 (genotype) ANOVA was conducted for cortex, cerebellum and liver. In each case, there were significant main effects of age and genotype and a significant interaction between the factors (P<0.001). Follow-up comparisons were then conducted to reveal where differences lay.
2.3.1 Cortex
There were significant effects of genotype at each age (P<0.05; Fig. 1a). At E20 and P1 gulo(−/−) mice had lower AA than gulo(+/+) littermates (P<0.05). An intermediate AA level in gulo(+/−) mice did not differ from either other genotype. In P10 mice, the difference between gulo(−/−) and the other two genotypes had increased (P<0.001), and was even greater at P18 at which point gulo(−/−) exhibited far lower levels than littermates (P<0.001). AA decreased from high prenatal levels in each genotype (P<0.001). In gulo(−/−) mice the decrease began after P1 with significant differences by P10 (P<0.001) and a further decrease at P18 that was not significant (P=0.37). For both gulo(+/−) and gulo(+/+) mice AA in the cortex remained high until the final measurement at P18 which was significantly lower than P10 (P<0.001).
2.3.2 Cerebellum
At E20 and P1 there were no differences among the littermates indicating that gulo(−/−) mice obtained sufficient AA from the dam in utero (Fig. 1b). At P10 and P18 gulo(−/−) had less stored AA than both gulo(+/+) and gulo(+/−) littermates (P<0.001). Post-natal AA decreases were seen in cerebellum in each genotype. In gulo(−/−) mice AA decreased at each age point measured compared to the previous measurements (P<0.05). In both gulo(+/−) and gulo(+/+) there were significant decreases from E20 to P1 (P<0.001). The smaller decrease noted at P10 was not different from P1 mice. There was a slight increase in cerebellar AA in P18 AA-synthesizing mice compared to P10 mice (P<0.001) although this was not different from P1 values (P>0.19). It is unknown whether this represents an upregulation in AA synthesis to deal with some additional stress in this area or not.
2.3.3 Liver
At E20 the only group difference was between gulo(−/−) and gulo(+/+) mice (P<0.01; Fig. 1c). At P1 there were no differences among the genotypes. At both P10 and P18 gulo(−/−) liver AA levels were significantly lower than gulo(+/−) or gulo(+/+) (P<0.001). Gulo(−/−) mice liver AA levels decreased with age but differences were not significant at any age. In gulo (+/−) mice significant increases were observed at P10 and at P18 (P<0.01). A similar increase was seen in gulo(+/+) mice from P1 to P10 (P<0.001) but no further change was observed at P18.
2.3.4 P18 mice
Blood serum AA did not vary among the genotypes (Ps >0.05; Fig. 1e). Of particular note is that gulo(+/−) and gulo(+/+) AA levels did not vary at any age in any tissue type.
2.4 MDA
In cortex from E20 mice, similar MDA levels were observed in tissue from fetuses and dams (P =0.738; Table 1). In contrast, MDA levels were elevated in cerebellar tissue from fetuses compared to dams and differed among fetal genotypes (P=0.004). In cerebellum the highest MDA levels were detected in gulo(−/−) and gulo(+/−) embryos, but MDA in gulo(+/+) fetuses was not elevated above dams. MDA levels in liver were higher in the fetuses than in the dam (P<0.05). There were no differences among the embryonic genotypes. MDA did not vary among the genotypes in the placenta (P=0.256). In P1 mice MDA levels were also similar across genotypes in each of the tissues measured (Ps >0.422). In P10 mice cortical MDA levels were similar among the three genotypes (P=0.480). In both cerebellum and liver MDA was elevated in gulo(−/−) pups compared to littermates (P<0.05). In P18 mice MDA levels did not differ among groups in the cortex or cerebellum despite the large differences in AA levels (Ps >0.22). In the liver MDA levels were significantly elevated in gulo(−/−) mice (P<0.01).
Table 1.
Malondialdehyde in Gulo(+/−) dams and offspring
| MDA/nmol/g | gulo(−/−) | gulo(+/−) | gulo(+/+) | gulo(+/−) dam | |
|---|---|---|---|---|---|
| E20 | Cortex | 0.42 (± 0.05) | 0.38 (± 0.06) | 0.33 (± 0.04) | 0.42 (± 0.06) |
| Cerebellum | 0.77 (± 0.09)a | 0.63 (± 0.07)a | 0.57 (± 0.04)a,b | 0.29 (± 0.03)b | |
| Liver | 0.79 (± 0.08)a | 0.70 (± 0.08)a,b | 0.72 (± 0.22)a,b | 0.27 (± 0.03)b | |
| Placenta | 0.31 (± 0.02) | 0.28 (± 0.03) | 0.35 (± 0.04) | ||
| P1 | Cortex | 0.56 (± 0.20) | 0.62 (± 0.26) | 0.31 (± 0.02) | |
| Cerebellum | 0.79 (± 0.06) | 0.82 (± 0.04) | 0.78 (± 0.06) | ||
| Liver | 0.85 (± 0.09) | 0.88 (± 0.14) | 0.69 (± 0.08) | ||
| P10 | Cortex | 0.31 (± 0.04) | 0.25 (± 0.02) | 0.27 (± 0.03) | |
| Cerebellum | 0.37a (± 0.02) | 0.25b (± 0.02) | 0.23b (± 0.02) | ||
| Liver | 0.66a (± 0.12) | 0.27b (± 0.04) | 0.33b (± 0.03) | ||
| P18 | Cortex | 0.69 (± 0.06) | 0.65 (± 0.05) | 0.62 (± 0.08) | |
| Cerebellum | 0.90 (± 0.08) | 0.78 (± 0.12) | 0.66 (± 0.19) | ||
| Liver | 0.60a (+0.05) | 0.44b (± 0.02) | 0.45b (± 0.03) |
Data shown are Mean (± S.E.M). E20 fetuses data are for 9 gulo(−/−), 9 gulo(+/−), 6 gulo(+/+) fetuses and 5 gulo(+/−) dams. P1 data are for 8 gulo(−/−), 8 gulo(+/−), and 7 gulo(+/−) pups. P10 data are for 5 gulo(−/−), 7 gulo(+/−), and 6 gulo(+/−) pups. Data are expressed per gram tissue (wet weight). Values in the same row that do not share superscript are significantly different.
