Abstract
Exogenous OPG gene modification appears a therapeutic strategy for osteolytic aseptic loosening. The feasibility and efficacy of a cell-based OPG gene delivery approach were investigated using a murine model of knee prosthesis failure. A titanium pin was implanted into mouse proximal tibia to mimic a weight-bearing knee arthroplasty, followed by titanium-particles challenge to induce periprosthetic osteolysis. Mouse fibroblast-like synoviocytes were transduced in vitro with either AAV-OPG or AAV-LacZ before transfused into the osteolytic prosthetic joint 3 weeks post surgery. Successful transgene expression at the local site was confirmed 4 weeks later after sacrifice. Biomechanical pull-out test indicated a significant restoration of implant stability following the cell-based OPG gene therapy. Histology revealed that inflammatory pseudo-membranes existed ubiquitously at bone-implant interface in control groups, while only observed sporadically in OPG gene-modified groups. TRAP+ osteoclasts and TNFα, IL-1β, CD68+ expressing cells were significantly reduced in periprosthetic tissues of OPG gene-modified mice. No transgene dissemination or tumorigenesis was detected in remote organs and tissues. Data suggest that cell based ex vivo OPG gene therapy was comparable in efficacy with in vivo local gene transfer technique to deliver functional therapeutic OPG activities, effectively halted the debris-induced osteolysis and regained the implant stability in this model.
Keywords: aseptic loosening, periprosthetic osteolysis, cell-based therapy, osteoprotegerin, implant stability
Introduction
Total joint replacement (TJR) is a highly successful procedure for the treatment of severe destructive arthritis. While failures of the surgery due to infections and errors have been greatly reduced in recent years, aseptic prosthetic loosening remains the most common complication of total joint replacement and represents a major problem for the long-term success and survival of prostheses.1 Wear debris-induced inflammation and osteoclastic resorption at the bone-implant interface are believed to play critical roles in the pathogenesis of aseptic loosening.2 It has been accepted that particles generated during the mechanical wear of prosthetic components are phagocytosed by macrophages and other inflammatory cells, resulting in cellular activation and release of pro-inflammatory mediators and cytokines such as interleukin-1 (IL-1β), tumor necrosis factor (TNFα), and IL-6.3 These cytokines in turn provoke cellular proliferation, promote osteoclastogenesis, and stimulate mature osteoclasts to absorb the adjacent bone.4,5 This process impacts bone remodeling around the implant and leads to osteolysis and aseptic loosening.6
The RANKL/OPG pathway plays a key role in bone metabolism and osteolysis. RANKL (receptor activator of nuclear factor NF-κB ligand) is mainly expressed by macrophages, osteoblasts, marrow stromal cells, and lymphocytes. RANKL binds to RANK, a receptor on the cell surface of osteoclasts and osteoclast precursors, resulting in proliferation, differentiation and maturation of osteoclasts, which can subsequently promote local osteolysis.7 In contrast, osteoprotegerin (OPG), a secreted protein with homology to members of the TNF superfamily, is considered to be a natural decoy receptor that profoundly modifies the effects of RANKL by inhibiting RANKL/RANK interaction.8,9 Mice genetically deficient for RANKL or RANK suffered severe osteopetrosis,10,11 and OPG transgenic mice share the same pathology,12 demonstrating that RANKL and RANK are essential for osteoclast development and OPG is a potent negative regulator for osteoclastogenesis.
Based on the anti-osteolytic nature of OPG, it appears to be a potential therapeutic agent to treat debris-associated periprosthetic bone resorption and aseptic loosening. However, the delivery of OPG to chronic periprosthetic osteolytic sites remains problematic. Current systemic anti-inflammation or anti-osteolysis therapies have several weaknesses including high dose requirements with modest efficiency, systemic side effects and the tendency for poor patient compliance13. Gene therapy, though still in its infancy, provides an attractive alternative to treat aseptic loosening since the transfer of genes may facilitate the continuous release of therapeutic proteins. Using a mouse calvaria model, Goater et al were the first to examine the potential of ex vivo OPG gene therapy with stably transfected fibroblast-like synoviocytes (FLS) expressing OPG in preventing wear debris-induced osteoclastogenesis 14. We recently reported that in vivo gene transfer of OPG effectively blocked osteoclastogenesis and reversed periprosthetic bone resorption using a newly characterized mouse model of knee prosthesis failure.15 The current study extended the investigation to evaluate the therapeutic effects of a cell-based OPG gene modification by delivering the stable OPG-transduced fibroblast-like synoviocytes (FLS) into the failing knee prosthesis, in comparison with the direct in vivo gene transfer approach. The biomechanical properties of failing knee pin-implantation following gene modification were also assessed.
