Abstract
One of the major fundamental causes for the aging of the immune system is the structural and functional involution of the thymus, and the associated decline in de novo naïve T-lymphocyte output. This loss of naïve T cell production weakens the ability of the adaptive immune system to respond to new antigenic stimuli and eventually leads to a peripheral T-cell bias to the memory phenotype. While the precise mechanisms responsible for age-associated thymic involution remain unknown, a variety of theories have been forwarded including the loss of expression of various growth factors and hormones that influence the lymphoid compartment and promote thymic function. Extensive studies examining two hormones, namely growth hormone (GH) and ghrelin (GRL), have demonstrated their contributions to thymus biology. In the current review, we discuss the literature supporting a role for these hormones in thymic physiology and age-associated thymic involution and their potential use in the restoration of thymic function in aged and immunocompromised individuals.
Keywords: GH, GH-R, Ghrelin, GHS-R, Thymus, Involution, Thymopoiesis, Aging, Hematopoiesis
Introduction
Immune function decreases with age, due to both quantitative and qualitative changes in the cells of the immune system and the lymphoid environment. The damaging effects of aging on immunity have been well studied and such changes are believed to lead to increased susceptibility to infection and autoimmunity, poor antibody and T-cell responses to vaccines and diminished immunosurveillance of malignant cells. The thymus is critical for the development, selection and maintenance of the peripheral T cell pool possessing a broad spectrum of TCR specificities. While the thymus lacks its own self-renewing pool of progenitors, it is continuously seeded with lymphoid progenitors emigrating from the bone marrow. The common lymphoid progenitors (CLP) and the early thymocyte progenitor (ETPs) decline markedly with age and the ETPs have a reduced proliferative capacity and an increased rate of apoptosis [1]. Thus, while the thymus is capable of generating T cells throughout the life span, with advancing age, the thymic space becomes progressively filled with adipocytes accompanied by a dramatic loss of progenitors, epithelial cells and differentiating thymocytes in the cortical and medullary areas leading to a reduction in naive T cell output. This process has been termed “thymic involution” [1-9].
The thymus is a critical organ in mammals as the lack of a thymus in humans (known as “DiGeorge's syndrome”) and in thymectomized neonatal mice leads to severe immunodeficiency due to paucity of mature naïve CD4+ and CD8+ T cells. During physiological aging, the total peripheral T cell pool is maintained by homeostatic expansion of preexisting memory T cells, predominantly CD8+ T cells, rather than replenishment by thymic export. Consequently, the long-lived naïve T cell repertoire is significantly reduced (diluted) with the expansion of these peripheral memory T cells possessing a more restricted T-cell receptor repertoire thereby limiting the host's ability to mount responses against challenges with new antigens. Moreover, defects in the activity of aged naïve T cells appear to be due to the chronologic age of the naïve cells themselves rather than the age of the host. Thus, the involution of the thymus with age and the paucity of newly formed naïve CD4+ and CD8+ T cells is believed to be responsible for much of the deterioration in adaptive immunity and the resultant immune dysfunction in the elderly [1-9].
While the precise mechanisms responsible for thymic involution remain to be identified, it is believed to be a complex programmed loss of multiple systems that cross communicate with each other and include the loss of the thymic architecture and epithelial cells to support and maintain thymopoiesis as well as the loss of various growth factors and hormones that assist in maintaining the thymic microenvironment. For many years, scientists have believed that, post-adolescence, the thymus simply becomes a fatty non-functional organ incapable of supporting T-cell production. However, more recent studies have demonstrated that, despite significant atrophy, the aged thymus still retains the capacity to promote T-cell differentiation and produce de novo naïve T cells when properly stimulated, albeit at a significantly reduced rate [2-6]. Thus, a greater understanding of the processes of thymic involution and strategies to restore thymic function and T cell export in the aged and immunosuppressed hosts remains an important and promising therapeutic goal. For a more thorough description of the various changes in the thymus with age and its impact on thymopoiesis and immunity [1-13].
There exists a complex system of communication between the neuroendocrine and immune systems. Through the use of shared ligands and receptors, these systems are capable of significant crosstalk that is believed to play an important role in physiological homeostasis [14-16]. Many hormones and neuropeptides have been shown to be potent immunoregulatory molecules that influence various aspects of immune function in healthy and diseased individuals. Many of the cells within the immune system possess receptors and demonstrate responses upon stimulation with neurohormones and similarly, neuronal and endocrine cells respond to immune-derived cytokines and growth factors under normal physiological and disease conditions. Further, immune and neuronal cells express or can be induced to express many similar cytokines, hormones and growth factors, thus further mediating cross communication between these systems within the body. Many attempts to define the molecular mechanisms responsible for thymic involution have focused on the examination of changing levels of expression of molecules, such as cytokines, growth factors, neuropeptides and their receptors, with aging [1]. Mediators that are known to have some influence on thymic involution include growth hormone (GH), insulin-like growth factor-1 (IGF1), keratinocyte growth factor (KGF), nerve growth factor (NGF), interleukin-7 (IL-7), gonadotropin releasing hormone (GnRH) [15-23] and increases in atrophic factors, such as IL-6 and transforming growth factor-β (TGFβ) [1-2]. Whether these changes in expression directly cause, or are a consequence of, involution remain unclear. In addition to direct thymic factors, aging also affects the bone marrow, demonstrating diminished lymphoid potential and reduced proliferative capacity or survival of hematopoietic stem cells (HSCs). This might also contribute to the involution process.
Several neuroendocrine hormones have long been associated with effects on immune cell function including growth-promoting effects on multiple immune cell lineages. GH is an important hormone with effects on the immune system. One of the first observations was that GH levels decreased with age, as thymic involution progresses and this impaired thymic function could be restored by the administration of GH [1; 23-27]. A number of subsequent reports have demonstrated both thymopoietic and hematopoietic effects for GH, IGF1, GH secretagogues (GHS) and, more recently ghrelin and leptin, during aging [15-16; 23; 26-27]. While the use of such hormones to promote immune reconstitution is indeed attractive considering their pleiotropic effects and low toxicity (in animals) after systemic administration, additional research is required to adequately evaluate their appropriateness as agents to promote T-cell recovery in human subjects. The remaining focus of this review shall examine the effects of two hormones, namely growth hormone (GH) and ghrelin (GRL) on T-cell and thymic functions and their potential use in boosting thymic activity in immunocompromised hosts.
Growth Hormone
Background
GH is a peptide hormone that is synthesized and secreted primarily by somatotrophic cells in the anterior pituitary gland. The production of GH is pulsatile and primarily nocturnal, and is controlled by several hypothalamic hormones including GH-releasing hormone (GHRH), hypothalamic GH release-inhibiting factor (GHRIF), and somatostatin (SMT). Circulating GH levels are highest during the neonatal period, decrease during childhood, peak again during puberty and fall dramatically with age [24-27]. Many of these GH effects appear to be mediated or influenced indirectly by the production of IGF1. Moreover, GH and IGF1 mediate the proliferation of a number of cell types including chondrocytes, fibroblasts, adipocytes, myoblasts and immune cells [23-29]. It is believed that the declining activity of this GH-IGF1 axis with advancing age may contribute to the decrease in lean body mass and the increase in mass of adipose tissue that occurs with aging. Based on these early correlations, it was initially believed that GH may serve as a potent therapeutic to boost the integrity and function of many organ systems in the elderly. Speculation regarding GH as an anti-aging treatment dates back to an early study where GH was used to treat 12 men over the age of 60 [30-31]. The study revealed that all of the subjects demonstrated significant increases in lean body mass and bone mineral density, while the control group failed to demonstrate such changes. While the authors did not claim that GH reversed the aging process, the results were so misinterpreted and hence the myth was born that GH actually reverses the aging process. Many subsequent studies have since been performed and failed to confirm the many benefits ascribed to GH [30-31]. A recent Stanford University survey of clinical GH studies revealed that the administration of GH to healthy elderly subjects increased muscle by only approximately 2 kg and decreased body fat by the same amount [32]. No effects on mineral bone density, cholesterol and other lipid levels, oxygen consumption, muscle strength or other fitness-associated factors were shown to be increased by GH administration. Moreover, there are also a number of side effects with GH administration including fluid retention, joint pain and nerve compression as well as concerns regarding an increased risk of developing diabetes or cancer. These unresolved issues have hindered the long term use of GH as a potential therapeutic. Thus, while GH is indeed not the magical elixir many had hoped for, hormone administration does have some beneficial effects on certain organ systems in the aged and immunosuppressed, more specifically for this review, the immune system.
GH and Immune Function
GH has been shown to exhibit a number of effects on a variety of cells and organ systems, including immune cells. GH mediates its effects through its cell surface receptors, GH-Rs, related to type I cytokine receptors, which have been shown to be present on the surface of peripheral blood mononuclear cells (PBMCs), T- and B-cells, NK cells, monocytes, thymocytes and hematopoietic cells in humans and/or other species [23; 26; 32-34]. B cells and monocytes appear to constitutively express GH-R mRNA and protein on their surface, while T-cells demonstrate much more variable patterns of expression [23; 32-34]. In many cell types, these receptors have been shown to be functionally active in that stimulation with purified or recombinant GH results in the activation of several cellular signaling pathways resulting in effects on the survival, differentiation and/or the proliferation of lymphoid cells [23-24; 26-28]. In addition to expressing functional GH-Rs, immune cell subsets have also been shown to express their own GH protein. GH mRNA and protein have been shown to be expressed in T- and B-lymphocytes, macrophages and NK cells in the blood and lymphoid organs of rats [23-24]. Subsequently, several laboratories have demonstrated that human PBMCs, lymphocytes, thymus, spleen, tonsils and T- and B-cell lines also express GH [23-24]. Human lymphocytes secreted GH both at rest and in response to mitogens. The presence of immune-derived GH suggests that, besides the classic endocrine pathways of hormone exposure and signaling, this hormone may also serve as an internal signal by which immune cells communicate with one another as well as with cells in the microenvironment via autocrine and/or paracrine pathways.
Interestingly, Wu and coworkers [35] as well as others [23-24; 26; 33] have reported that GH mRNA expression was significantly higher in B cells than T cells suggesting that B cells may be a major source of GH and possibly a site of action. Moreover, GH was also found to enhance the production of IgG1, IgG2, IgG3, IgG4, IgA1, IgA2, and IgM by human tonsillar B cells, which upon stimulation with GH and IGF-I induced IgE and IgG4 production by class switching via an IL-4- and IL-13-independent mechanism [23; 36]. While these studies are interesting, the precise mechanistic role of GH in B cell development and function remains to be fully defined. In addition, GH has also been shown to have a number of other biological effects on various inflammatory cell subsets, including neutrophils, monocytes and macrophages. Several investigators have also reported that GH is capable of priming macrophages and neutrophils for superoxide and/or hydrogen peroxide production in response to cellular stimulation [23; 26]. Moreover, GH has also been shown to have effects on neutrophil and monocyte adhesion and migration and to play a role in regulating apoptosis in human T- and B-cell and monocyte lines and in primary neutrophils and monocytes [23; 26; 37-39]. In these studies, GH treatment has been found to diminish Fas-mediated cell death via increasing the expression of Bcl-2 and inhibiting the activation of caspase 3 [38] and to protect lymphocytes from irradiation- [39] and methyl methanesulfonate- [40] induced cell death. Several excellent reviews summarize the various effects of GH on immune cells and function [23-27; 41-44].