P<0.05
P<0.001
2.5 Other measures of oxidative stress in P18 mice
2.5.1 GSH
Total GSH was measured to assess antioxidant response to oxidative stress load. Higher total GSH levels were observed in gulo(−/−) mice in cortex, cerebellum, and liver (Ps <0.05), and in gulo(+/−) mice in cortex and liver (Table 2). GSH is oxidized to GSSG under conditions of oxidative stress so we also measured GSSG and assessed the GSH/GSSG ratio as a measure of oxidative stress. GSH/GSSG ratios did not differ systematically among genotypes in any area tested (data not shown).
Table 2.
Further measures of oxidative stress in P18 mice.
| gulo(−/−) | gulo(+/−) | gulo(+/+) | ||
|---|---|---|---|---|
| Total glutathione | Cortex | 59.24c (± 1.32) | 54.50c (± 0.92) | 40.0d (± 3.02) |
| nmol/g | Cerebellum | 75.17a (± 2.12) | 69.45ab (± 3.03) | 63.02b (± 2.34) |
| Liver | 51.09a (± 2.98) | 50.24a (± 2.59) | 39.22b (± 2.85) | |
| F2-Isoprostanes | Cortex | 6.83a (± 0.58) | 5.02b (± 0.25) | 5.49b (± 0.15) |
| ng/g | ||||
| F4-Neuroprostanes | Cortex | 115.58 (± 9.83) | 108.60 (± 14.70) | 134.39 (± 10.62) |
| ng/g | ||||
| Protein Carbonyls | Cortex | 1.58 (± 0.06) | 1.62 (± 0.03) | 1.64 (± 0.10) |
| nmol/mg protein | Cerebellum | 1.99 (± 0.25) | 2.11 (± 0.17) | 2.19 (± 0.17) |
| Liver | 2.85 (± 0.12) | 2.61 (± 0.12) | 2.51 (± 0.08) | |
| Sulfhydryls | Cortex | 922.39 (± 25.6) | 931.13 (± 27.26) | 906.02 (± 29.95) |
| nmol TNB/mg | Cerebellum | 911.73 (± 25.15 | 910.34 (± 32.42) | 919.59 (± 39.57) |
| protein | Liver | 1435.89 (± 64.44) | 1489.21 (± 67.85) | 1483.72 (± 55.57) |
Data shown are Mean (± S.E.M) for 8 gulo(−/−), 10 gulo(+/−), and 8 gulo(+/+) pups for AA, MDA and GSH and 5 of each genotype for F2-Isoprostanes and F4-Neuroprostanes and 6 gulo(−/−), 7 gulo(+/−), and 5 gulo(+/+) pups for GSH/GSSG ratio, protein carbonyls and sulfhydryls. Data are expressed per gram tissue (wet weight) or per mg of protein as noted. Values in the same row that do not share superscript are significantly different.
P<0.05
P<0.001
2.5.2 F2-isoprostanes
Greater levels of F2-isoprostanes were seen in gulo(−/−) mice than in gulo(+/−) or gulo(+/+) littermates (P<0.05; Table 2), but F4-neuroprostanes did not differ among the genotypes (P=0.32).
2.5.3 Protein carbonyls and sulfhydryls
High oxidative stress is marked by elevated protein carbonyl measurements, but lower numbers of sulfhydryl thiol groups reacting with DTNB. In P18 mice, these measures were not significantly different among tissue types in any of the tissues measured (P>0.05; Table 2). However, there was a trend towards increased carbonyls and reduced sulhydryl measurements in the liver in gulo(−/−) mice, similar to the changes observed with MDA, although in this case, the differences were not significant.