Results
OPG and LacZ gene transduction efficacy in FLS
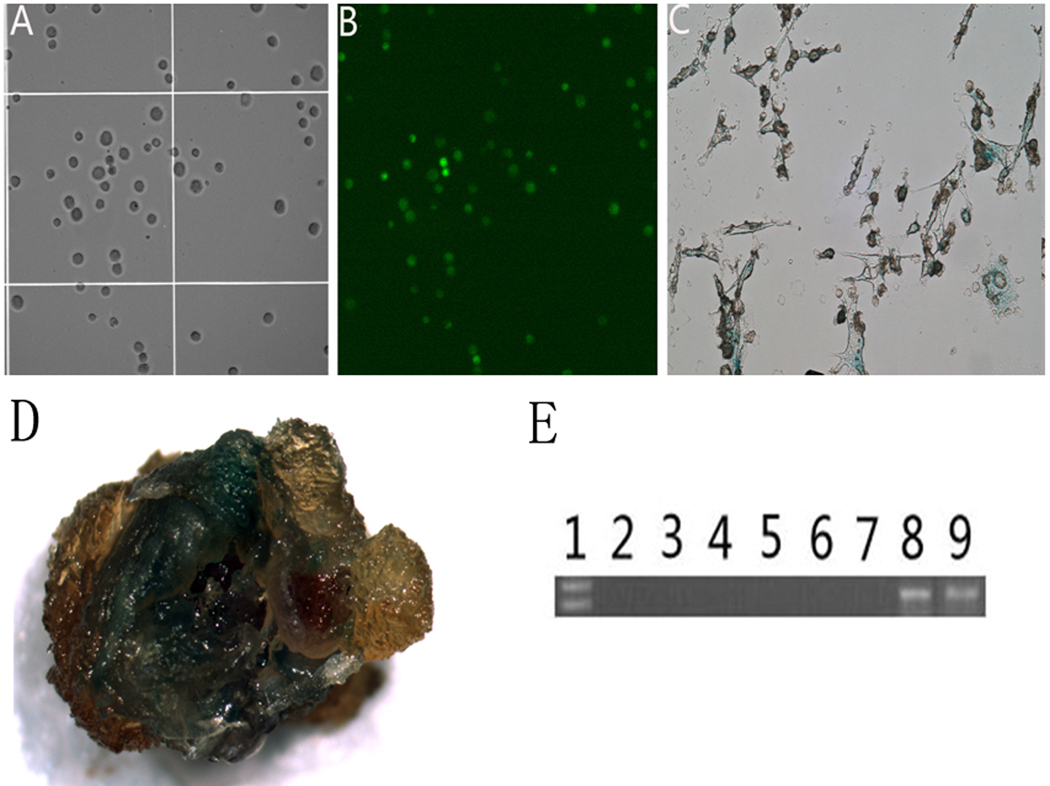
In preparation for the ex vivo gene therapy experiments, target cells stably expressing OPG and β-galactosidase (LacZ) were engineered by infecting the primary fibroblast-like synoviocytes (FLS) with AAV-OPG-EGFP or AAV-LacZ, respectively. Fluorescent microscopy identified green fluorescent signals on cells transduced with AAV-OPG-EGFP. X-gal stain was utilized to trace LacZ gene transduced cells. The transduction efficiencies in the fibroblast-like synoviocytes for both vectors were higher than 90% at day 3 and were maintained for at least 4 weeks (Figure 1A, B and C).
Figure 1.
Transgene expression in FLS cells. Panel A: bright light microscopic appearance of mouse FLS cells 3 days after AAV-OPG-EGFP transduction; Panel B illustrates fluorescent microscopy of the same cells for green fluorescent protein emission. Panels C and D show X-gal staining of in vitro transduction with AAV-LacZ on FLS cells (1C) and a macroscopic joint sample 4 weeks following cell-based gene modification. Panel E illustrates an agarose gel of conventional PCR products revealing OPG gene expression in tissues from FLS-AAV-OPG treated mice: Lane 1: DNA ladder; 2 – liver, 3 – lung, 4 – lymph node, 5 – muscle, 6 – kidney, 7 – spleen; Lane 8: prosthetic tissue homogenate; Lane 9: tissue with in vivo AAV-OPG transduction as positive control.