Additional reports have also suggested that GH may have direct effects on cytokine expression, although this literature is a bit controversial. Several studies have shown that either GH treatment of PBMCs or monocytes directly or in combination with LPS enhanced the production of interferon and proinflammatory cytokines [23; 26; 45-46]. Similarly, GH administration into short stature children also was found to increase serum levels of several cytokines [47]. In contrast, additional reports have demonstrated that GH treatment of LPS-stimulated human monocytes and macrophages [23; 26] results in diminished levels of TNFα and other proinflammatory cytokines. Patients with GH deficiency have been shown to possess increased plasma and cell-derived levels of TNFα and IL-6 [48], while administration of GH back into these subjects reduced such cytokine expression. Similar results demonstrating an ability of GH to inhibit cytokine expression have been reported in treated patients with cardiomyopathy [49] and in postmenopausal women with abdominal obesity [50]. Furthermore, in some studies, no differences were observed [51]. Given the variability in the reports to date, it is difficult to assign a specific function for GH in regulating cytokine expression. However, more recent studies [23; 26; 52-55] have suggested that GH treatment of lymphoid cells results in a rapid dose-dependent increase in suppressor of cytokine signaling 3 (SOCS3) mRNA expression. If indeed induced and active, then GH would be expected to suppress proinflammatory cytokine expression. However, as GH has failed to demonstrate any consistent effect on cytokine expression, it is likely that GH activation and signaling is much more complicated than simply inducing alterations in SOCS expression.
Growth Hormone Effects on Thymus
By far, the most published and predominant observations regarding GH and its interactions with the immune system involve its role in thymus development and function. Earlier observations documented that thymic atrophy was often associated with ablation of the pituitary gland and mice with the dwarf mutation had reduced immune functions [23-24; 26; 32]. Hypophysectomized rats or Snell dwarf mice (dw/dw), which are both defective in the production of GH (and also of prolactin and thyroid hormones) displayed deficiencies in lymphocyte development and function, which were corrected upon administration of exogenous GH to these animals. Similarly, administration of GH was found to enhance the development of the thymus and promote the engraftment of murine or human T cells in SCID mice [23; 26]. Among the most promising immune effects of GH specifically relevant to aging is its potential to improve thymic function, promote thymic and bone marrow engraftment and stimulate hematopoiesis in immunosuppressed and aged animals [23; 26; 32; 56]. Administration of GH or IGF-1 has also been shown to enhance T cell recovery in syngeneic and allogeneic HSCT recipients as well as in aged BMT recipients [23-26; 57-58]. It should be noted that many of the effects of GH in vivo may be indirectly mediated by IGF1, which is synthesized in the liver in response to GH stimulation. Similar to GH, administration of IGF1 in vivo has also been shown to partially reverse age-associated thymic involution, enhance thymopoiesis in aged rodents and also accelerate the reconstitution of the immune system in immunodeficient animals and in mice post transplant [24-27; 58-60]. Furthermore, GH and IGF1 can synergize with other cytokines such as GM-CSF in hematopoiesis [25-26], possibly leading to the various hematopoietic effects attributed to GH. Additional studies have revealed that both GH and IGF1 can act directly on isolated fetal thymic tissue to expand the number of thymocytes [26]. Together, these data support the use of GH and/or IGF1 as therapeutic treatments for patients post transplantation and during states of immunosuppression including aging.
Kelley and colleagues have previously reported on the effects of GH on thymic growth in 16 and 22 month old rats implanted with a pituitary tumor that produced both GH and PRL [23; 26-27; 61]. After two months, these rats demonstrated detectable thymus glands, while little to no thymus tissue was detectable in the untreated animals. These rats also possessed more T cells and demonstrated greater lectin-induced proliferative responses. More recently, this same group [61] performed a more extensive series of studies using 24 month old rats that received the same type of GH-producing tumor cells or recombinant GH. In addition to the dramatic restorative effects on GH on the aged thymic tissue, they also observed that GH increased hematopoietic precursor cells and the quantity of adipocytes in the bone marrow and resulted in more extensive extramedullary hematopoiesis. These results suggest that GH indeed has profound effects on the thymus and bone marrow of aged mice and rats. While these studies are interesting, there are few rodent or human studies to date, focusing primarily on aging, that have performed an extensive analysis examining the effects of GH on the various thymic progenitors, thymic T cell output and the impact of GH infusion on peripheral T cell receptor (TCR) repertoire. To this end, we have included some of our own unpublished observations here using GH in aged mice to support some of our hypotheses and conclusions. We have found that GH infusion for 2 weeks not only induced a significant increase in thymic cellularity of old (but not young) BALB/c mice but it also led to a greater than two-fold increase in ETPs in thymus (p<0.05) with no significant change in DN2 (linlowC-kit+CD25+) DN3 (linlowC-kit-CD25+) or DN4 populations (Figure 1, panel A; D. Taub unpublished observations). Moreover, GH infusion also significantly increased (p<0.05) the number of bone marrow linlowsca1+ckit+ (LSK) populations in old but not young mice (panel B). GH infusions also increased the number of recent thymic emigrants in aged mice (panel C) and induced significant and reproducible alterations in the TCR diversity as assessed by CDR3 length PCR analysis (panel D). The T cells examined from GH-infused mice were found to possess much more dramatic Vβ CDR3 differences in their Gaussian profiles when compared to age-matched sham controls. In agreement with previous studies [1; 16; 23; 26], GH also appears to exert positive effects on the numbers of cortical and medullary epithelial cells when infused into aging mice, which may in part account for some of the reorganization and more defined structures observed in the infused aged thymus (data not shown). In addition, there is an age-associated reduction in LSKs from bone marrow, most likely contributing to the resulting in the unavailability of T cell progenitors to seed the thymus. Thus, it is not surprising that the thymus loses its capacity to continue support for thymopoiesis over time. As GH treatment results in a significant increase in both the LSK and ETP populations as well as promoting thymic epithelial cell re-growth [1; 16; 24-26], the aged thymus begins to reorganize and compartmentalize, becoming a much more optimal organ for thymocyte growth and development, and thus enhancing the output of newly selected naïve T cells possessing a more diverse TCR repertoire compared to untreated age-matched animals.
Figure 1. GH enhances thymic cellularity and thymopoiesis in aged mice.
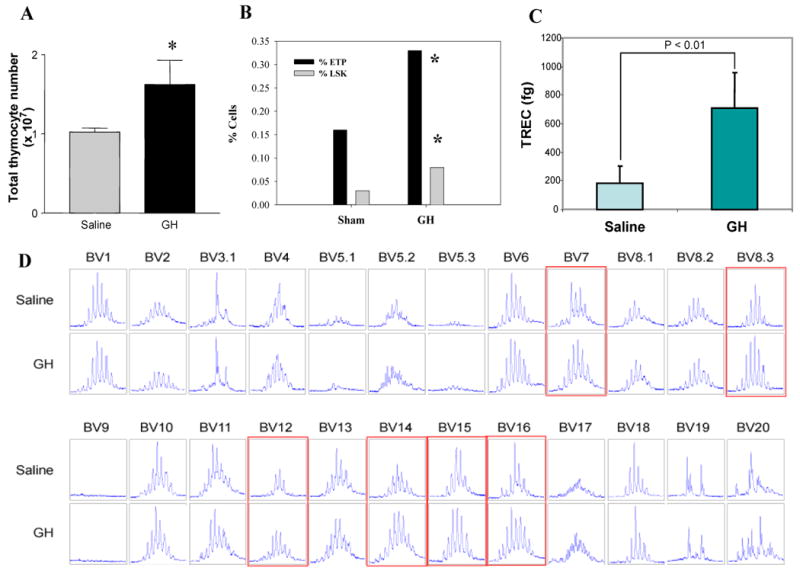
(A) GH infusion for 2 weeks via s/c osmotic pumps causes a significant increase in thymic cellularity in 14m old BALB/c mice. (B) GH infusion in old mice led to a >2 fold increase in ETPs in thymus (p<0.05) with no significant change in DN2 (linlowC-kit+CD25+) DN3 (linlowC-kit-CD25+) or DN4 populations (data not shown). In addition, the bone marrow cells were analyzed for linlowsca1+ckit+ (LSK) populations where GH infusion also significantly increased (p<0.05) the bone marrow LSK cells in old but not young mice. Data is represented at mean ± SEM (n = 6) of 14m old mice. (C) GH infusions also increased the number of recent thymic emigrants in aged mice. (D) GH induced significant and reproducible alterations in the TCR diversity as assessed by CDR3 length PCR analysis. In comparison to ghrelin and leptin, GH was found to induce much more dramatic changes in the Gaussian profiles of infused mice compared to sham controls. The CDR3 sizes are shown in x-axis and the peak fluorescence intensity is shown on the y-axis. While not shown, similar results were observed using 6m and 18-20m old mice but not using 2m old mice suggesting that this hormone mediates it optimal effects when immunological space is available and age-associated thymic atrophy or involution has initiated. Moreover, an increase in spleen cellularity was also observed in the aged mice infused with GH vs. vehicle (data not shown). The GH doses utilized in these studies did not result in significant weight gain or loss demonstrating that the effects on immune parameters occur using doses of GH that do not induce appreciable anabolic effects. It should also be noted that it is quite possible that the mechanisms by which GH exerts its pro-thymopoietic effects may be via the production of other hormonal/cytokine survival factors such as IGF1, KGF or possible even GRL.
A very interesting and informative series of studies regarding the direct and indirect effects of GH on thymocytes and thymic microenvironment have come from studies by Savino, Dardenne and colleagues [62-68]. They have identified several GH-specific intrathymic effects on thymocyte development including that (1) GH upregulates the proliferation of thymocytes and thymic epithelial cells (TECs), (2) GH-transgenic mice and animals treated with exogenous GH demonstrate enhanced thymic cellularity, (3) GH stimulates the secretion of thymic hormones, cytokines, and chemokines within the thymic microenvironment and (4) GH induces the production of extracellular matrix proteins in the thymus resulting in increased thymocyte migratory responses as well as increased intrathymic trafficking and thymic T cell output. They have suggested that some of the observed effects could be attributed to the production of IGF1, most likely systemic production as well as intrathymic production.