2.6 Immunohistochemistry
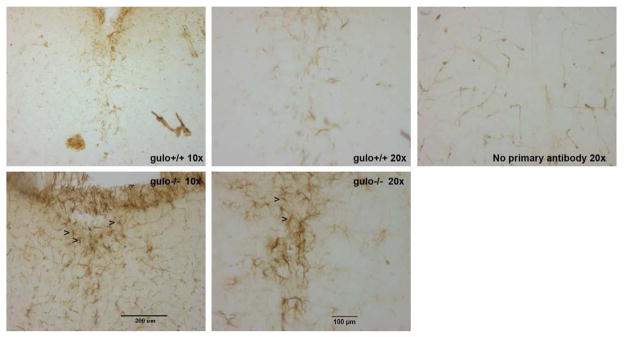
Astrocytes, stained positive for GFAP, were clearly discernible throughout the brain. Large numbers were seen in hippocampus and cerebellum among other areas but did not differ between gulo(−/−) and gulo(+/+) littermates. More GFAP-positive cells were seen in gulo(−/−) mice compared to gulo(+/+) mice in brain stem although the distribution was not formally quantified (Fig. 3).
Figure 3. Immunohistochemical staining for glial fibrillary acidic protein (GFAP) in brain stem of P18 gulo(−/−) and gulo(+/+) littermates.
The area shown is the area under the 4th ventricle in (a, b, c) gulo(+/+) and (d, e) gulo(−/−) mice, at 10x (a, d) and 20x (b, c, e) magnifications. Some staining of blood vessels by the secondary antibody is also visible (c) but astrocytes are clearly discernable, multiple exemplars are marked with arrows (>) in panels d and e.
2.7 Behavioral measures
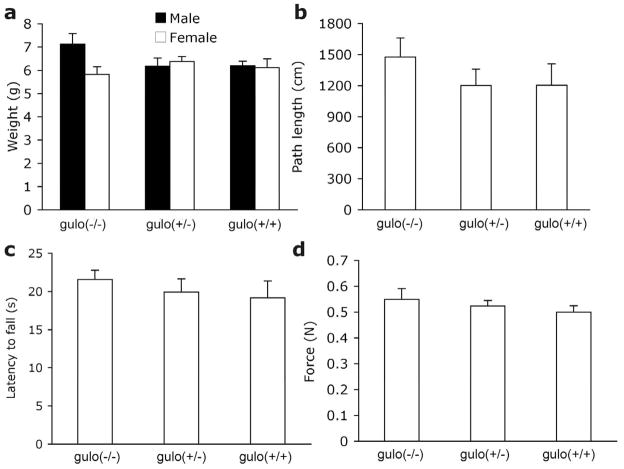
There was no effect of gender on any of the following measures so data were collapsed across gender (Ps>0.43). Weight did not differ among the genotypes (P=0.903; Fig. 2a) despite differing AA levels, indicating that the gulo(−/−) mice were not suffering from scurvy. Data were unavailable from six mice in the locomotor activity chambers (four gulo(+/−) and two gulo(+/+)) due to corrupted data files. Distance traveled, mean speed, and time spent resting were also similar among the genotypes (Ps>0.40; Fig. 2b). Some mice were unable to perform the inverted screen (one gulo(−/−), one gulo(+/−) and one gulo(+/+)) and were excluded from analyses. There were no differences among littermates in agility and strength as measured by the inverted screen and grip tasks (Ps>0.54; Fig. 2c-d).
Figure 2. Behavioral data from P18 mice.
18-day-old mice did not differ according to genotype on measures of (a) Weight, (b) path length, (c) inverted screen latency to fall, (d) grip strength.
3. Discussion
In weanling gulo(−/−) mice that, like humans, cannot synthesize AA there is a direct relationship between AA levels and oxidative stress in brain and liver. These changes occurred without weight loss, indicating potentially damaging increases in oxidative stress in the absence of clinical signs of scurvy. In fact scurvy may be avoided with only a very small amount of AA intake (10 mg/day in humans). The more subtle roles of AA for antioxidant protection, neuromodulation and regulation of gene expression clearly require greater amounts of AA (Grano and De Tullio, 2007; Rebec and Pierce, 1994; Rice, 2000).
Gulo(+/−) and gulo(+/+) offspring have two sources of AA; from the dam (via placenta and later presumably via milk) and synthesis in liver. In keeping with the diminished antioxidant capacity in gulo(−/−) mice lower AA levels were observed in gulo(−/−) E20 fetuses in both cortex and cerebellum. Higher MDA was seen in cerebellum of the gulo(−/−) fetuses. This relationship between low AA and higher MDA was preserved at post-natal day 10 in cerebellum and liver where AA levels were 2.5- and 4.7-fold lower, respectively, in gulo(−/−) than in gulo(+/+) littermates. Later in the post-natal developmental period, in P18 mice, MDA levels were elevated in gulo(−/−) liver, but no longer in brain despite the fact that gulo(−/−) cortex and cerebellum AA levels were approximately 30% of gulo(+/+) littermates. Total GSH increased in gulo(−/−) compared to gulo(+/+) mice in cortex, cerebellum and liver, with intermediate increases in gulo(+/−) pups. GSH synthesis increases under conditions of oxidative stress such as low AA, presumably in order to regulate antioxidant capacity (Meister, 1992) and thus the increases seen here represent a protective response in the face of oxidative challenge. The increase in reduced GSH provides a probable explanation for the lack of change in GSH/GSSG ratio despite presumed increases in GSH reduction to GSSG. Confirmation of the increase in oxidative stress in the brain was seen in significantly elevated F2-isoprostanes in cortex of gulo(−/−) mice. F4-neuroprostanes did not differ among genotypes. There is some redundancy of antioxidant systems in the body to allow these compensation mechanisms to exist together and prevent permanent damage arising from small changes in intake or oxidant stressors in the environment. It is not known whether the oxidative stress changes reported here could have been rectified with another antioxidant supplement or if AA itself is required to maintain homeostasis.