Outcome of surgical procedures
A custom-made titanium-pin was implanted into mouse proximal tibia to form a weight-bear knee implant. The mice tolerated surgery well and ambulated with the implanted limbs within 3 days after surgery. Injections of titanium particles and FLS into the prosthetic knees appeared to exert no influence on their daily activity. The macroscopic examination of the prosthetic joints at sacrifice revealed that the metal pins positioned in proximal tibiae with no signs of scratching or inflammation on opposing articulate surfaces. There were no obvious surgical differences between the groups to the naked eye, except that a few pin-implants from non-treated or LacZ-treated groups could be rotated or moved by hand due to loss of the fixation.
Transgene expression and dissemination
RNA extracted from the treated joints and other organs/tissues from animals with ex vivo OPG gene therapy were examined by conventional RT-PCR with human OPG primers. No positive PCR products were detected, except for the periprosthetic tissues where the ex vivo gene transfer was conducted (Figure 1E), where results were similar to in vivo OPG treatment.15 All prosthetic joint samples with ex vivo LacZ gene transfer exhibited dark blue coloration (Figure 1D). ELISA confirmed that OPG protein production in FLS-AAV-OPG culture medium was 4.20ng/72 hr/106 cells, where OPG levels in peri-prosthetic tissues from ex vivo OPG-treated groups were 2.60ng/mg total protein at sacrifice, comparable with levels observed during in vivo OPG treatment.
Implant stability assessment using the Pull-out test
The implanted pin pull-out test was performed to examine the mechanical stability of the implant following wear debris challenge and gene therapy. The average peak interfacial shear strength against pulling was 10.34±2.05, 8.14±1.23 and 7.32±1.35 N in AAV-LacZ, FLS-AAV-LacZ and virus-free non-treated groups respectively. There was no statistical difference between the three groups. However, the ex vivo and in vivo OPG gene therapies significantly increased the implant stability. The pulling forces of 21.56±2.44 and 18.19±2.10 N were required to dissociate the pin implants from tibiae, respectively (Figure 2). There was no statistical difference between the two OPG gene-modified groups.
Figure 2.
Summary of maximum pulling force required to pull out the implants at 4 weeks following treatments (* p<0.05). The insert illustrates an example trace of pulling forces applied to extract the pin implant from the surrounding bone.
Histological analysis
Histological evaluation on cross-sections of prosthetic tibiae revealed ubiquitous presence of inflammatory fibrous membranes at the bone-implant interface in both FLS-AAV-LacZ and non-treated control groups. However, the interface membranes in sections from the FLS-AAV-OPG group were dramatically thinner, and even resolved in many of the samples. Figure 3 illustrates a typical micrograph of peri-implant pseudo-membranes among the groups, and Figure 3F summarizes the measurements of membrane thickness, indicating that both in vivo and ex vivo OPG gene transfer significantly blocked the periprosthetic membrane formation. There was no significant difference between the two OPG treated groups with respect to interfacial membrane thickness (Figure 3). Modified Trichrome staining was performed to reveal changes in bone collagen content.16 While the periprosthetic bone tissues from AAV-LacZ, FLS-AAV-LacZ and virus-free groups generally exhibited weak staining of Trichrome blue coloration, OPG-gene modification using in vivo or ex vivo delivery markedly enhanced the staining as seen previously.17 Quantified intensity analysis by computerized image analysis system confirmed the statistical significance (P<0.01, Figure 4).
Figure 3.
Histological appearance of the pin-implanted tibiae at 4 weeks following in vivo and ex vivo gene modifications: (A) in vivo AAV-OPG treated; (B) cell-based FLS-AAV-OPG therapy; (C) in vivo AAV-LacZ injected; (D) FLS-AAV-LacZ transfused; (E) virus-free non-treated control. Panel (F) illustrates the comparison of periprosthetic membrane thickness among the five groups (** p< 0.01).
Figure 4.