GH has also been shown to enhance the production of the chemokine CXCL12 by TECs leading to increased chemotaxis through CXCR4 signaling [69]. This finding is fascinating in that interactions between CXCL12 and its receptor CXCR4 are important not only in maintaining thymic function but also for the interactions between stem cells and the stromal cells within the bone marrow. The interaction of CXCL12 with CXCR4 is known to be mediated through the JAK/STAT pathway [26], which is inhibited by SOCS3 expression [70]. Thus, during conditions where SOCS3 levels are elevated (such as during inflammation or upon GH exposure), signaling through the CXCL12-CXCR4 pathway may be impaired and thus may interfere with T cell generation and activity. Recent work by Pello and coworkers [71] has demonstrated that GH administration induces the mobilization of stem cells from the bone marrow into the circulation, and that this results in the increased expression of SOCS1 and SOCS3. It is believed that the increase in SOCS expression in the bone marrow cells inhibits the CXCL12-mediated chemotactic signaling leading to the release of stem cells. Such mobilization could prove invaluable therapeutically in studies examining transplantation and stem cell biology. Thus, a greater understanding of how these GH signals interact with the SOCS proteins, chemokines and other cytokines may yield valuable insight into therapeutic methods to promote stem cell mobilization, cellular engraftment and hematopoiesis.
There has also been considerable interest in using GH and IGF1 to treat HIV-infected patients, as the HIV-infected thymus is quite atrophied even at an early age and T cell output is quite low [5-6]. There have been several studies performed looking at these parameters since 1995 yielding many mixed results, and are summarized by Kelley and others [72-73]. Based on these studies, HIV patients typically required 5-10 fold higher “pharmacological” doses of GH to achieve results that could be obtained in non-HIV infected GH-deficient patients using physiological quantities. It was subsequently reported that AIDS patients demonstrate GH resistance, which was believed to be related to ongoing inflammation [26; 72-73]. Today, with the use of highly aggressive anti-retroviral therapy (HAART) and more control of the ongoing inflammation in these subjects, there have been more studies showing that GH can improve the immune status of HIV patients. Several recent studies by Napolitano and coworkers [74] have demonstrated a prothymic role for GH upon administration into human subjects. They initially examined the effects of GH on the thymus of 5 HIV-1–infected adults receiving GH as part of a lipodystrophy study. They observed several promising findings including a marked increase in thymic mass and circulating CD4+ T cells in all GH recipients suggesting an enhancement of thymic function. However, due to the small sample size, the patients' lipodystrophy, the lack of more detailed immunological markers of thymic activity and the absence of an internal control arm, drawing any formal conclusions from these results was difficult. Thus, these investigators subsequently have been performed in a prospective randomized study specifically designed to assess the effects of GH on thymopoiesis in HIV-1-infected adults with persistently low CD4+ T cell counts despite virologic suppression with effective anti-retroviral therapy [75]. Of the 22 HIV-1 infected subjects being examined, one half of study participants were randomly assigned to continue their usual HIV therapy and to receive GH in the first year, while the other half of the subjects continued their usual HIV therapy without GH treatment (control). In the second year of the study, the treatment was reversed and the control participants received GH, while the prior GH recipients had GH treatment stopped. The results were very encouraging as Napolitano's team found that GH treatment markedly increased thymic mass and appeared to double the number of newly made T-cells. On average, GH treatment was associated with a 30% increase in CD4+ T-cells (approximately 2.4 fold higher than the control group). These gains continued to increase at least 3 months beyond GH discontinuation and appeared to persist for at least one year after GH discontinuation. Pires and coworkers [76-77] have also shown that rhGH given to patients also receiving HAART resulted in increased T cell numbers, their in vitro antigen-specific responsiveness and the number and function of CD56Bright NK cells, which are typically depleted in patients receiving HAART alone. Interestingly, Geenan and his colleagues [78] have revealed similar prothymic activities upon GH administration in subjects with adult GH deficiency (AGHD). They found that withdrawal of GH treatment of AGHD patients (who routinely receive GH) results in diminished thymic T cell output as well as intrathymic proliferation. These thymopoietic parameters were found to be partially restored upon resumption of GH treatment.
In all of these in vivo studies, the persistence of these thymic gains upon the removal of administered GH is interesting as they suggest that GH may be conditioning the atrophied thymus to become more permissive to progenitor thymocyte entry, thymocyte and TEC cell growth and T-cell output. An atrophied thymus, especially with age and in AIDS patients, loses much of its structure and compartmentalization and it can be difficult to find well-defined cortical and medullary regions where thymocyte development, selection and maturation can occur. The effects of GH on thymic stroma and epithelial cells as well as its adipolytic activities may make these thymi more permissive to progenitor entry and thymocyte growth. Obviously, the ideal scenario would be to find a long term means to maintain the thymus and retain these prothymic effects. Most likely, combinatorial conditioning regiments with GH, IGF1 and/or other growth factors, alone or in parallel, may be necessary to optimally maintain thymic function and integrity on a long term basis, especially in the aged, diseased and/or immunosuppressed subjects.
While it would be remarkable to be able to state that “GH works all of the time” and that it is universally accepted as the “ideal means to promote thymic regeneration”, there have been several red flags raised regarding the legitimacy and reproducibility of the use of GH in vivo. First, there have been studies that have failed to demonstrate similar positive prothymic and pro-engraftment effects using GH. For example, eight children with growth hormone deficiency (ages 1-17 years) were examined before and during treatment with human growth hormone for 12 to 16 months [79]. A number of immunological parameters were examined including immunoglobulins, cell surface markers, mitogen responses, and polymorphonuclear cell function. Before treatment immune functions were normal in all children and during treatment, GH did not significantly affect serum immunoglobulins, polymorphonuclear cell function, or percent T cells. However, a significant loss of B cells was noted in 7 out of 8 patients, the T helper/suppressor ratios decreased in all patients and the mitogen responses decreased to below normal in all. While the depression of immune functions did not result in an increased rate of infections during the observation period, these researchers had some concern about the effects of prolonged GH treatment and therefore cautioned against the indiscriminate use of human growth hormone. Moreover, studies reported by Dorshkind and Horseman [24] have shown little evidence that GH is required for primary lymphopoiesis or for B and T cells to generate humoral- or cell-mediated immune responses in GH and GH receptor deficient mice. Indeed, the failure to observe a “loss of function” in these deficient mice does make one wonder if the mere presence of hormones and their receptors by immune cells is simply an evolutionary artifact of no significant value or benefit or if their presence and activity require certain metabolic or environmental conditions to observe their role in lymphopoiesis and thymopoiesis. Dorshkind and colleagues [80] have suggested that the non-obligate role of GH in immunity could be reconciled with its potential augmentation of the immune system if one considered the primary role of GH to be to counteract negative immunoregulatory signals such as can be seen during states of stress. Under these circumstances, these hormones could stimulate immune cell development and function and the secretion of GH may be increased to counteract the negative immunoregulatory effects of glucocorticoids, for example. The idea that stress may influence the effect of GH on a host are supported by a joint study by Dorshkind and Murphy [81] where substantial differences in the T cell development of the Snell Dwarf (dw/dw) mouse strain (which are deficient in the production of anterior pituitary hormones) were observed between the laboratories. Several laboratories have demonstrated a dependence on GH for optimal T cell development within these Dwarf mice, while others have observed normal thymopoiesis. In an effort to understand this discrepancy, these investigators examined these mice in separate facilities and under distinct housing conditions. They found that when female dw/dw mice were housed together with their normal-sized littermates, thymic cellularity and the frequency of double positive thymocytes were markedly reduced. Administration of GH into these mice reversed these effects suggesting that stress may be the unifying parameter that may explain the disparate results using not only the dw/dw mouse model but also in the reported effects of GH on the T-cell development in various rodent models and human studies.
In summary, based on the predominance of the data described so far, it would appear that GH administration into aged or immunosuppressed human subjects and in many rodent models facilitates thymic re-growth by expanding the number of thymocytes within the thymus, possibly via the recruitment of de novo T cell progenitors from the bone marrow or by enhancing their proliferation. We have shown in this review that GH infusion into aged mice does indeed promote an increase in LSKs in the bone marrow as well as ETPs in the thymus compared to age-matched vehicle-infused control animals (Figure 1). Further, while the typical aged thymus has lost many of the boundaries separating the specific compartments responsible for T cell development and selection, GH administration favors a much more defined thymus by stimulating the growth of thymic epithelial cells (TEC), the production of extracellular matrix proteins and creating a more hospitable microenvironment leading to more defined cortical and medullary structures capable of supporting thymocyte development. Moreover, GH infusion also results in a loss of thymic fat. While it is unclear if thymic fat has any specific function other than filling the perivascular space where thymocyte used to reside, adipocyte entry and/or development in the old thymus appears to correlate with the loss in thymic mass, thymocyte and thymic epithelial cell numbers as well as a loss in T-cell output. Thus, the ability of infused GH to reduce thymic fat may open the available immunological space promoting re-growth and restructuring and may also serve as one of the key measures of an active prothymic, anti-aging hormonal interventional strategy. Several additional findings have demonstrated GH-mediated increases in intrathymic thymocyte trafficking and thymic cytokine/chemokine expression are also likely in play in such rejuvenation processes. While it is indeed possible that all of the above described effects of GH administration are solely due to GH-GHR interactions, it seems more likely many of the findings with GH are mediated by additional hormonal or growth factor intermediates such as IGF1 or KGF, etc. More extensive studies are necessary to more fully understand the various autocrine, paracrine and endocrine communication pathways involved in these processes. Finally, as exogenously-administered GH mediates several positive effects on the aging thymus, this begs the question “what is the precise role and function of intrathymic GH in normal thymic physiology?” Thymic GH levels appear to diminish with advancing age (Figure 2), in a manner quite similar to systemic GH levels, and thus it is possible that thymus-associated GH levels are required for thymic maintenance and with the accumulation of damage with advancing age, homeostasis is lost and the thymus and its microenvironment begins to implode and atrophy over time. It is also of interest to know if the intrathymic hormone levels are systemically controlled and the loss of peripheral signals with aging may have a similar impact on thymic homeostasis.
Figure 2. Intrathymically-expressed GH and acylated GRL proteins appear to co-localize in the murine thymus and this co-expression appears to diminish with age.