Greater MDA levels were observed in cerebellum than cortex at E20, P1 and P18, despite higher AA in cerebellum at E20 and P18. Elevated MDA was not observed in the cortex at any age despite large discrepancies in AA levels among genotypes. Thus cortex and cerebellum may have different protective and/or oxidative stress mechanisms. Each assay, F2-isoprostanes, F4- neuroprostanes, MDA, protein carbonyls and sulfhydryls measures a different endpoint in oxidative stress processes and thus may be differentially affected in different tissue types. The markers of lipid peroxidation, MDA and especially isoprostanes are much more sensitive measures of oxidative damage, particularly in the earlier stages of oxidative damage. A key antioxidant protecting lipid membranes is vitamin E (tocopherol). Oxidized tocopherol radicals are recycled by AA, thus AA-deficiency will also impact the tocopherol antioxidant defense system and lead to additional damage to lipids. The brain has far greater levels of docosahexaenoic acid, of which F4- neuroprostanes are the end point in oxidative damage, than arachidonic acid, of which the end point of oxidative damage is production of F2-isoprostanes. This disparity is reflected in the 20-fold differences in levels of the two prostanoid derivatives seen in this study and thus, simple quantitative differences may preclude detection of change except under even greater oxidant stressor situation. A low AA diet in young guinea pigs decreased AA levels in tissues and increased MDA in the brain, although not in liver, while protein carbonyls increased in both tissues (Lykkesfeldt et al., 2007). Overall antioxidant homeostasis was also disrupted. Together, these data strongly suggest that sufficient AA intake is required during critical developmental periods in order to protect against neuronal damage due to oxidative stress.
Astrocytes have an important protective role in the brain under conditions of oxidative stress, including the recycling of dehydroascorbic acid back to the reduced form of vitamin C (reviewed (Wilson, 1997)). They may therefore be expected in higher numbers in areas undergoing oxidative stress. We found evidence of GFAP-positive cells throughout the brains of both gulo(−/−) and gulo(+/+) mice but genotypes only seemed to differ in the brain stem. These data suggest the possibility that low AA and increased oxidative stress lead to activated microglia and inflammatory response. We did not quantify these differences and neither did we assay all different regions of the brain. This would be an appropriate area for future research in order to fully understand how neuronal function is compromised by nutritional deficiency, and whether it can be reversed.
Brain AA was higher in gulo(+/+) fetuses than dams and did not approach adult levels until the end of the developmental period studied. Gulo(+/+) E20 brain AA was slightly higher than those reported in rats of the same developmental age (Kratzing et al., 1985) and P1 cerebellum AA in gulo(−/−) and gulo(+/+) pups was similar to those reported in guinea pigs on the first day of birth (Pate, 1996). Although there appear to be slight differences across the different rodent species, the pattern of elevated AA in brain tissues during early development persists in each case. Liver AA levels were only slightly lower in gulo(+/+) and gulo(+/−) mice at P18 compared to P10. These levels were higher than is typically seen in adult wild-type mice (0.8–1 μmol/g (Harrison and May, 2009; Harrison et al., 2008)) suggesting that young mice synthesize higher levels of AA than adults, further supporting an important role for AA in development. Gulo (+/−) dams were supplemented with 0.33g/l AA during the pregnancy to ensure adequate supply under conditions of increased demand. AA levels in liver in dams were at least two-fold greater than usually seen in non-pregnant wild- type mice (~0.8–1.0 μmol/g). This increase is greater than would be expected from the level of supplementation alone and indicates increased AA synthesis during pregnancy (Corpe et al., 2010). This up-regulation of AA synthesis is presumably to cope with the demands of fetuses which, even in gulo(+/−) and gulo(+/+) fetuses are reliant on maternal AA supply until at least E16 or E17 (Kratzing and Kelly, 1986).
Gulo(−/−) pups, incapable of synthesizing AA at any point during their life, were for the most part protected in utero by a sufficient supply of AA from the mother via the placenta and umbilical cord. The gulo(+/−) dam was able to synthesize some AA, plus received AA supplements in the water, and therefore all placental tissues were adequately supplied. All three genotypes had similar placental AA levels and MDA levels did not differ among littermates, reflecting adequate maternal AA status. Critical for comparison purposes, both humans and mice have the same type of placenta, hemochorial, in which the maternal blood comes into direct contact with the chorion, permitting enhanced transfer of proteins and nutrients to the fetus. P1 pups were sacrificed within 12 hours of birth and the data show that the AA stored in tissues during in utero development persisted at the same high level during this time. Therefore, any potential oxidative stress generated during delivery was not severe enough to cause any differences in AA or MDA levels among genotypes of P1 littermates.
