Abstract
Objectives
Surrogate markers of HIV disease progression are HIV RNA in plasma viral load (VL) and CD4 cell count (immune function). Despite improved international access to antiretrovirals, surrogate marker diagnostics are not routinely available in resource-limited settings. Therefore, the objective was to assess effects of economic and diagnostic resourcing on patient treatment outcomes.
Methods
Analyses were based on 2333 patients initiating highly active antiretroviral therapy (HAART) from 2000 onwards. Sites were categorized by World Bank country income criteria (high/low) and annual frequency of VL (≥ 3, 1–2 or <1) or CD4 (≥ 3 or <3) testing. Endpoints were time to AIDS/death and change in CD4 cell count and VL suppression (<400 HIV-1 RNA copies/mL) at 12 months. Demographics, Centers for Disease Control and Prevention (CDC) classification, baseline VL/CD4 cell counts, hepatitis B/C coinfections and HAART regimen were covariates. Time to AIDS/death was analysed by proportional hazards models. CD4 and VL endpoints were analysed using linear and logistic regression, respectively.
Results
Increased disease progression was associated with site-reported VL testing less than once per year [hazard ratio (HR)=1.4; P=0.032], severely symptomatic HIV infection (HR=1.4; P=0.003) and hepatitis C virus coinfection (HR=1.8; P=0.011). A total of 1120 patients (48.2%) had change in CD4 cell count data. Smaller increases were associated with older age (P<0.001) and `Other' HIV source exposures, including injecting drug use and blood products (P=0.043). A total of 785 patients (33.7%) contributed to the VL suppression analyses. Patients from sites with VL testing less than once per year [odds ratio (OR)=0.30; P<0.001] and reporting `Other' HIV exposures experienced reduced suppression (OR=0.28; P<0.001).
Conclusion
Low measures of site resourcing were associated with less favourable patient outcomes, including a 35% increase in disease progression in patients from sites with VL testing less than once per year.
Keywords: antiretroviral therapy, Asia, CD4 counts, diagnostic monitoring, viral load
Introduction
Highly active antiretroviral therapy (HAART) suppresses HIV viral load (VL) resulting in enhanced patient immune function and reduced risk of opportunistic infections and death [1,2]. Disparities remain in patient access to antiretrovirals (ARVs), however, the challenges of treatment coverage and health system capacity are being progressively addressed [3]. As a result, more HIV-infected patients in developing and transitional economies have the opportunities of decreased morbidity and longer survival as have been observed in developed economies [4–6].
Predictive biomarkers of disease progression are HIV RNA in plasma (VL) and CD4 cell count (immune function) [7]. HIV RNA informs knowledge of trends in viral replication and gives advance notice of non-adherence, treatment regimen failure and HIV drug resistance (HIVDR) [8,9]. CD4 cell counts provide quantitative measures of immunocompetence and current clinical status [10]. Furthermore, international patient management guidelines recommend periodic collection of HIV RNA and CD4 cell counts to determine indications for treatment and the monitoring of therapeutic response [11,12].
Still, in developing countries access to disease staging diagnostics has lagged considerably behind the availability of anti-HIV medications [13]. Consequently, monitoring of patient status via surrogate markers, thereby identifying optimal therapy initiation periods and when treatment should be changed, is not available in resource-limited settings at a level comparable to that found in developed economies [13–15]. Plasma VL commercial assay kits and CD4 reagents remain expensive. Assays require dedicated space and equipment and infrastructure costs are prohibitive. Further, the lack of physical resources, such as uninterrupted electricity and water, and the cost and availability of maintenance impact upon whether valid results of patient prognostic status are obtained even when infrastructure is in place [13,16]. Currently, there is little information on how the lack of economic and, particularly, diagnostic resourcing affects patient health outcomes. Therefore, our objective was to determine whether clinical resourcing, measured as country income and site-reported frequencies of CD4 and VL diagnostic testing, impacted on patient treatment outcomes.
Methods
Sites
The TREAT Asia (Therapeutics Research, Education, and AIDS Training in Asia) HIV Observational Database (TAHOD) is a multicentre prospective cohort of HIV-infected patients, established since September 2003. Data are shared with the International Epidemiologic Databases to Evaluate AIDS (IeDEA). One objective of TAHOD is to evaluate the natural history of HIV disease in ARV-experienced and -naïve patients in the Asia-Pacific region. Seventeen clinical sites (see Appendix A) are included in TAHOD based upon capacity to fulfil data submission requirements and with a view to retaining sites representative of the region [5]. Ethics approvals were obtained from local Institutional Review Boards and each site sequentially enrolled approximately 200 patients. Where available, sites provided retrospective data for enrollees and clinical interventions and testing procedures were implemented according to local practices.
Patient data
Average follow-up for TAHOD patients in the 12-month period from September 2005 to September 2006 was 86%.
Since not all TAHOD patients are taking ARVs, our sampling frame was HIV-infected patients initiating HAART, any combination of three or more ARVs, from 2000 onwards. Eligible patients were also required to have at least one subsequent clinical visit or result recorded in the database, post-therapy, at the time of analysis. Patient covariates included demographics (age at entry to cohort, gender, HIV source exposure), indices of illness severity [Centers for Disease Control and Prevention (CDC) classification, baseline CD4 lymphocyte count and HIV RNA], hepatitis B and C coinfections and prescribed HAART regimen. Retrospective and prospective data were included.