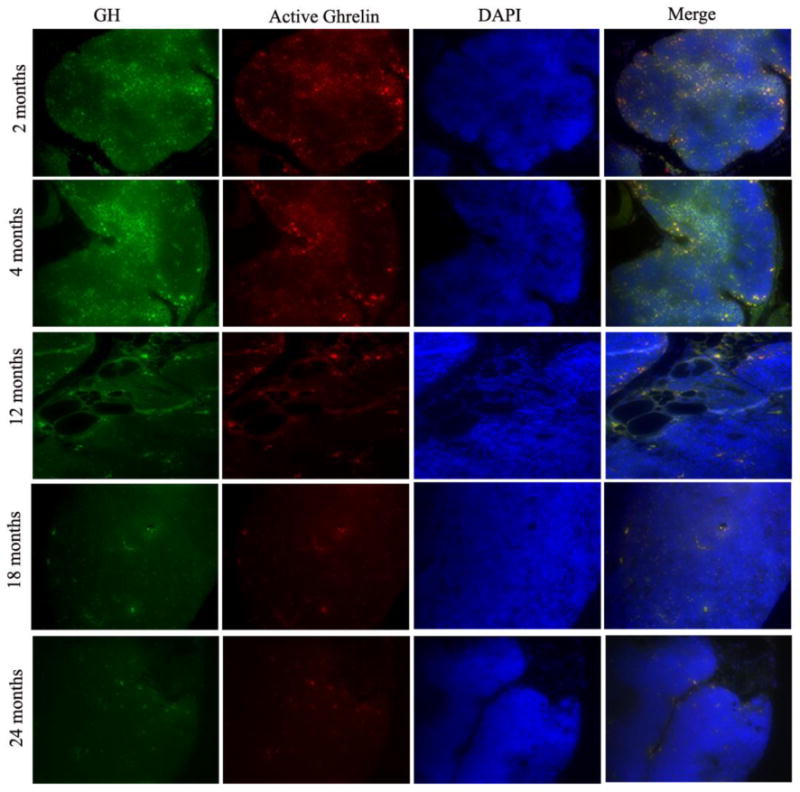
(Upper Panel) GH (green) and acylated GRL (red) immunopositivity appears to be quite strong in both the cortical and medullary regions of the 2- and 4-month old thymi shown here. The majority of the thymic GH staining appears to co-localize with the acylated GRL protein (yellow). Over time, the thymic GH and acylated GRL levels start to diminish. In 18- and 24-month old mice (Lower Panel), the total intrathymic GH and acylated GRL levels are almost entirely absent in these sections. These results have since been confirmed using real time RT-PCR analysis of whole thymi and thymocytes derived from mice at different ages (data not shown). These sections are representative of 2 or more thymi in each group,
Ghrelin
Background
The story of ghrelin biology actually starts with the discovery of its receptor and a search for synthetic analogues that mimic the positive effects of GH. Early work by Cyril Bowers and coworkers revealed that several synthetic opioid analogues elicited significant GH release from pituitary cells upon binding to unknown receptor(s) distinct from known endogenous opioid and growth hormone releasing hormone (GHRH) binding sites [82]. This class of synthetic nonpeptidyl compounds eventually became known as GH secretagogues (GHS) based on their ability to promote GH release [83-84]. It was thought that GHS could be utilized therapeutically to mimic the in vivo effects of GH but without the toxicities associated with long-term administration. Recent preclinical and clinical studies have demonstrated the efficacy of GHS in facilitating muscle development and the strengthening bones in the elderly [84]. Critical studies by Howard and colleagues [84] revealed that GHS promote the release of GH by acting on a specific G protein-coupled receptor, which they called the “growth hormone secretagogue receptor” (GHS-R). However, the biological significance of this receptor, other than being a target for a variety of GHS compounds, was for the most part unknown until the discovery of its natural endogenous ligand, ghrelin, in 1999 [82-88].
The ghrelin gene encodes a 117 amino acid peptide, pre-pro- ghrelin, which is subsequently cleaved into the mature 28 amino acid form in which it is secreted [85-88]. It is acylated on its third serine residue and this octanoylation appears to be critical for the binding of ghrelin to GHS-R. More recently, lysophospholipase-1 found in blood and expressed by various cells is believed to cleave the acyl linkage rendering the hormone inactive [86; 89-90]. Early reports have suggested that the des-acyl form of ghrelin is biologically inactive, despite being present at 3-times higher levels than acylated ghrelin in stomach. Interestingly, des-ghrelin has can be re-acylated in vivo by ingested long chain fatty acids giving rise to the speculation that there is a balance between the circulating levels of des-acyl and acylated ghrelin in the body [88]. While des-acyl ghrelin does not bind GHS-R1a, both the acylated and des-acyl form of ghrelin bind to common sites on cardiomyocytes and endothelial cells in vitro and work similarly to inhibit apoptosis [23; 85-88; 90] suggesting that both forms of ghrelin may bind and signal through a novel receptor other than GHS-R1a. Moreover, Ariyasu and colleagues (2005) have reported that transgenic mice with up to 44-fold higher levels of plasma des-ghrelin compared to wild-type mice exhibit a dwarf phenotype with a suppressed GH-IGF-1 axis [91]. This phenotype was actually quite surprising in that it is exactly opposite phenotype of what one would observe with acylated ghrelin transgenic mice. These opposite phenotypes suggest a possible role for des-ghrelin as a counterbalance to the effects of acylated ghrelin.
Similar to GHS, ghrelin function was initially described by its ability to strongly stimulate the GH release from the pituitary upon binding to the GHS-R [84-88]. Subsequently, ghrelin was also found to be a potent inducer of food intake and also increases host adiposity [84-88]. Ghrelin is predominantly expressed by the X/A-like cells of the stomach and during states of fasting, ghrelin is released from the stomach into the circulation where it mediates it effects on the feeding centers in hypothalamus to induce hunger. Ghrelin mediates its orexigenic effects via inhibition of leptin expression and the signaling of the neuropeptide Y (NPY), agouti-related peptide (AGRP) and orexin pathways. Ghrelin administration typically stimulates food intake within an hour of administration, and continuous ghrelin treatment results in sustained feeding eventually leading to increased bodyweight and adiposity. Ghrelin is believed to be the major regulator in humans as a pre-prandial rise in serum ghrelin levels induces feeding behavior [82-85]. Also, while ghrelin is considered to be a potent GHS, it is unclear whether this property is physiologically important and it has been hypothesized that ghrelin may actually serve as an adjuvant enhancing the magnitude of GH pulse in response to the growth hormone releasing hormone (GHRH). In addition to these functions, ghrelin has also been shown to mediate or influence a number of other biological actions, including effects on hormone secretion, glucose homeostasis, pancreatic function, gastrointestinal motility, cardiovascular function, neurogenesis, immunity, inflammation, cell proliferation and survival, bone metabolism, reproductive organ functions, memory, sleep, gastric emptying and gastric acid secretion, and more [84-88]. While the physiologic relevance of many of these actions remains to be defined, the role of ghrelin in energy homeostasis is currently considered its most important functions. Ghrelin administration has also been shown to attenuate cachexia associated with chronic heart failure in rats [92], whiles the GHS-R analogue, GHRP-2 counteracts protein hypercatabolism, skeletal muscle proteolysis and osteoporosis in critically ill patients with wasting condition [93]. Despite ghrelin's participation in a variety of biological functions, the initial reports failed to note any significant metabolic or physiological abnormalities were found in the ghrelin or GHS-R knockout mice [84]. However, a more recent study [94] has concludes that adult congenic ghrelin KO and GHS-R KO mice are not resistant to diet-induced obesity but under conditions of negative energy balance demonstrate impairment in maintaining glucose homeostasis. These results support a role for ghrelin modulating glucose sensing and insulin sensitivity, rather than directly regulating energy intake and energy expenditure. Perhaps more detailed studies in these mice using various disease and stress models or with progressive aging or metabolic interventions may reveal more specific defects in various organ compartments.
GRL and Immune Function
While the stomach is considered the major source of peripheral ghrelin, recent studies have demonstrated ghrelin to be widely distributed in body throughout several major organ systems including the brain, jejunum, duodenum, colon, lungs, liver, fat and placenta as well as the spleen, lymph nodes and thymus [84-88; 95-97]. We have recently described ghrelin expression by peripheral human T cells, monocytes and dendritic cells [96; D Taub, personal observations]. Moreover, ghrelin has also been shown to be constitutively expressed by a number of cancers influencing their growth and motility and it has been hypothesized that the ghrelin-GHS-R axis may serve an autocrine-paracrine role in cancer biology [88; 98-99]. In addition, GHS-R mRNA and protein has also been shown to be present on a number of human and rodent T- and B-cell and myeloid cell lines as well as by human and rodent T cells, B cells, monocytes and neutrophils, although there are wide individual variations between donors and with the state of cellular activation [23; 84; 87-88]. We have recently demonstrated that GHS-R protein in present in the lipid rafts on human T cell subsets and monocytes using immunofluorescence and flow cytometric staining [96]. In addition, human T lymphocytes also express the pre-pro form of ghrelin, and both acylated and des-ghrelin are produced and secreted upon T cell activation [96]. We also found that immune-derived ghrelin was present in a polarized fashion in association with lipid rafts, in a fashion similar to GHS-R expression in activated T cells. This raft localization of ghrelin may assist in the targeting of acylated ghrelin to the sites where its receptors are abundant and also to areas where T cell signaling is taking place. These data suggest that, similar to the GH, ghrelin has the capacity to act and signal on a wide various types of immune cells that possess ghrelin-specific receptors and thus may be stimulated via classical endocrine-derived ligands derived from the circulation and tissues and/or by autocrine/paracrine ligands produced within the immune microenvironment.
Immune cells are highly sensitive to changes in the energy balance in a tissue microenvironment and thus require proper receptors and ligands to sense such metabolic and physiological changes, which may influence immune responses and cytokine production. Conversely, cytokines and growth factors derived from immune cells also serve to act on the cells within the endocrine and central nervous systems to control food intake and energy homeostasis. Given the wide distribution of functional GHS-R on various immune cell subsets and their ability to express both the des-acyl and acylated forms of ghrelin, Taub and colleagues in 2004 [96] hypothesized that ghrelin may exert immunoregulatory effects on immune cell subpopulations. Our laboratory found that acylated but not des acylated ghrelin exerted potent inhibitory effects on the mRNA and protein expression of the proinflammatory cytokines, IL-1β, IL-6 and TNFα, by activated T cells, monocytes, dendritic cells and NK cells [D. Taub, personal observations] upon binding to cell surface GHS-Rs. This regulatory effect was found to be both dose- and time-dependent. It is currently unclear how ghrelin mediates its inhibition of cytokine mRNA and protein levels, although several studies have suggested direct effects on cytokine mRNA transcription or stability or possibly effects on protein translation. Interestingly, we have also found that ghrelin significantly attenuates leptin-induced proinflammatory and Th1-responses in human mononuclear and T cells [96] and ghrelin infusion also attenuates age-associated inflammatory cytokine expression in mice [100]. Moreover, knockdown of ghrelin expression in human T cells using siRNA resulted in increased levels of IFNγ, IL-17 and other proinflammatory cytokines and chemokines upon cellular activation suggesting that endogenous ghrelin expression influences and possibly regulates the cytokine expression pattern of a given T cell [100].