Human milk contains AA and is sensitive to maternal intake levels (Bates et al., 1983; Friel et al., 2002; Salmenpera, 1984), as is guinea pig milk (Bates et al., 1988). Mouse milk contains much smaller amounts of AA, presumably owing to the ability of wild-type pups to synthesize their own (FE Harrison, ME Meredith & JM May, unpublished data). With the loss of the gulo gene, humans and other gulo-lacking animals may have evolved ways to compensate for the loss of this ability in order to protect developing offspring. The approximately 70% that comes from in situ synthesis in gulo(+/+) mice (based on the difference between gulo(−/−) and gulo(+/+) littermates) is critical to maintaining the high AA needed during development. It appears that some AA was passed to the gulo(−/−) pups because 21 days of absolute AA deprivation in an adult gulo(−/−) mouse results in cortex AA levels approaching 20 % of those seen in the P18 gulo(−/−) mice (FE Harrison & JM May unpublished data). Although AA levels continued to decrease in gulo(−/−) mice, they were not scorbutic, and the decrease in gulo (−/−) cortex levels over this time was less than that of gulo(+/+) littermates (~1.0 μmol/g compared to 3.7 μmol/g). AA in gulo(−/−) liver approached zero and thus it is possible that the brain was siphoning AA away from other organs to protect brain levels, but also that some AA was still being ingested, presumably from the milk. These hypotheses are supported by the finding of no difference among groups in serum AA levels. Serum AA levels often show high variability due to the hemolysis in the sample. Nevertheless, gulo(−/−) mice had similar serum AA levels to their littermates indicating that there was some AA intake in these animals. It is estimated that 12 % of AA in the CSF and 2 % of AA in the brain is replaced per hour through diffusion from CSF into blood stream and the serum values may therefore represent this recirculation of AA (Spector, 1977). Alternatively, AA from other organs may be transported back into blood stream in order for it to be made accessible to CSF.
We have previously shown that gulo(−/−) pups from gulo(−/−) dams have motor skills deficits at 11–14 weeks, despite adequate AA intake following weaning (Harrison et al., 2008). In the present study, P18 gulo(−/−) pups from gulo(+/−) dams were not impaired relative to littermates despite low AA in brain and liver. It is possible that any differences were obscured at this early age owing to poor strength and performance in all P18 pups. Mean fall latencies on the inverted screen were only 20 s, even though the apparatus had been adapted for the small size of pups. Another possibility is that the lack of deficit in these gulo(−/−) pups represents a critical difference in the ability to supply sufficient AA between gulo(−/−) dams in the original study (Harrison et al., 2008) and gulo(+/−) dams in the present study, in which greater protection is offered in both pre-natal and the immediate post-natal period. In the earlier study where the entire litter were gulo(−/−), from a gulo(−/−) dam, a situation was created with maximum stress on maternal reserves of AA and thus greater oxidative damage to offspring. Low AA in young developing guinea pigs leads to spatial memory impairments in the Morris water maze and reduced neuron numbers in the hippocampus (Tveden-Nyborg et al., 2009). In fact, a large number of changes in pre-natal environment, including hypoxia, can lead to critical changes in brain development in the fetus and result in neurological dysfunction throughout the lifespan. The changes in oxidative stress reported here represent markers for damage or change, and not a disease state itself. Future investigations must focus on the possible effects of low AA and high oxidative stress such as reduced neurogenesis or apoptosis (Verity, 1994).
We report here a direct relationship between AA and oxidative stress in young gulo(−/−) mice. We show that when the dam has a sufficient supply of AA, then the maternal environment offers good antioxidant protection and even gulo(−/−) fetuses can maintain high AA levels. This protection persists into the immediate post-natal period but not throughout the nursing period where it appears the majority of AA is normally obtained from in situ synthesis creating an unmet need in the gulo(−/−) pups. Protective mechanisms, in the form of increased GSH were observed against conditions of low AA and oxidative stress. Together, the data show that insufficient dietary AA intake while nursing can lead to oxidative damage in the brains of developing offspring, even in the absence of clinical signs of scurvy.
4. Methods
4.1 Animals
Heterozygous gulo(+/−) mice were obtained from Mutant Mouse Regional Resource Centers (http://www.mmrrc.org, #000015-UCD) and were maintained on a C57BL/6J background (stock #000664; Jackson laboratories, USA). Animals were housed in breeding pairs in tub cages in a temperature- and humidity-controlled vivarium. Mice were kept on a 12:12-hour light:dark cycle with lights on at 6 AM. Mice had free access to food and water for the duration of the experiment. The use of gulo(+/−) dams ensured that normal maternal environment was maintained, since adult gulo(+/−) mice have the same AA levels as gulo(+/+) mice. Gulo(+/−) dams were also supplemented with AA in the water to make up any potential shortfall due to the additional requirements of pregnancy and nursing. Deionized water was supplemented with 0.33 g/L ascorbic acid (Sigma, USA) with 20 μl EDTA to increase stability of AA in solution. This is the standard supplement level that provides adult (non-pregnant) gulo(−/−) mice with wild-type levels of AA in tissues (Harrison et al., 2008; Maeda et al., 2000). In the present study we investigated AA levels in the offspring from matings of heterozygous gulo mice (gulo(+/−)). Each litter comprised gulo(−/−), gulo(+/−) and gulo(+/+) pups and thus permitted us to assess AA and oxidative stress levels resulting from the different genotypes from the same maternal environment, during both pre- and post-natal development.