Quantitive bone collagen changes determined by integrated optical density (IOD) of Trichrome staining quantified using a computerized image analysis system (Left plot, ** p<0.01). Plot on the right side summarizes the numbers of TRAP+ cells in periprosthetic membranes among groups (** p<0.01).
Immunohistochemistry analysis and TRAP staining
A profound accumulation of CD68+ macrophages, and TNFα and IL-1 expressing cells were observed at the interface between the pin and surrounding bone in sections from both FLS-AAV-LacZ and virus-free non-treated groups. In contrast, significantly less positive cells were present on tissue sections from OPG gene modified groups (Figure 5). There was also a marked reduction of TRAP+ cells in the sections from OPG gene modified animals in comparison with LacZ-treated and virus-free groups (P<0.01, Figure 4).
Figure 5.

IHC stained periprosthetic tissue sections to detect mouse IL-1β (upper panels), TNFα (middle panels) and CD68 (lower panels).
Discussion
Despite successful outcomes of total joint replacement (TJR) surgery, aseptic prosthetic loosening remains the single most common long-term complication. Implant wear is widely recognized as the major initiating event in the development of periprosthetic osteolysis and aseptic loosening18. Evidence includes observations that osteolysis was correlated with higher wear rates19 and vast numbers of wear particles were found in periprosthetic interfacial membranes removed during revision surgery20,21. Studies indicate that wear debris particles may promote periprosthetic inflammation and subsequent bone resorption to lead to the prosthesis failure17,22.
Osteoclasts, derived from hematopoietic precursors of the monocyte/macrophage lineage, are multinucleated giant cells that specialize in resorbing bone. Osteoclast precursors interact with osteoblasts and stromal cells to differentiate into mature osteoclasts.23 Receptor activator of NFκB ligand (RANKL) is a cytokine-like protein expressed by osteoblasts, stromal cells and some lymphocytes24 that appears central to the promotion of osteoclast maturation and activity through its binding to RANK, the physiological receptor located on surface of osteoclasts and their progenitor cells. OPG is a soluble protein that may competitively bind to RANKL to negatively regulate the interaction between RANKL and RANK. It is the balance between the expression of the stimulator of osteoclastogenesis, RANKL, and the inhibitor, OPG that dictates the quantity of bone resorption and remodeling.25 Thus, OPG may be an effective therapeutic candidate to retard the process of debris-associated inflammatory osteolysis and aseptic loosening of the prosthetic joint. The delivery of the potential therapeutic agent (OPG) to the chronic periprosthetic osteolytic site remains problematic. While conventional systemic administration of biological drugs relies on vascular perfusion to the local sites of loosening (requiring relatively high doses and repeated administrations), the delivery of therapeutic genes to sites of disease appears appropriate to achieve the expression of therapeutic proteins in a persistent and localized manner. We and others have evaluated in vivo gene therapy for aseptic loosening by directly injecting viral vectors encoding therapeutic protein and/or markers using various models15–17,22,26. However, concerns remain over the safety of these approaches due to the lack of control over the phenotype of infected cells and the level of incorporated transgene. Potential side effects of in vivo administration include adverse immunological reactions, vector-mediated cytotoxicity, vector-mediated transgene dissemination, and oncogenesis. Ex vivo strategies using cell-based gene delivery may circumvent some of these obstacles. Using fibroblast-like synovial cells from homologous donor mice, the stable OPG gene was successfully transduced in vitro, in agreement with the results elsewhere14. When the gene-modified FLS were returned to the joint tissues, they localized in knee joint periprosthetic tissue and expressed the target protein. Therefore, we have demonstrated the feasibility of ex vivo gene transfer technique and the cell-base OPG therapy on the mouse model of titanium particle-induced knee prosthesis loosening.
In vitro gene modification of fibroblast-like synovial cells resulted in elevated and persistent OPG protein in FLS culture medium 3 days after viral infection. Following the cell transfer to the prosthetic joint, OPG levels appeared comparable to in vivo OPG-treated mice. The data indicates that the exogenous OPG gene was stably transduced into FLS and the transgene-engineered cells survived in the prosthetic joints to subsequently secrete OPG protein. The transgene-modified cells appeared to persist at the local transfusion site in mice, since PCR amplification of nucleic acid from the remote organs and tissues did not indicate disseminated transgenes. Macroscopic and microscopic examinations did not reveal any neoplasms in mice throughout the experimental course.