Several additional reports have supported a potential role for ghrelin in various proinflammatory disease states in humans and in animal models including increased levels of ghrelin in ulcerative colitis (UC), Crohn's disease (CD), ankylosing spondylitis, sepsis, pancreatitis, colitis, and possibly arthritis [23; 84; 87-88; 101-105]. However, it is unclear how nutritional status, the degree of inflammation and the timing at which ghrelin levels were measured may influence its association with the status of the disease. Given the influence of ghrelin on proinflammatory cytokine expression, it seems likely that changes in the systemic levels of ghrelin may be influenced by the inflammatory status of the host as well as the effects on of an inflammatory signal on the host's energy balance. This point seems a bit controversial in that endotoxin and TNFα administration have been shown both to induce and suppress ghrelin levels in humans and rodents [106-111]. However, ghrelin administration decreases the mortality of septic shock in rats and mice [112-117]. Additional studies have demonstrated that ghrelin attenuates TNFα and peroxide-induced cytokine expression by human endothelial cells [118], pancreatic damage in a rat model of cerulein-induced pancreatitis [119], hepatopancreatic injury and inflammation in rats induced by pancreaticobiliary duct ligation [120], inflammatory pain in carrageenan-induced hyperalgesia in rats [121], the clinical severity of the trinitrobenzene sulfonic acid-induced colitis in mice [122], experimental allergic encephalomyelitis (EAE) development in a mouse model [123] and arthritis [124]. Moreover, Granata and coworkers [125] have demonstrated that ghrelin treatment also prevents the apoptosis and nitric oxide release induced by interferon and TNFα from human pancreatic cells and Waseem and colleagues [126] have reported that GRL treatment of LPS-stimulated RAW 264.7 macrophage line not only inhibited the production of the pro-inflammatory cytokines IL-1β and TNFα but also resulted in an inhibition of NFκB activation and an increase in IL-10 production. These data suggested that GRL exerts its anti-inflammatory effects via the regulation of both pro- and anti-inflammatory cytokine expression. We have since confirmed and extended these data using purified in human and murine macrophages [D. Taub, personal observation]. A number of other effects of ghrelin on immune function can be found in the following reviews [23; 26; 82-88; 127]. Together, these data suggest that ghrelin possesses potent anti-inflammatory properties, and that such changes in the systemic levels of ghrelin during disease and inflammatory states may be a means to maintain the energy balance by controlling the body's response to systemic inflammation and stress and also to a degree, immune activation. The ability of ghrelin to control cytokine expression and the reciprocal regulatory effects of this hormone on leptin-induced activity on immune cells suggests the existence of a novel immunoregulatory network controlling cytokine expression, cellular activation and trafficking, and apoptosis have implications on the control of the cellular and inflammatory responses. These findings also have implications on the degree of “crosstalk” between the neuroendocrine, metabolic, and immune systems.
Ghrelin Effects on the Thymus
In addition to effects on inflammation, it was believed that the ability of GHS and ghrelin to stimulate the GH-IGF-1 axis may also influence the thymus and T cell output. To this end, Koo and colleagues treated 14-month old mice with a synthetic GHS-R agonist for three weeks and then subsequently examined the effects on the thymus and bone marrow [128]. The results revealed that GHS administration significantly increased the cellularity of the thymus in these older mice and also demonstrated an improvement in bone marrow engraftment using a SCID model. These investigators proposed that the observed effects were being mediated through GH induction in response to the GHS administration. This is supported by a study by Poppi and coworkers in 2002 [129] that demonstrated through the use of synthetic GHS-R analogues that GH secretion can be induced in peripheral immune cells as well as systemically. However, the precise role for GH or IGF1 in mediating these effects was never defined. Thus, these GHS effects may have been mediated alone or in combination with endocrine- or immune-derived GH or IGF1. More recent studies by Taub and colleagues [97] have demonstrated that both ghrelin, GHS-R and GH levels decline with progressive thymic aging (e.g., Figure 2). The loss of thymic acylated ghrelin protein expression in the thymus with age strongly correlated with thymic involution as well as with the loss the loss of thymic GH expression. As the loss of ghrelin and ghrelin receptor expression appeared to correlate with the aging and the involution process, additional studies were performed to assess if restoration of acylated ghrelin in the aged thymus may rejuvenate thymic function in aged mice. Administration of acylated but not des-acylated ghrelin into aged (14 to 22m) but not young (2 to 4m) mice resulted in a partial reversal of age-associated thymic involution. Infusion of ghrelin into young mice failed to induce any significant effects on thymic size or cellularity. This effect appeared to be independent of IGF1 as ghrelin infusion failed to induce an increase in IGF1 levels, suggesting that these effects are direct and not mediated through GH-IGF-I pathways. However, more detailed studies would be necessary to support this claim. Surprisingly, the actions of ghrelin infusion on the aging thymus appeared to be quite similar to the effects observed using its metabolic counterpart, leptin [97]. They both appeared to partially reverse age-associated thymic involution and increase thymic T cell out and peripheral T-cell diversity; however, only ghrelin specifically inhibited thymic TNFα levels and enhanced SCF expression, while leptin infusion increased the peripheral IGF1 levels and upregulated KGF expression in the aged thymus. These data suggest that while both leptin and ghrelin exert pro-thymic effects in aged but not young mice, they appear to mediate these effects through distinct mechanisms based on the differential induction of IGF1, KGF and SCF. Such a distinction is also suggested by other studies using leptin in an endotoxin model of thymic atrophy [130]. Thus, a balance between these two metabolic hormones may be critical in the regulation of thymopoiesis.
Besides increasing thymocyte numbers and weight, infusion of acylated ghrelin also significantly improved the thymic architecture and more defined cortical and medullary regions and a loss of a large percentage of thymus adiposity were observed post infusion. Interestingly, ghrelin also facilitated an increase in the numbers of both cortical and medullary epithelial cells, possibly playing a role in the restructuring of the aged thymi. Ghrelin infusions also increased the number of thymic and peripheral recent thymic emigrants (as assessed by examining the numbers of T-cell receptor excision circle [TREC] positive cells as well as the peripheral memory/naïve T-cell ratios), an improvement in the peripheral TCR diversity of both CD4+ and CD8+ T cells, increased numbers of early thymocyte progenitors (ETP) in the thymus as well as increased numbers of bone marrow-derived pluripotential stem cells and the common lymphoid progenitors (CLP) in the bone marrow [97]. Studies using GRL-and GHS-R-deficient mice revealed enhanced thymic involution with age compared to control animals along with reduced thymopoiesis, contraction of bone marrow stem cells and major perturbations in the TCR repertoire of peripheral T lymphocytes. These mice also appear to express diminished levels of the transcription factor, Pit-1 in their pituitary gland and in the thymus, which may account for the lower pituitary GH and prolactin levels as well as diminished levels of GH and IGF1 expressed in the circulation and the thymus [131]. The lower GH levels in these mice may also contribute to the premature thymic aging phenotype observed in these deficient mice. More recently, Dixit and colleagues [132] have demonstrated that caloric restriction also promotes greater T cell output in aged mice and that this increased output correlates with increased ghrelin levels. Moreover, these investigators [133] as well as our studies [1; 88; 97] have also shown that adipose development in the mouse thymus can be influenced by ghrelin infusion and signaling. Together, these results reveal a novel role for ghrelin and its receptor in thymic biology and T cell development.
Conclusions
The interplay between these hormones and their receptors in the thymic and BM compartments appear to play an important role in rejuvenation of thymic output in old animals. These data support the existence of a functional immunoregulatory network involving neuroendocrine hormones that appear to play a significant role in cytokine regulation, cellular activation and survival. Immune-associated expression of GH, GRL, IGF1 and even leptin as well as their receptors appear to significantly diminish with age in specific immune subsets and lymphoid organs, including the thymus. While the relevance of immune-derived hormones remains to be defined, we believe that the loss of lymphoid expression with age may be associated with a loss of homeostatic control of cytokine and/or growth factor expression, possibly resulting in immune dysfunction and increased levels of proinflammatory cytokines (as is commonly observed in older frail subjects). Re-administration of these hormones into older subjects may, in part, restore hematopoietic, thymic and peripheral immune activity. While the ultimate goal of many regenerative studies is to restore lymphoid function and cell numbers to those matching much younger individuals, partial restoration or recovery may be all that is possible as the accumulation of age-associated damage in DNA, lipids and proteins, the loss and/or diminished function of cellular repair pathways, the increased levels of oxidative damage, adipocyte accumulation in the primary lymphoid organs and the changes in cellular and host metabolism with age do not provide the “youthful” environment for optimal cellular regeneration, differentiation and maturation. The proposed hormonal strategies may be able to target one or more tissues and thus may have an impact in certain organ environments, such as lymphoid tissues. However, the treated individual is still aging and many other organ systems in the body remain damaged.
The age-associated reduction in LSKs and CLPs from the bone marrow and the resultant inaccessibility of sufficient ETP progenitors to restock the thymus may be one of several reasons for the loss of thymopoiesis with age. Both GH and GRL infusions appear to influence this process and induce significant increases in these hematopoietic progenitor populations, resulting in increased trafficking and accumulation of ETP numbers in the thymus and hence greater de novo T-cell output. These data suggest that these hormones play a role in the maintenance of the hematopoietic stem cell pool. In addition, the recent findings that GH administration induces the mobilization of stem cells from the bone marrow into the circulation falls in line with this theory and could be quite important to clinical studies to facilitate bone marrow engraftment and thymic regeneration [26; 71]. This study has also suggested that GH mediates an increase in SOCS protein expression post infusion, which in turn inhibits CXCL12-CXCR4 signaling resulting in the release of stem cells from their stromal layers. Whether IGF1 or GRL mediates similar effects on SOCS or related factors remains to be determine but the observed increases in LSK and CLP numbers post infusion with these hormones suggest some similarities to GH. Such hormone-mediated stem cell and progenitor mobilization could prove invaluable therapeutically in bone marrow transplants, where bone marrow and stem cell engraftment and thymic re-growth are necessary for the host's survival.
One obvious question arises from several of the studies discussed in this review, namely why does GRL appear to distinctly regulate cellular activation and inflammatory cytokine expression compared to GH and IGF1? GRL is anti-inflammatory by nature, while GH appears to be much more variable in its ability to influence cytokine expression (although a number of reports appear to favor a potentiating effect for GH on immune activation and cytokine expression). More consistently, IGF1 has been shown to be a potent immune activator and anti-apoptotic hormone and increases cytokine expression. As GRL is a GH secretagogue, one might expect GRL infusion to induce GH expression and thus the combination of these hormones might yield some similar or more uniform activities on immune function. GH is known to induce IGF1 as one of its effector intermediates, while GRL does not appear to induce such expression, at least based on our studies in middle aged and old mice infused with GRL. Perhaps additional distinct intermediates and/or paracrine mediators influence the activities of GRL or these other hormones and serve to distinguish the activities of these hormonal mediators. Or the differences in the activities between GH and GRL may simply be due to differential receptor expression on immune subsets and their associated downstream signaling pathways activated upon ligand binding. Moreover, as the expression of these hormones and their receptors diminish with age (especially on lymphoid cells), the efficacy of exogenous hormone administration may become more limited in aged vs. younger subjects, thus making clinical interventions more problematic in the older patient cohorts. While each hormone has it's advantages and disadvantages, the anti-inflammatory nature of GRL and GRL mimetics make them optimal for clinical use in the transplant setting in that GRL not only facilitates bone marrow and thymus engraftment and activity, but also suppresses age- and disease-related inflammation as well as and the cytokine storm associated with graft-vs.-host disease and graft rejection. In addition to age, a number of other factors may also influence hormonal responsiveness in vivo and in vitro including the activation and differentiation state of the target cell populations, the nutritional-metabolic status of the donor and/or host and the host's sensitivity to the hormones being administered (as hormone insensitivities occur during states of inflammation and metabolic imbalance). A greater understanding of the inter-relationships between these various hormones, their receptors and the downstream signaling pathways and the variables associated with hormonal responsiveness may provide more ideal treatment regiments for use in immunosuppressed subjects.