P18 mice (post-natal day 18) that were used in behavioral assays were transferred with the dam and entire litter to a vivarium in the Vanderbilt Neurobehavioral Core Facility 1 week prior to testing to allow them to habituate to the new surroundings. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
4.2 Behavioral procedures
Behavioral testing was undertaken only with P18 mice. Prior to testing the dam was removed from the cage and the home cage was used to transport the pups to the behavioral testing room. This served to minimize the stress that would be caused by transferring the litter to a clean cage when removed from the dam. Tasks were conducted in the order described below. Total test time per mouse was less than 15 minutes and testing took place across a 2-hour period to minimize the effects of tiredness in the mice. Following testing, the dam was returned to the home cage and the pups and dam were left undisturbed for at least 2 hours before sacrifice. Genotyping was conducted post-mortem and thus the experimenter was blind to genotype during behavioral testing. Behavioral testing was undertaken in eight gulo(−/−) mice, 19 gulo(+/−) mice and 13 gulo(+/+) mice.
Weight
Mice were weighed on a standard gram scale and identified as male or female.
Locomotor activity
Mice were then placed in commercially-available activity monitors (27 × 27 cm; Med Associates, Georgia, VT) and activity was monitored for 10 min. via the breaking of infrared beams. Total distance moved, time stationary, and speed of movement were calculated.
Grip strength
Grip strength was measured using a grip strength system designed for use with mice (San Diego Instruments, San Diego, CA). Each mouse was given five trials in which the mouse was suspended by the tail over a wire grid until able to grab hold of the wire bars with their front paws. The mouse was then pulled gently backwards in a horizontal plane and the maximum grip force created was measured automatically by the equipment.
Inverted screen
Mice were suspended from an inverted wire mesh and latency to fall was recorded. The protocol was identical to that described in (Harrison et al., 2008) except that a smaller wire mesh (0.5 × 0.5 cm) was used for the pups than that used for adult mice. Mice were given three trials with a maximum trial time of 60 s. and an inter-trial interval of approximately 3 min.
4.3 Sacrifice and tissue removal
Pups were sacrificed at embryonic day 20 (E20), or post-natal day 1, 10 or 18 (P1, P10, P18). To obtain samples from E20 mice, pregnant dams were fully anaesthetized using inhaled isoflurane and sacrificed by decapitation. Embryos were delivered by caesarian section and placed on a Petri dish on ice. Embryos were then quickly decapitated. P1 pups were placed in iced water to dull nociception and induce hypothermia prior to decapitation. P10 and P18 pups were anaesthetized using inhaled isoflurane and sacrificed by decapitation. Liver, cortex and cerebellum were removed from each animal and tissues were quickly frozen and stored at −80 °C until needed. In addition to these tissues the placenta for each E20 animal was also removed and stored as above. Trunk blood was obtained from P18 mice following decapitation and was kept on ice for 1 h before it was spun for 20 m at 13 000 rpm at 4 °C. Serum was removed and stored at -80 °C. Due to the small size of tissue samples in E20 and P1 animals ‘cortex’ comprised the whole brain excluding the cerebellum and olfactory bulbs, in all other mice the cortex itself was dissected from other brain tissues. Tail was also removed from each pup for post-mortem determination of genotype.
4.4 Neurochemical methods
Ascorbic acid
Neurochemical measurements were conducted in 5–9 mice per group. AA levels were measured in cortex, liver and blood serum. Concentrations were measured by ion pair HPLC and electrochemical detection as previously described with tetrapentyl ammonium bromide used as the ion pair reagent (Harrison et al., 2008; Pachla and Kissinger, 1979).
F2-Isoprostanes and F4-Neuroprostanes
These were measured in cortical tissue using methods previously described by (Roberts et al., 1998). Small pieces of tissue was dissected on ice and immediately frozen in liquid nitrogen and stored at −80 °C until measurements were made. Briefly, esterified F4-neuroprostanes were quantified by stable isotope dilution negative ion chemical ionization gas chromatography mass spectrometry (GC/MS) using [2H4]-15-F2t- isoprostane as an internal standard.
Malondialdehyde
MDA was assessed as thiobarbituric acid reactive substances and was quantified to detect any short-term changes in lipid peroxidation following treatments using methods based on (Sabharwal and May, 2008) but modified for use with 96 well microtiter plates as in (Harrison et al., 2009).
Glutathione (GSH and GSSG)
Total glutathione (GSH + oxidized form glutathione disulfide (GSSG)) was measured according to the methods of (Rahman et al., 2006) using a spectrophotometric/microplate reader assay method. Briefly, weighed samples were homogenized in 0.5 ml of homogenization buffer; liver in ice-cold 5% metaphosphoric acid and brain in 0.6% sulfosalicylic acid–Triton-X solution. Samples were centrifuged and the supernatant diluted 1 in 4 with homogenization fluid. Sample or GSH standard (20 μl) was added to separate wells of a 96- well microtiterplate. 5,5′-dithiobis-(2-nitrobenzoic acid) DTNB and glutathione reductase solution (250 units ml−1, 120 μl) was added to the sample and after allowing 30 s for conversion of GSSG to GSH. NADPH (60 μl) was added and GSH was measured on a Spectramax M5 microplate reader (Molecular Devices, USA) at 412 nm using a kinetic measurement program that took one reading every 30 s for 2 ½ min. The same tissue homogenates were used to measure GSSG alone. 2 μl of 2-vinylpyridine was added to 100 μl of sample and left to react (to derivatize GSH) for 1 hour. 6 μl of triethanolamine was added to the sample and left for 10 min. for neutralization. These samples were then assayed for GSH as described above, but since any GSH originally in the sample should have been removed by the 2-vinylpyridine step, only GSSG then converted to GSH should be measured.