The ex vivo OPG gene therapy resulted in a successful therapeutic influence by reversing inflammatory bone resorption, increasing new bone formation, preserving bone collagen content, and blocking osteoclast infiltration and maturation, with the significant reduction in TRAP-positive cells following OPG-modified FLS transfusion supporting this observation. We consistently found that over expression of OPG also decreased the accumulation of TNFα and IL-1 expressing cells at the interface between the pin and surrounding bone in both OPG gene treated groups.15 CD68+ macrophages in the periprosthetic membrane were also reduced compared with other control groups. The mechanism of this anti-inflammation phenomenon is currently unclear. Although there is no evidence that OPG directly influences local inflammatory cascades, reversion of debris-induced periprosthetic osteolysis and reduction of micro-motion of prosthetic implant component as the results of RANK/OPG rebalance may subsequently ameliorate and prevent micromotion-induced local inflammation and periprosthetic pseudo-membrane formation.
Implant stability is the major determinant of interfacial strength, and testing the interfacial strength by pull-out tests represents a useful means to evaluate implant stability. Chang et al27 used pull-out test to measure peak load duration in a similar study of bone–implant interface; demonstrating that the interfacial shear load values, peak, and its duration were influenced by the bone bonding and could be indications for implant stability. Using a custom jig and dental cementing techniques, we have successfully developed a pull-out test for the mouse pin-implantation model. Both the in vivo and ex vivo OPG gene therapies dramatically restored the implant stability, to the comparable level of the non-particle-challenged stable implantation. In spite of the small size of mouse tibiae and the custom pin-implants, the biomechanical pullout data were consistent and reproducible.
One limitation of this study is that only one time point (28 days post surgery) was evaluated for the efficacy and safety of the cell-based gene therapy. Dynamic and long-term studies are warranted to evaluate the therapeutic influence, the fate of the engineered cells and the potential side effects.
However, this study suggests that ex vivo gene modification inhibited Ti particle-induced local osteolysis by blockage of osteoclastogenesis and alteration of inflammatory cell infiltration. There was no significant difference at this evaluation time point between the ex vivo and in vivo therapeutic approaches. Overall, the murine model of the knee prosthesis appears an excellent tool to evaluate therapeutic approaches to debris-associated joint prosthesis failure at biomechanical, histological, biochemical and molecular levels.
Materials and methods
Establishment of the mouse model of knee pin implantation
The Institutional Animal Care and Use Committee (IACUC Wichita State University) approved all the animal surgery and care procedures. The mouse model was established as described previously.13 Sixty BALB/c mice aged 10–12 weeks were obtained from Jackson Laboratories (Bar Harbor, ME) and quarantined in our animal facility for 2 weeks prior to experimentation. All mice weighed at least 20 grams at surgery. Mice were anesthetized by i.p. injection of a mixture of Xylazine at 10 mg/kg and Ketamine at 120 mg/kg. The left hind limb area was shaved using an electric animal clipper and scrubbed with Proividine followed by a rinse of 70% alcohol. A 5mm medial parapatellar incision was made to expose patellar ligament. The patella was dislocated laterally to expose the tibial plateau. The cartilage of the tibial plateau was partially removed by a #11 scalpel. The center of the tibial plateau was reamed to form a circular indent of 1.2 mm diameter and 0.3 mm depth. The proximal 5 mm of the tibial intramedullary canal was reamed with a 0.7 mm dental drill through the center of the plateau. A custom-made titanium-alloy pin of 0.8mm in diameter and 5mm in length with a round flat top of 1.2 mm diameter (the generous gift of Stryker Orthopedics Corporation) was press-fit inserted into the canal in a fashion that the surface of the pin head was flush with the cartilaginous surface of the tibial plateau. To mimic wear debris-associated prosthesis failure, 20µl of titanium particle suspension was injected into the tibia canal before insertion of the pin. The wound was cleaned and rinsed with PBS containing Penicillin G (500 unit/ml) and Streptomycin (500 µg/ml), before closure of the layers using sutures.