Based on the various publications discussed in this report, GH, IGF1 and GRL appear to function in three main areas on thymic function, namely (1) progenitor output, (2) the restoration of thymic structure/architecture and subsequently T cell output and (3) peripheral immune function. The use of hormones is a “big plus” for systemic therapeutic administration as they are typically secreted into the circulation in a steady or pulsatile manner, exposing many different organ systems to their presence, albeit at physiologic levels, without any significant toxicities. Moreover, hormones have longer half lives in vivo when compared to various cytokines and growth factors, and these agents are typically more toxic at the levels necessary to mediate therapeutic effects. In addition, cytokines and growth factors tend to be more rapidly cleared when administered systemically. Thus, the use of these hormones or bioactive mimetics, alone or in combination with other hormones, cytokines or growth factors, may assist in conditioning the bone marrow and thymus in patients pre- and post transplant or in aged subjects to enhance lymphoid trafficking, engraftment and function and in the end, optimal immune responses to pathogens and antigens. Such combinations are supported by studies by Van den Brink [58] who demonstrated that IL-7 in combination with IGF1 demonstrated an additive effect above IGF1 alone in increasing the numbers of pro-, pre-, and mature B cells and myeloid cell populations in the spleens of allo-BMT recipients on day 28 without aggravating GVHD. It would appear that these agents operate through distinct signaling mechanisms, which augment the responses when utilized in combination. Perhaps a combination of hormonal treatments in conjuction with specific cytokines and/or growth factors with specific thymotropic cytokines or growth factors may also prove more effective as interventions for aged and immunosuppressed.
Figure 3. Acylated GRL increases thymic size and cellularity in aging mice.
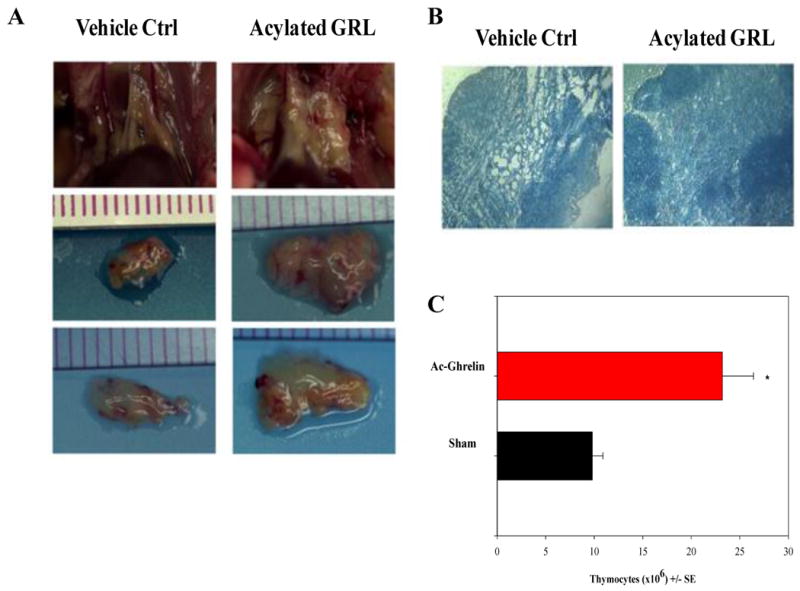
(A) Acylated GRL is infused for 2 weeks via s/c osmotic pumps after which thymic size, weight and cellularity is examined. A significant increase in thymic size in 20m mice infused with acylated GRL compared to vehicle controls. (B) GRL infusion is also associated with increased cellularity and much clearer delineation of cortex from medulla as well as a well defined CMJ, the site where progenitors enter the thymus. (C) Moreover, acylated GRL infusion into aged (20m) mice increases the total thymocyte numbers and thymic weight (not shown) compared to vehicle (Sham) control animals. More detailed studies on the prothymic effects of GRL can be found in Ref. 97.
Acknowledgments
We wish to thank former NIA post-doctoral fellows, Drs. Dixit and Yang, and our excellent technical staff members, Ana Lustig, Arnell Carter and Gary Collins for their significant efforts in the studies shown here for both the GH and GRL infusions. This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
- 1.Taub DD, Longo D. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]; This is a comprehensive review of the various theories of age-associated thymic involution and the potential mediators that can reverse and restore thymic function.
- 2.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 3.Fry TJ, Mackall CL. Current concepts of thymic aging. Springer Semin Immunopathol. 2002;24:7–22. doi: 10.1007/s00281-001-0092-5. [DOI] [PubMed] [Google Scholar]
- 4.Gill J, Malin M, Sutherland JS, Gray DHD, Hollander G, Boyd RL. Thymic generation and regeneration. Immunol Rev. 2003;195:28–50. doi: 10.1034/j.1600-065x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 5.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17(5):468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 2005;22(7):589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 8.Chidgey A, Dudakov J, Seach N, Boyd R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin Immunol. 2007;19(5):331–340. doi: 10.1016/j.smim.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp Gerontol. 2008;43(7):700–705. doi: 10.1016/j.exger.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Pfister G, Savino W. Can the immune system still be efficient in the elderly? An immunological and immunoendocrine therapeutic perspective. Neuroimmunomodulation. 2008;15(4-6):351–364. doi: 10.1159/000156477. [DOI] [PubMed] [Google Scholar]
- 11.Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, Pini E, Monti D, Franceschi C. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15(4-6):224–240. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- 12.Shanley DP, Aw D, Manley NR, Palmer DB. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009;30(7):374–381. doi: 10.1016/j.it.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30(7):366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5(6):575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 15.Taub DD. Neuroendocrine Interactions in the Immune System. Cell Immunol. 2008;252(1-2):1–6. doi: 10.1016/j.cellimm.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel K, Taub DD. Role of neuropeptides, hormones, and growth factors in regulating thymopoiesis in middle to old age. F1000 Biology Reports. 2009;1:42–46. doi: 10.3410/B1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland AM, van den Brink MR. Rejuvenation of the aging T cell compartment. Curr Opin Immunol. 2009;21(4):454–459. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp Gerontol. 2008;43(7):700–705. doi: 10.1016/j.exger.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30(7):366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leposavić G, Perisić M. Age-associated remodeling of thymopoiesis: role for gonadal hormones and catecholamines. Neuroimmunomodulation. 2008;15(4-6):290–322. doi: 10.1159/000156473. [DOI] [PubMed] [Google Scholar]
- 21.Fülöp T, Larbi A, Hirokawa K, Mocchegiani E, Lesourds B, Castle S, Wikby A, Franceschi C, Pawelec G. Immunosupportive therapies in aging. Clin Interv Aging. 2007;2(1):33–54. doi: 10.2147/ciia.2007.2.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol. 2008;252(1-2):122–138. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Hattori N. Expression, regulation and biological actions of growth hormone(GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19(3):187–197. doi: 10.1016/j.ghir.2008.12.001. [DOI] [PubMed] [Google Scholar]; This is an excellent review of the current literature concerning GH and ghrelin on immune function and the clinical applications of hormone administration on inflammatory cytokine production and disease processes.
- 24.Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21(3):292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]; An excellent review and commentary regarding the effects of GH, Prolactin, IGF1 and thyroid hormones and their receptors on lymphocytes and the caveats of such studies based on the lack of an overt immune phenotype in hormone ligand and receptor KO mice.
- 25.Welniak LA, Sun R, Murphy WJ. The role of growth hormone in T-cell development and reconstitution. J Leukoc Biol. 2002;71(3):381–387. [PubMed] [Google Scholar]
- 26.Redelman D, Welniak LA, Taub D, Murphy WJ. Neuroendocrine hormones such as growth hormone and prolactin are integral members of the immunological cytokine network. Cell Immunol. 2008;252(1-2):111–121. doi: 10.1016/j.cellimm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an up-to-date review discussing the various hypotheses and uses for GH and GRL in the clinical setting, including transplant models and on various aspects of immune activity.
- 27.O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol. 2008;252(1-2):91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another excellent and comprehensive review by Dr. Kelley regarding the role of IGF1 on immune activation, apoptosis and cytokine expression.