Protein Carbonyls
Weighed samples were homogenized by hand in 20 mM sodium phosphate buffer (pH 7.4) containing 140 mM potassium chloride (10 μl/mg). Samples were then centrifuged at 750 g for 10 min. at 4 °C. Quantification of protein carbonyls was then performed by allowing samples to react with 2,4-Dinitrophenylhydrazine (DNPH) and measuring the absorbance at 370 nm on a Spectramax M5 microplate reader (Molecular Devices, USA) following the methods described in (Hawkins et al., 2009). Protein concentration of each sample was also recorded at 280 nm in control samples that were not exposed to DNPH and calculated based on a standard curve prepared with BSA standards.
Sulfhydryls
Sulfhydryls were calculated by reaction between the thiol groups and DTNB. Oxidation reactions damage the sulphur-hydrogen bonds of the thiol groups and thus less TNB is found in oxidized samples. Measurements were made using the same tissue homogenate that was used to protein carbonyl measurements, based on the methods described in (Aksenov and Markesbery, 2001; Sgaravatti et al., 2009) and modified for use with 96 well microtiter plates. 12 μl of sample (diluted 1:1 with homogenization buffer) or blank (no tissue) was added to two separate wells. 240 μl of PBS buffer (pH 7.4) containing 1 mM EDTA was then also added to each well. 7.2 μl of 10 mM DTNB dissolved in 0.2 M potassium phosphate solution (pH 8) was added to one of each pair of wells, and potassium phosphate solution alone was added to the second well. Samples were incubated in the dark at room temperature for 30 min. TNB absorbance was then measured on a Spectramax M5 microplate reader (Molecular Devices, USA) at 412 nm, and protein concentration was also measured at 280 nm.
Immunohistochemistry
Whole brains were removed from P18 mice and immersion-fixed in 10x formalin for 5 days. They were then rinsed and stored in 1x PBS. Prior to sectioning brains were cryoprotected in 30 % sucrose. Brains were sectioned on a sliding, freezing microtome (Leica) at 45 microns and sections were stored in cryoprotectant solution (30% ethylene glycol with 25% glycerol) at −20 °C until stained.
Glial fibrillary acidic protein (GFAP) is found specifically in the brain in astrocytes and indicates a response to inflammatory damage. All washes and reactions were performed at room temperature on a shaker unless stated otherwise. Free-floating sections were first washed for five 5- min. washes in PBS. Endogenous peroxidase activity was quenched for 10 min. with hydrogen peroxide in PBS and methanol, followed by a further five 5-min. washes in PBS. Sections were incubated in a basic blocking solution (1 % horse serum and 0.5 % Tween 20 in PBS containing a blocking antibody - donkey/anti-mouse IgG; Jackson ImmunoResearch #715-505-150) for 1 hour. Sections were then placed in the primary antibody (mouse monoclonal anti-GFAP antibody (IgG1 isotype; Sigma G3893) used at a 1:10,000 dilution) for 24 hours at 4 °C. Following incubation with the primary antibody sections were washed five times for 5-min. in PBS. The secondary antibody was a biotinylated horse anti-mouse IgG (Vector laboratories #BA-2000) at a final concentration of 10 μg/ml in PBS with horse serum. Incubation with the secondary antibody was for 30 min. followed by a further three 5-min. washes in PBS. Sections were then treated with Vectastain Elite ABC reagent kit, covered, and left to incubate for 1 hour. Following three 5-min. washes in PBS sections were treated with Vectastain DAB substrate kit for 2 min. until color developed. Sections were then given several quick washes in PBS to prevent further reaction and two final 5-min. washes. Sections were then mounted on slides, cover-slipped and left to dry.
4.4 Statistics
All data were analyzed using SPSS 16.0 for Windows. Pup genotype demographics were also examined for each age group using a Chi2 test (χ2) against expected Mendelian ratio of 1:2:1. Separate Univariate ANOVAs were run for AA for each tissue type at age E20 including fetal and maternal tissues, and each age and for MDA and total GSH. GSH/GSSG ratio, protein carbonyls, sulfhydryls, weight, serum AA and behavioral tests were only measured at P18. For P1, P10 and P18 mice gulo genotype ((−/−), (+/−) and (+/+)) was the between subjects variable. For E20 mice a 4th group, the gulo(+/−) dam, was also included. Separate ANOVAs were conducted on AA levels in cortex, cerebellum and liver with age and genotype as between groups factors in order to assess the change in AA levels with age. Following significant omnibus ANOVA post hoc analyses were conducted using Bonferroni-corrected t-tests.
Acknowledgments
All behavioral testing was conducted using the facilities available in the Vanderbilt Murine Neurobehavioral Core. Immunohistochemical staining was performed by the Molecular Neurobiology and Genomics Core Service, with support from the John F. Kennedy Center for Research on Human Development. This work was supported by a grant from the National Institute of Health (NS057674 to James M May).