The titanium particles (50mg/ml Ti-6Al-4V) used in this experiment were kindly provided by Zimmer, Inc. (Warsaw, IN) and the size of the particles (mean particle size 2.3 um with a range of 0.1–68 µm) was similar to debris retrieved from periprosthetic tissues of patients revised for aseptic loosening.15 50 µl of debris particles was intra-articularly injected into the prosthetic joint again 4 weeks later.
Viral vectors and in vivo gene transfer
The recombinant adeno-associated virus coding for OPG was constructed using multiple sub-cloning steps as detailed previously,17 and packaged by Gene Core Facility, University of North Carolina (UNC) at Chapel Hill, NC, who prepared the purified rAAV-OPG-IRES-EGFP using type 2 rAAV. The rAAV-LacZ control vectors were obtained from the Gene Core Facility, UNC. For in vivo gene transfer groups, 50µl of rAAV-OPG-EGFP or AAV-LacZ at titers of 107 pfu were injected respectively into the prosthesis joints at 3 weeks post pin-implantation surgery when Ti-particle induced periprosthetic osteolysis and pseuo-domembrane formation was established.13
Mouse Fibroblast-like Synoviocyte (FLS) isolation and cell-based gene transfer
FLS were isolated from the knees of adult BALB/c mice as described elsewhere.28 After careful removal of the skin and muscles, the tissue of the knee joint was minced, digested with 1mg/ml of collagenase (Sigma, St. Louis, MO) in serum-free RPMI 1640 (Life Technologies, Rockville, MD) for 2h at 37°C, filtered through nylon mesh, and washed extensively. Cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified 5% CO2 atmosphere. After overnight culture, non-adherent cells were discarded and adherent cells were cultured in DMEM-10% FBS. The cells were sub-cultured at 1:5 ratios when the cultures reached confluence, and FLS at passage 2 or 3 were used for gene transduction when confluence of 30–40% was achieved. Cells were co-cultured with 107 particles/ml titer of AAV-OPG-EGFP and AAV-LacZ, and a second dose of viral vectors at titer of 107 was added into culture six hours later,. Transduction efficacy was determined by fluorescent microscopy (for the emission of GFP) and X-gal staining (for LacZ transduction) as previously described.17 Stable transduced FLS were injected into the implanted joints of mice at 3 weeks post-operation.
Experimental course
The mice were divided into 5 groups: (1) in vivo AAV-OPG group given an intra-articular injection of AAV-OPG-EGFP at titer of 106 in 50µl medium (n=12); (2) ex vivo FLS-AAV-OPG group given an intra-articular injection of 50µl medium containing 106 of OPG-engineered FLS cells (n=12); (3) in vivo AAV-LacZ group given an intra-articular injection of AAV-LacZ at titer of 106 in 50µl medium (n=12); (4) 106 LacZ-transduced FLS cells in 50 µl medium was intra-articularly injected into the implanted joints of FLS-AAV-LacZ control mice (n=12); (5) 12 non-treated control mice received an intra-articular injection of 50 µl virus-free medium.
Mice were sacrificed at 4 weeks after gene therapy. Ten implanted knee joints from each group were harvested for pull-out testing and histological evaluation, while the other two implanted joints, along with the lungs, liver, spleen, kidneys, muscles from contralateral limb, and lymph nodes of subiliac and axillary regions were snap-frozen and stored for transgene detection.
Interfacial shear strength test
The limb with the implanted titanium pin was removed by disarticulating from knee joint following sacrifice. All soft tissue around the prosthetic joint was carefully removed to expose the implanted pin surface and proximal tibia. Approximately 10 m of the distal tibia was cemented into a custom-designed jig using dental cement (Garreco, Herber Springs, AR). Proper alignment was achieved by positioning the tibia titanium implant vertically for pull-out testing. The dental cement was allowed to cure for a minimum of 30 minutes prior to testing. Another custom fixture was designed to align the long axis of the orthopedic implant with the loading axis of the MTS 858 Bionix material testing system (MTS Model 858, Eden Prairie, MN) (Figure 6). After the fixture was attached to the loading cell, the MTS 858 actuator pulled the implant out of the bone at a rate of 2.0 mm/minute under displacement control. Actuator positions and loading data were recorded.
Figure 6.
Implant sample mounted on the MTS 858 for the pull-out test. The insert shows the pin head captured between the blades of the holding jig.