- 28.Welniak LA, Tian ZG, Sun R, Keller JR, Richards S, Ruscetti FW, Murphy WJ. Effects of growth hormone and prolactin on hematopoiesis. Leuk Lymphoma. 2000;38(5-6):435–445. doi: 10.3109/10428190009059263. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res. 2009;71(2):36–40. doi: 10.1159/000192434. [DOI] [PubMed] [Google Scholar]
- 30.Bartke A. Growth hormone and aging: a challenging controversy. Clin Interv Aging. 2008;3(4):659–665. doi: 10.2147/cia.s3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146(2):104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 32.Welniak LA, Sun R, Murphy WJ. The role of growth hormone in T-cell development and reconstitution. J Leukoc Biol. 2002;71(3):381–387. [PubMed] [Google Scholar]
- 33.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–4291. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 34.Savino W. Neuroendocrine control of T cell development in mammals: role of growth hormone in modulating thymocyte migration. Exp Physiol. 2007;92(5):813–817. doi: 10.1113/expphysiol.2007.038422. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Wang J, Cacioppo JT, Glaser R, Kiecolt-Glaser JK, Malarkey WB. Chronic stress associated with spousal caregiving of patients with Alzheimer's dementia is associated with downregulation of B-lymphocyte GH mRNA. J Gerontol A: Biol Sci Med Sci. 1999;54:M212–M215. doi: 10.1093/gerona/54.4.m212. [DOI] [PubMed] [Google Scholar]
- 36.Sumita K, Hattori N, Inagaki C. Effects of growth hormone on the differentiation of mouse B-lymphoid precursors. J Pharmacol Sci. 2005;97:408–416. doi: 10.1254/jphs.fpj04054x. [DOI] [PubMed] [Google Scholar]
- 37.Kähler CM, Pischel AB, Haller T, Meierhofer C, Djanani A, Kaufmann G, Wiedermann CJ. Signal transduction pathways in directed migration of human monocytes induced by human growth hormone in vitro. Int Immunopharmacol. 2001;1:1351–1361. doi: 10.1016/s1567-5769(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 38.Mitsunaka H, Dobashi H, Sato M, Tanaka T, Kitanaka A, Yamaoka G, Tokuda G, Matoba K, Hiraishi T, Ishida T. Growth hormone prevents Fas induced apoptosis in lymphocytes through modulation of Bcl-2 and caspase-3. Neuroimmunomodulation. 2001;9:256–262. doi: 10.1159/000054288. [DOI] [PubMed] [Google Scholar]
- 39.Lempereur L, Brambilla D, Scoto GM, D'Alcamo M, Goffin V, Crosta L, Palmucci T, Rampello L, Bernardini R, Cantarella G. Growth hormone protects human lymphocytes from irradiation-induced cell death. Brit J Pharmacol. 2003;138:1411–1416. doi: 10.1038/sj.bjp.0705173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold RE, Weigent DA. The inhibition of apoptosis in EL4 lymphoma cells overexpressing growth hormone. Neuroimmunomodulation. 2004;11:149–159. doi: 10.1159/000076764. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee A, Helbert M, Davis J, Shalet S. Immune function in hypopituitarism; time to reconsider. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03751.x. [DOI] [PubMed] [Google Scholar]
- 42.Borghetti P, Saleri R, Mocchegiani E, Corradi A, Martelli P. Infection, immunity and the neuroendocrine response. Vet Immunol Immunopathol. 2009;130(3-4):141–162. doi: 10.1016/j.vetimm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnard A, Layton D, Hince M, Sakkal S, Bernard C, Chidgey A, Boyd R. Impact of the neuroendocrine system on thymus and bone marrow function. Neuroimmunomodulation. 2008;15(1):7–18. doi: 10.1159/000135619. [DOI] [PubMed] [Google Scholar]
- 44.Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252(1-2):16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Uronen-Hansson H, Allen ML, Lichtarowicz-Krynska E, Aynsley-Green A, Cole TJ, Höidén-Guthenberg I, Fryklund L, Klein N. Growth hormone enhances proinflammatory cytokine production by monocytes in whole blood. Growth Horm IGF Res. 2003;13:282–286. doi: 10.1016/s1096-6374(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 46.Sodhi A, Tripathi A. Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p-38 MAP kinase, STAT3 and NF-kappaB. Cytokine. 2008;41:162–173. doi: 10.1016/j.cyto.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Bozzola M, De Benedetti F, De Amici M, Jouret B, Travaglino P, Pagani S, Conte F, Tauber M. Stimulating effect of growth hormone on cytokine release in children. Eur J Endocrinol. 2003;149:397–401. doi: 10.1530/eje.0.1490397. [DOI] [PubMed] [Google Scholar]
- 48.Serri O, St-Jacques P, Sartippour M, Renier G. Alterations of monocyte function in patients with growth hormone (GH) deficiency: effect of substitutive GH therapy. J Clin Endocrinol Metab. 1999;84:58–63. doi: 10.1210/jcem.84.1.5374. [DOI] [PubMed] [Google Scholar]
- 49.Adamopoulos S, Parissis JT, Paraskevaidis I, Karatzas D, Livanis E, Georgiadis M, Karavolias G, Mitropoulos D, Degiannis D, Kremastinos DT. Effects of growth hormone on circulating cytokine network, and left ventricular contractile performance and geometry in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2003;24:2186–2196. doi: 10.1016/s0195-668x(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 50.Franco C, Andersson B, Lönn L, Bengtsson BA, Svensson J, Johannsson G. Growth hormone reduces inflammation in postmenopausal women with abdominal obesity: a 12-month, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;92:2644–2647. doi: 10.1210/jc.2007-0068. [DOI] [PubMed] [Google Scholar]
- 51.Zarkesh-Esfahani SH, Kolstad O, Metcalfe RA, Watson PF, von Laue S, Walters S, Revhaug A, Weetman AP, Ross RJ. High-dose growth hormone does not affect proinflammatory cytokine (tumor necrosis factor-alpha interleukin-6 and interferon-gamma) release from activated peripheral blood mononuclear cells or after minimal to moderate surgical stress. J Clin Endocrinol Metab. 2000;85:3383–3390. doi: 10.1210/jcem.85.9.6823. [DOI] [PubMed] [Google Scholar]
- 52.Greenhalgh CJ, Alexander WS. Suppressors of cytokine signalling and regulation of growth hormone action. Growth Horm IGF Res. 2004;14(3):200–206. doi: 10.1016/j.ghir.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Mol Endocrinol. 2007;21:2503–2515. doi: 10.1210/me.2006-0498. [DOI] [PubMed] [Google Scholar]; This article describes the ability of GH to regulate SOCS3 expression, which may have a significant impact on its ability to regulate cytokine activity as well as stem cell mobilization.
- 54.Tollet-Egnell P, Flores-Morales A, Stavreus-Evers A, Sahlin L, Norstedt G. Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology. 1999;140:3693–3704. doi: 10.1210/endo.140.8.6878. [DOI] [PubMed] [Google Scholar]
- 55.Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol Endocrinol. 2006;20:241–253. doi: 10.1210/me.2005-0170. [DOI] [PubMed] [Google Scholar]
- 56.Dardenne M, Smaniotto S, de Mello-Coelho V, Villa-Verde DM, Savino W. Growth hormone modulates migration of developing T cells. Ann N Y Acad Sci. 2009;1153:1–5. doi: 10.1111/j.1749-6632.2008.03977.x. [DOI] [PubMed] [Google Scholar]
- 57.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 58.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O'Reilly RJ, van den Brink MR. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 59.Sirohi B, Powles R, Morgan G, Treleaven J, Kulkarni S, Horton C, Saso R, Rolfe D, Cook G, Shaw C, Wass J. Use of physiological doses of human growth hormone in haematological patients receiving intensive chemotherapy promotes haematopoietic recovery: a double-blind randomized, placebo-controlled study. Bone Marrow Transplant. 2007;39:115–120. doi: 10.1038/sj.bmt.1705545. [DOI] [PubMed] [Google Scholar]
- 60.Montecino-Rodriguez E, Clark R, Dorshkind K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology. 1998;139:4120–4126. doi: 10.1210/endo.139.10.6263. [DOI] [PubMed] [Google Scholar]
- 61.French RA, Broussard SR, Meier WA, Minshall C, Arkins S, Zachary JF, Dantzer R, Kelley KW. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143:690–699. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 62.Savino W, Smaniotto S, De Mello-Coelho V, Dardenne M. Is there a role for growth hormone upon intrathymic T-cell migration? Ann N Y Acad Sci. 2000;917:748–754. doi: 10.1111/j.1749-6632.2000.tb05439.x. [DOI] [PubMed] [Google Scholar]
- 63.Smaniotto S, Mendes-da-Cruz DA, Carvalho-Pinto CE, Araujo LM, Dardenne M, Savino W. Combined role of extracellular matrix and chemokines on peripheral lymphocyte migration in growth hormone transgenic mice. Brain Behav Immun. 2010;24(3):451–461. doi: 10.1016/j.bbi.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Dardenne M, Smaniotto S, de Mello-Coelho V, Villa-Verde DM, Savino W. Growth hormone modulates migration of developing T cells. Ann N Y Acad Sci. 2009 Feb;1153:1–5. doi: 10.1111/j.1749-6632.2008.03977.x. [DOI] [PubMed] [Google Scholar]
- 65.Smaniotto S, de Mello-Coelho V, Villa-Verde DM, Pléau JM, Postel-Vinay MC, Dardenne M, Savino W. Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology. 2005;146(7):3005–3017. doi: 10.1210/en.2004-0709. [DOI] [PubMed] [Google Scholar]
- 66.Smaniotto S, Ribeiro-Carvalho MM, Dardenne M, Savino W, de Mello-Coelho V. Growth hormone stimulates the selective trafficking of thymic CD4+CD8- emigrants to peripheral lymphoid organs. Neuroimmunomodulation. 2004;11(5):299–306. doi: 10.1159/000079410. [DOI] [PubMed] [Google Scholar]
- 67.Savino W, Mendes-da-Cruz DA, Silva JS, Dardenne M, Cotta-de-Almeida V. Intrathymic T-cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 2002;23(6):305–313. doi: 10.1016/s1471-4906(02)02224-x. [DOI] [PubMed] [Google Scholar]
- 68.de Mello Coelho V, Villa-Verde DM, Farias-de-Oliveira DA, de Brito JM, Dardenne M, Savino W. Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells. Neuroendocrinology. 2002;75(2):139–150. doi: 10.1159/000048230. [DOI] [PubMed] [Google Scholar]
- 69.Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, Peault B, Bordignon C. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 70.Soriano SF, Hernanz-Falcon P, Rodriguez-Frade JM, De Ana AM, Garzon R, Carvalho-Pinto C, Vila-Coro AJ, Zaballos A, Balomenos D, Martinez AC, Mellado M. Functional inactivation of CXC chemokine receptor 4-mediated responses through SOCS3 up-regulation. J Exp Med. 2002;196:311–321. doi: 10.1084/jem.20012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pello OM, Moreno-Ortiz Mdel C, Rodriguez-Frade JM, Martinez-Munoz L, Lucas D, Gomez L, Lucas P, Samper E, Aracil M, Martinez C, Bernad A, Mellado M. SOCS up-regulation mobilizes autologous stem cells through CXCR4 blockade. Blood. 2006;108:3928–3937. doi: 10.1182/blood-2006-02-006353. [DOI] [PubMed] [Google Scholar]; An interesting manuscript discussing the ability of SOCS proteins to inhibit CXCR4 signaling and function, which in turn releases hematopoietic stems cells from the bone marrow stroma permitting them to enter the circulation.
- 72.Kelley KW. From hormones to immunity: the physiology of immunology. Brain Behav Immun. 2004;18:95–113. doi: 10.1016/j.bbi.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Tesselaar K, Miedema F. Growth hormone resurrects adult human thymus during HIV-1 infection. J Clin Invest. 2008;118:844–847. doi: 10.1172/JCI35112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Napolitano LA, Lo JC, Gotway MB, Mulligan K, Barbour JD, Schmidt D, Grant RM, Halvorsen RA, Schambelan M, McCune JM. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS. 2002;16:1103–1111. doi: 10.1097/00002030-200205240-00003. [DOI] [PubMed] [Google Scholar]
- 75.Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert El, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, Li K, Killian ML, Bacchetti P, McCune JM. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118:1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript describes the prothymic effects of GH infusion on thymic regeneration and output in a controlled study of HIV-1 infected subjects and includes detailed measurements of de novo thymic activity.