Abbreviations
- AA
Ascorbic acid; vitamin C
- MDA
Malondialdehyde
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett. 2001;302:141–5. doi: 10.1016/s0304-3940(01)01636-6. [DOI] [PubMed] [Google Scholar]
- Bates CJ, et al. Growth, ascorbic acid and iron contents of tissues of young guinea-pigs whose dams received high or low levels of dietary ascorbic acid or Fe during pregnancy and suckling. Br J Nutr. 1988;60:487–97. doi: 10.1079/bjn19880121. [DOI] [PubMed] [Google Scholar]
- Bates CJ, et al. The effect of vitamin C supplementation on lactating women in Keneba, a West African rural community. Int J Vitam Nutr Res. 1983;53:68–76. [PubMed] [Google Scholar]
- Casanueva E, et al. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: a randomized trial. Am J Clin Nutr. 2005;81:859–63. doi: 10.1093/ajcn/81.4.859. [DOI] [PubMed] [Google Scholar]
- Corpe CP, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010 doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel JK, et al. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res. 2002;51:612–8. doi: 10.1203/00006450-200205000-00012. [DOI] [PubMed] [Google Scholar]
- Grano A, De Tullio MC. Ascorbic acid as a sensor of oxidative stress and a regulator of gene expression: the Yin and Yang of vitamin C. Med Hypotheses. 2007;69:953–4. doi: 10.1016/j.mehy.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Harrison FE, et al. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, et al. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CL, et al. Quantification of protein modification by oxidants. Free Radic Biol Med. 2009;46:965–88. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366–70. doi: 10.1080/07315724.1998.10718777. [DOI] [PubMed] [Google Scholar]
- Kratzing CC, Kelly JD. Tissue levels of ascorbic acid during rat gestation. Int J Vitam Nutr Res. 1982;52:326–32. [PubMed] [Google Scholar]
- Kratzing CC, Kelly JD. Ascorbic acid synthesis by the mammalian fetus. Int J Vitam Nutr Res. 1986;56:101–3. [PubMed] [Google Scholar]
- Kratzing CC, et al. Ascorbic acid in fetal rat brain. J Neurochem. 1985;44:1623–4. doi: 10.1111/j.1471-4159.1985.tb08804.x. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, et al. Vitamin C deficiency in weanling guinea pigs: differential expression of oxidative stress and DNA repair in liver and brain. Br J Nutr. 2007;98:1116–9. doi: 10.1017/s0007114507787457. [DOI] [PubMed] [Google Scholar]
- Madruga de Oliveira A, et al. Plasma concentrations of ascorbic acid in parturients from a hospital in Southeast Brazil. Clin Nutr. 2008;27:228–32. doi: 10.1016/j.clnu.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Maeda N, et al. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000;97:841–6. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGanity WJ, et al. Vanderbilt cooperative study of maternal and infant nutrition. J Am Med Assoc. 1958;168:2138–45. [PubMed] [Google Scholar]
- Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol. 1992;44:1905–15. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- Pate SKL, BP, Kipp DE. Tissue vitamin C levels of guinea pig offspring are influenced by maternal vitamin C intake during pregnancy. Nutritional Biochemistry. 1996;7:524–528. [Google Scholar]
- Poston L, et al. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–54. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- Rahman I, et al. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43:537–65. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–16. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, et al. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–12. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2005:CD004072. doi: 10.1002/14651858.CD004072.pub2. [DOI] [PubMed] [Google Scholar]
- Sabharwal AK, May JM. alpha-Lipoic acid and ascorbate prevent LDL oxidation and oxidant stress in endothelial cells. Mol Cell Biochem. 2008;309:125–32. doi: 10.1007/s11010-007-9650-z. [DOI] [PubMed] [Google Scholar]
- Salmenpera L. Vitamin C nutrition during prolonged lactation: optimal in infants while marginal in some mothers. Am J Clin Nutr. 1984;40:1050–6. doi: 10.1093/ajcn/40.5.1050. [DOI] [PubMed] [Google Scholar]
- Sgaravatti AM, et al. Effects of 1,4-butanediol administration on oxidative stress in rat brain: study of the neurotoxicity of gamma-hydroxybutyric acid in vivo. Metab Brain Dis. 2009;24:271–82. doi: 10.1007/s11011-009-9136-7. [DOI] [PubMed] [Google Scholar]
- Sotiriou S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–7. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- Spector R. Vitamin homeostasis in the central nervous system. N Engl J Med. 1977;296:1393–8. doi: 10.1056/NEJM197706162962409. [DOI] [PubMed] [Google Scholar]
- Tveden-Nyborg P, et al. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.27954. [DOI] [PubMed] [Google Scholar]
- Verity MA. Oxidative damage and repair in the developing nervous system. Neurotoxicology. 1994;15:81–91. [PubMed] [Google Scholar]
- Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75:1149–63. [PubMed] [Google Scholar]
- Woods JR, Jr, et al. Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol. 2001;185:5–10. doi: 10.1067/mob.2001.115868. [DOI] [PubMed] [Google Scholar]