Histological and immunohistochemical (IHC) analysis
Following the pull-out test, the peri-implant proximal tibiae were formalin-fixed and decalcified using formic acid/sodium citrate solution before paraffin-embedding and mounting with either cross or longitudinal orientation for cutting 6um sections. Sections were stained with hematoxylin & eosin to examine new bone formation or bone erosion around the prosthetic pin, and the evidence of debris-associated inflammation (periprosthetic tissue formation and cellular infiltration). The thickness of the periprosthetic membrane was measured using Image-Pro+ software (Media Cybernetics, Silver Spring, MD).
Modified Trichrome staining was performed to examine bone collagen changes.17 Briefly, sections were deparaffinized and hydrated before equilibrating in Bouin’s solution (70% picric acid, 5% glacial acetic acid and 10% formaldehyde) at 56°C for 1 hr. The sections were incubated in phosphomolybdic (0.21% w/v)–phosphotungstic acid (0.21% w/v) for 10 min, followed by aniline blue solution (2.5% aniline blue in 2% acetic acid) stain for 5 min, and then incubated in 1% acetic acid for 4 minutes before dehydration in graded alcohol. The bone collagen acquired a blue stain, with the stain density proportionate to the collagen content.17
IHC was performed to reveal IL-1β, TNFα, and CD68 expressions in peri-implant tissues.29 Briefly, tissue sections were deparaffinized, re-hydrated, and microwaved before blocking with 1.5% normal goat serum for 1 hour. The sections were incubated with primary antibodies anti-mouse TNFα(Abcam, Cambridge, MA), IL-1β or CD68 (Santa Cruz,Santa Cruz, CA) (diluted in 1.5% normal goat serum/50mM Tris buffer, PH7.6) over night in a moisturized chamber at 4°C. Biotin-conjugated secondary antibody (goat anti-rabbit IgG) was followed for 30 minutes after 3x washes. Avidin biotin enzyme reagent was applied onto sections for 30 minutes. The color was developed by adding 3.3’-diaminobezidine tetrahydrochloride (DAB) or alkaline phosphatase (AP). In negative control sections, the primary antibody was replaced by non-immune rabbit sera at the same concentration as the primary antibody. Digital images of IHC stained sections and modified Trichrome stained sections were captured. The levels of the positive stains and localizations were evaluated in four random fields per section and expressed as integrated optical density (IOD), using the Image-Pro Plus software.17
Tartrate-resistant Acid Phosphatase (TRAP) stain for osteoclasts
A commercial kit (Sigma 387) was used for histological TRAP staining on paraffin-sectioned prosthetic joints after pin removal. Following deparaffinization and rehydration, the sections were permeated in a microwave oven for 30 seconds before brief fixation in buffered acetone. The sections were then incubated at 37 °C for 1 hour in 0.1 M acetate buffer (pH 5.2), containing 0.5 mM naphthol AS-BI phosphoric acid, 2.2 mM Fast Garnet GBC and 10 mM sodium tartrate. The reaction was stopped by washing in several changes of distilled water. TRAP+ cells in the periprosthetic membrane were detected by the presence of dark purple staining granules in the cytoplasm.
Transgene expression assessment
Conventional reverse transcription polymerase chain reaction (RT-PCR) was performed to detect OPG gene expression at the local delivery site and the remote organs/tissues. Hard and soft tissues were minced on dry ice followed by Polytron homogenization (PT-MR2100; Kinematica, Lucerne, Switzerland). Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's protocol. Extracted total RNA was treated with deoxyribonuclease I to eliminate genomic DNA according to the vendor’s instructions. One microgram of total RNA, after DNase treatment, was reverse-transcribed to cDNA in a Perkin-Elmer Thermal cycler (Perkin Elmer, CT).17 The detection of OPG transgene was performed by conventional PCR using 2µl of cDNA template, 0.4µM of forward and backward primers, 2.5 U Taq DNA polymerase, 0.2mM dNTPs, and 1.5mM MgCl2. The PCR was run for 35 cycles with denaturing at 94°C for 1 minute, annealing at 60°C for 55 seconds, and elongation at 72°C for 60 seconds. The PCR product was electrophoresed on 1.5% agarose gels to confirm the amplification of OPG gene. The transgene product, human OPG protein levels in FLS-AAV-OPG culture medium and variant prosthetic joint tissue were determined by a quantitative sandwich enzyme immunoassay technique (ELISA) utilizing capture and detection monoclonal antibody pairs against different epitopes of OPG (R&D systems, Minneapolis, MN), and the standardized protocol was detailed previously.17,30
Statistical analysis
Data from three independent experiments were analyzed for this study. A total of 60 animals were investigated and were distributed at twelve animals for each group. Statistical analysis among groups was performed by one-way ANOVA test; with the Schafer formula for post hoc multiple comparisons (SPSS v10, Chicago, IL). A p-value of less than 0.05 was considered as significant difference. Data are expressed as Mean±Standard Errors of the Mean.