- 76.Pires A, Pido-Lopez J, Moyle G, Gazzard B, Gotch F, Imami N. Enhanced T-cell maturation, differentiation and function in HIV-1-infected individuals after growth hormone and highly active antiretroviral therapy. Antivir Ther. 2004;9:67–75. [PubMed] [Google Scholar]
- 77.Goodier MR, Imami N, Moyle G, Gazzard B, Gotch F. Loss of the CD56hiCD16- NK cell subset and NK cell interferon-gamma production during antiretroviral therapy for HIV-1: partial recovery by human growth hormone. Clin Exp Immunol. 2003;134:470–476. doi: 10.1111/j.1365-2249.2003.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrhaye G, Kermani H, Legros JJ, Baron F, Beguin Y, Moutschen M, Cheynier R, Martens HJ, Geenen V. Impact of growth hormone (GH) deficiency and GH replacement upon thymus function in adult patients. PLoS One. 2009;4(5):e5668. doi: 10.1371/journal.pone.0005668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rapaport R, Oleske J, Ahdieh H, Solomon S, Delfaus C, Denny T. Suppression of immune function in growth hormone-deficient children during treatment with human growth hormone. J Pediatr. 1986;109(3):434–439. doi: 10.1016/s0022-3476(86)80113-5. [DOI] [PubMed] [Google Scholar]
- 80.Dorshkind K, Horseman ND. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays. 2001;23(3):288–294. doi: 10.1002/1521-1878(200103)23:3<288::AID-BIES1039>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 81.Dorshkind K, Welniak L, Gault RA, Hixon J, Montecino-Rodriguez E, Horseman ND, Gertner JM, Murphy WJ. Effects of housing on the thymic deficiency in dwarf mice and its reversal by growth hormone administration. Clin Immunol. 2003;109(2):197–202. doi: 10.1016/s1521-6616(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 82.Tannenbaum GS, Bowers CY. Interactions of growth hormone secretagogues and growth hormone-releasing hormone/somatostatin. Endocrine. 2001;14(1):21–27. doi: 10.1385/ENDO:14:1:021. [DOI] [PubMed] [Google Scholar]
- 83.Frutos MG, Cacicedo L, Fernández C, Vicent D, Velasco B, Zapatero H, Sánchez-Franco F. Insights into a role of GH secretagogues in reversing the age-related decline in the GH/IGF-I axis. Am J Physiol Endocrinol Metab. 2007;293(5):E1140–1152. doi: 10.1152/ajpendo.00236.2007. [DOI] [PubMed] [Google Scholar]
- 84.Smith RG, Jiang H, Sun Y. Developments in ghrelin biology and potential clinical relevance. Trends Endocrinol Metab. 2005;16:436–442. doi: 10.1016/j.tem.2005.09.004. [DOI] [PubMed] [Google Scholar]; This is an excellent review of some of the current literature regarding ghrelin and its receptor and the biological and molecular functions of this hormone and GHS in various organ systems and in the clinical setting.
- 85.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 86.Kojima M, Kangawa K. Ghrelin structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 87.Dixit VD, Taub DD. Ghrelin and immunity: a young player in an old field. Exp Gerontol. 2005;40:900–910. doi: 10.1016/j.exger.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Taub DD. Novel Connections between the Neuroendocrine and Immune Systems: The Ghrelin Immunoregulatory Network. Vitam Horm. 2007;77:325–346. doi: 10.1016/S0083-6729(06)77014-5. [DOI] [PubMed] [Google Scholar]
- 89.Shanado Y, Kometani M, Uchiyama H, Koizumi S, Teno N. Lysophospholipase I identified as a ghrelin deacylation enzyme in rat stomach. Biochem Biophys Res Commun. 2004;325(4):1487–1494. doi: 10.1016/j.bbrc.2004.10.193. [DOI] [PubMed] [Google Scholar]
- 90.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, Kangawa K, Nakao K. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146(1):355–364. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- 92.Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, Yu XX, Guo S, Chen MC, Chen C. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289(4):H1643–651. doi: 10.1152/ajpheart.01042.2004. [DOI] [PubMed] [Google Scholar]
- 93.Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, Bowers CY, Bouillon R. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab. 1999;84(4):1311–1323. doi: 10.1210/jcem.84.4.5636. [DOI] [PubMed] [Google Scholar]
- 94.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988–2993. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 96.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first paper describing the anti-inflammatory effects of acylated ghrelin on proinflammatory cytokine expression induced by cellular activation and leptin treatment both in vitro and in vivo.
- 97.Dixit VD, Yang H, Sun Y, Weeraratna AT, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117(10):2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first paper describing the prothymic effects of acylated ghrelin and leptin on murine hematopoiesis and thymopoiesis in aged but not young mice.
- 98.Dixit VD, Weeraratna AT, Yang H, Bertak D, Cooper-Jenkins A, Riggins GJ, Eberhart CG, Taub DD. Ghrelin and the growth hormone secretagogue receptor constitute a novel autocrine pathway in astrocytoma motility. J Biol Chem. 2006;281(24):16681–16690. doi: 10.1074/jbc.M600223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeffery PL, Herington AC, Chopin LK. The potential autocrine/paracrine roles of ghrelin and its receptor in hormone-dependent cancer. Cytokine Growth Factor Rev. 2003;14(2):113–122. doi: 10.1016/s1359-6101(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 100.Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009;113(21):5202–520. doi: 10.1182/blood-2008-09-181255. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript describes the ability of infused acylated ghrelin to inhibit age-associated proinflammatory cytokine expression including IL-6 and IL-17 levels and also demonstrates the effects of knocking down endogenous ghrelin expression in human T cells on cellular activation and cytokine expression.
- 101.Katergari SA, Milousis A, Pagonopoulou O, Asimakopoulos B, Nikolettos NK. Ghrelin in pathological conditions. Endocr J. 2008;55(3):439–53. doi: 10.1507/endocrj.k07-106. [DOI] [PubMed] [Google Scholar]
- 102.Kouroumalis Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 103.Toussirot E, Streit G, Nguyen NU, Dumoulin G, Le Huédé G, Saas P, Wendling D. Adipose tissue, serum adipokines, and ghrelin in patients with ankylosing spondylitis. Metabolism. 2007;56:1383–1389. doi: 10.1016/j.metabol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Maruna P, Gürlich R, Frasko R, Rosicka M. Ghrelin and leptin elevation in postoperative intra-abdominal sepsis. Eur Surg Res. 2005;37:354–359. doi: 10.1159/000090336. [DOI] [PubMed] [Google Scholar]
- 105.Kerem M, Bedirli A, Pasaoglu H, Unsal C, Yilmaz TU, Ofluoglu E, Sahin TT. Role of ghrelin and leptin in predicting the severity of acute pancreatitis. Digest Dis Sci. 2007;52:950–955. doi: 10.1007/s10620-006-9150-0. [DOI] [PubMed] [Google Scholar]
- 106.Otero M, Nogueiras R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Chronic inflammation modulates ghrelin levels in humans and rats. Rheumatology. 2004;43:306–310. doi: 10.1093/rheumatology/keh055. [DOI] [PubMed] [Google Scholar]
- 107.Vila G, Maier C, Riedl M, Nowotny P, Ludvik B, Luger A, Clodi M. Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J Clin Endocrinol Metab. 2007;92:3930–3934. doi: 10.1210/jc.2007-1194. [DOI] [PubMed] [Google Scholar]
- 108.Basa NR, Wang L, Arteaga JR, Heber D, Livingston EH, Taché Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci Lett. 2003;343:25–28. doi: 10.1016/s0304-3940(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 109.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, Taché Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 110.Hataya Y, Akamizu T, Hosoda H, Kanamoto N, Moriyama K, Kangawa K, Takaya K, Nakao K. Alterations of plasma ghrelin levels in rats with lipopolysaccharide-induced wasting syndrome and effects of ghrelin treatment on the syndrome. Endocrinology. 2003;144:5365–5371. doi: 10.1210/en.2003-0427. [DOI] [PubMed] [Google Scholar]
- 111.Endo M, Masaki T, Seike M, Yoshimatsu H. Involvement of stomach ghrelin and hypothalamic neuropeptides in tumor necrosis factor-alpha-induced hypophagia in mice. Regulat Pept. 2007;140:94–100. doi: 10.1016/j.regpep.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 112.Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C. Therapeutic effects of ghrelin on endotoxic shock in rats. Eur J Pharmacol. 2003;473:171–176. doi: 10.1016/s0014-2999(03)01972-1. [DOI] [PubMed] [Google Scholar]
- 113.Wu R, Dong W, Zhou M, Cui X, Hank Simms H, Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318–326. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 114.Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, Wang P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu R, Zhou M, Das P, Dong W, Ji Y, Yang D, Miksa M, Zhang F, Ravikumar TS, Wang P. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–E1702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 116.De Winter BY, De Man JG, Seerden TC, Depoortere I, Herman AG, Peeters TL, Pelckmans PA. Effect of ghrelin and growth hormone-releasing peptide 6 on septic ileus in mice. Neurogastroenterol Motil. 2004;16(4):439–46. doi: 10.1111/j.1365-2982.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 117.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008 Jun 15;180(12):8369–77. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 118.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 119.Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Stachura J, Konturek SJ, Konturek PC. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54:561–573. [PubMed] [Google Scholar]
- 120.Kasımay O, Iseri SO, Barlas A, Bangir D, Yegen C, Arbak S, Yegen BC. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res. 2006;36:11–19. doi: 10.1016/j.hepres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 121.Sibilia V, Lattuada N, Rapetti D, Pagani F, Vincenza D, Bulgarelli I, Locatelli V, Guidobono F, Netti C. Ghrelin inhibits inflammatory pain in rats: involvement of the opioid system. Neuropharmacology. 2006;51:497–505. doi: 10.1016/j.neuropharm.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 123.Theil MM, Miyake S, Mizuno M, Tomi C, Croxford JL, Hosoda H, Theil J, von Hörsten S, Yokote H, Chiba A, Lin Y, Oki S, Akamizu T, Kangawa K, Yamamura T. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J Immunol. 2009;183(4):2859–2866. doi: 10.4049/jimmunol.0803362. [DOI] [PubMed] [Google Scholar]
- 124.Granado M, Priego T, Martín AI, Villanúa MA, López-Calderón A. Antiinflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:E486–E492. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- 125.Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghè C, Isgaard J, Papotti M, Ghigo E, Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 30,50-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3- kinase/Akt signaling. Endocrinology. 2007;148:512–529. doi: 10.1210/en.2006-0266. [DOI] [PubMed] [Google Scholar]
- 126.Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143(3):334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008 Oct;84(4):882–92. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koo GC, Huang C, Camacho R, Trainor C, Blake JT, Sirotina-Meisher A, Schleim KD, Wu TJ, Cheng K, Nargund R, McKissick G. Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166:4195–4201. doi: 10.4049/jimmunol.166.6.4195. [DOI] [PubMed] [Google Scholar]; The first paper demonstrating the use of a GHS to influence both thymic and bone marrow function within aged mice, presumably via the release of GH and IGF1.
- 129.Poppi L, Dixit VD, Baratta M, Giustina A, Tamanini C, Parvizi N. Growth hormone secretagogue (GHS) analogue, hexarelin stimulates GH from peripheral lymphocytes. Exp Clin Endocrinol Diab. 2002;110:343–347. doi: 10.1055/s-2002-34991. [DOI] [PubMed] [Google Scholar]
- 130.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177(1):169–76. doi: 10.4049/jimmunol.177.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang H, Dixit VD, Patel K, Vandanmagsar B, Collins G, Sun Y, Smith RG, Taub DD. Reduction in hypophyseal growth hormone and prolactin expression due to deficiency in ghrelin receptor signaling is associated with Pit-1 suppression: relevance to the immune system. Brain Behav Immun. 2008;22(8):1138–1145. doi: 10.1016/j.bbi.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009;183(5):3040–52. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Youm YH, Yang H, Sun Y, Smith RG, Manley NR, Vandanmagsar B, Dixit VD. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem. 2009;284(11):7068–7077. doi: 10.1074/jbc.M808302200. [DOI] [PMC free article] [PubMed] [Google Scholar]