Acknowledgement
This work was supported by an NIH grant 5R03AR054929 to S.-Y.Y. The authors wish to thank Ms. Zheng Song and Dr. Bin Wu for their advice and technical support.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240–252. doi: 10.1097/BLO.0b013e31804b4147. [DOI] [PubMed] [Google Scholar]
- 2.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Nishiyama T, Hasuda K, Fujishiro T, Niikura T, Hayashi S, et al. Effect of etidronate on COX-2 expression and PGE(2) production in macrophage-like RAW 264.7 cells stimulated by titanium particles. J Orthop Sci. 2007;12:568–577. doi: 10.1007/s00776-007-1180-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Chiba J, Rubash HE. In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. J Bone Joint Surg Am. 1994;76:172–180. doi: 10.2106/00004623-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty. 1995;10:498–506. doi: 10.1016/s0883-5403(05)80152-4. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RA, McAllister CM, Borden LS, Bauer TW. Polyethylene debris-induced osteolysis and loosening in uncemented total hip arthroplasty. A cause of late failure. J Arthroplasty. 1992;7:285–290. doi: 10.1016/0883-5403(92)90050-z. [DOI] [PubMed] [Google Scholar]
- 7.Bell NH. RANK ligand and the regulation of skeletal remodeling. J Clin Invest. 2003;111:1120–1122. doi: 10.1172/JCI18358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256:449–455. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim N, Odgren PR, Kim DK, Marks SC, Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000;97:10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 13.Yang SY, Yu H, Gong W, Wu B, Mayton L, Costello R, et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. J Orthop Res. 2007;25:603–611. doi: 10.1002/jor.20342. [DOI] [PubMed] [Google Scholar]
- 14.Goater JJ, O'Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–173. doi: 10.1016/S0736-0266(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Yu H, Gong W, Zhang L, Jia T, Wooley PH, et al. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrich-Vinther M, Carmody EE, Goater JJ, K Sb, O'Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002;84-A:1405–1412. doi: 10.2106/00004623-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum. 2002;46:2514–2523. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]
- 18.Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001:66–70. doi: 10.1097/00003086-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 20.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 21.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. Hss J. 2006;2:102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH, et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther. 2004;11:483–491. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 23.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer LC. Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol. 1999;141:195–210. doi: 10.1530/eje.0.1410195. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Wu B, Mayton L, Evans CH, Robbins PD, Wooley PH. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res. 2002;51:342–350. doi: 10.1007/pl00000313. [DOI] [PubMed] [Google Scholar]
- 27.Chang YS, Kobayashi M, Li ZL, Oka M, Nakamura T. Significance of peak value and duration of the interfacial shear load in evaluation of the bone-implant interface. Clin Biomech (Bristol, Avon) 2003;18:773–779. doi: 10.1016/s0268-0033(03)00138-4. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Sanz I, O'Keefe RJ, Schwarz EM. NF-kappa B regulates VCAM-1 expression on fibroblast-like synoviocytes. J Immunol. 2000;164:5990–5997. doi: 10.4049/jimmunol.164.11.5990. [DOI] [PubMed] [Google Scholar]
- 29.Geiler T, Kriegsmann J, Keyszer GM, Gay RE, Gay S. A new model for rheumatoid arthritis generated by engraftment of rheumatoid synovial tissue and normal human cartilage into SCID mice. Arthritis Rheum. 1994;37:1664–1671. doi: 10.1002/art.1780371116. [DOI] [PubMed] [Google Scholar]
- 30.Wooley PH, Petersen S, Song Z, Nasser S. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res. 1997;15:874–880. doi: 10.1002/jor.1100150613. [DOI] [PubMed] [Google Scholar]