The CDC classification for TAHOD was modified from the 1993 Center for Disease Control and Prevention case definition in that it does not differentiate between presumptive and definitive diagnoses [17]. The most severe pre-HAART CDC category recorded was used as the baseline clinical status. Hepatitis B (C) positive status was defined as being HBsAg (HCV-Ab) positive and patients were assumed to be coinfected for the duration of follow-up. HIV RNA copies/mL and CD4 cell counts up to 91 days prior to HAART initiation were considered for inclusion as baseline values. Where multiple assay results existed, the value closest to the target date was selected.
Resource covariates
For classifying TAHOD sites with respect to clinical site resourcing, the four-category World Bank criterion (gross national income per capita) was dichotomized into high (upper-middle and upper: >USD 3705) and low (lower-middle and lower: ≤USD 3705) [18]. The annual frequencies of VL and CD4 monitoring of patients reported between December 2006 and February 2007 were also included as measures of site resourcing. VL monitoring was classified into three categories: at least three times per year, one to two times per year and less than once per year. Since most sites reported that patients were CD4 tested at least annually, CD4 monitoring was classified into two categories: at least three times and fewer than three times per year. The two exceptions monitored patients at least annually when resources were available to do so.
Endpoints
Clinical disease progression was determined as a new diagnosis of an AIDS-defining illness (CDC category C) or death from any cause. Patient follow-up commenced at HAART initiation and ended at date of death, AIDS-defining illness or most recent contact, whichever was the earliest. Surrogate endpoints were HIV RNA viral suppression (<400 copies/mL) and change in CD4 cell count from baseline at 12 months post-HAART. Surrogate marker values closest to the target date were selected from windows of 9–15 months.
Statistical analysis
Patients contributing data to each analysis are shown in Fig. 1. For eligible patients, baseline comparisons by country income (χ2, Fisher's exact or Cochrane – Armitage test for trend) were performed as appropriate. Determinants of 12-month HIV RNA suppression and change in CD4 cell count were assessed via logistic regression and linear regression, respectively. Proportional hazards models were used to evaluate predictors of time to progression to new AIDS-defining illness or death. Analyses were based on an intention to continue treatment approach in that we did not take into account regimen changes, interruptions or failure post-HAART. Forward stepwise techniques were used to determine the best fitting models. To identify significant variables and important confounders, binary covariate P-values and multi-categorical parameter P-values (from tests for trend/heterogeneity) of <0.2, in univariate analyses, were considered for inclusion in multivariate models. Final multivariate models consisted of covariates remaining significant at the 0.05 level.
Fig. 1.
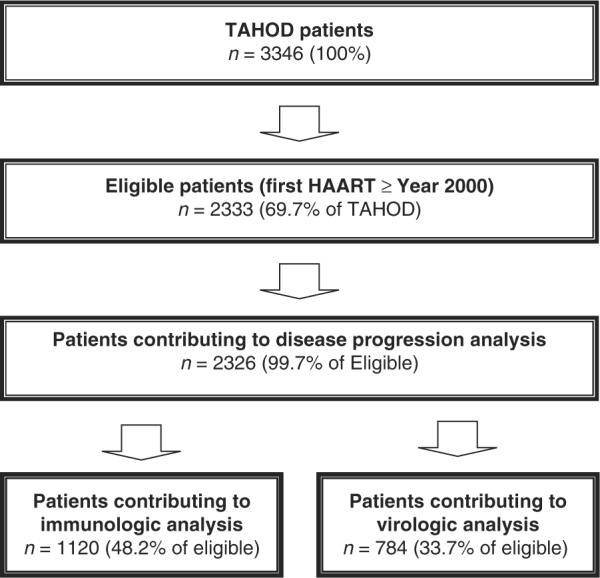
Patients contributing data for disease progression, immunologic and virologic analyses.
Note: A total of 531 (23%) of the eligible patients contributed data to both the immunologic and virologic analyses.
For each endpoint, a base predictive patient model was determined from significant patient covariates. Then, because of our a priori interest in the role of site resourcing on outcomes, individual estimates of country income and reported frequencies of VL and CD4 testing were assessed for statistical significance after adjustment for the base patient model. Analyses were performed using SAS software version 9.1.3 (SAS Institute Inc., Cary, NC, USA) and STATA software version 8.2 (STATA Corp., College Station, TX, USA).
Results
Of 3346 patients recruited to TAHOD, 2333 (69.7%) fulfilled the inclusion criteria. Of these, 79% had at least 6 months of retrospective data available and 13% were mono- or dual-ARV experienced. Patient demographics, clinical parameters and prescribed HAART regimen are summarized in Table 1. One hundred and seventy-six of the monoand dual-experienced patients recycled one or two previously used ARVs in the HAART regimen. Thirteen of these patients reported a virological or clinical treatment failure on the previous regimen.
Table 1.
Patient characteristics at highly active antiretroviral therapy (HAART) initiation by country income
| Low income [n (%)] | High income [n (%)] | |
|---|---|---|
| Age (years) at entry | ||
| Median (range) | 35.5 (6.8–70.9) | 37.7 (18.6–81.6) |
| < 30 years | 292 (20.4) | 163 (18.0) |
| 30–40 years | 709 (49.6) | 371 (41.0) |
| >40 years | 420 (29.4) | 371 (41.0) |
| Unknown | 7 (0.5) | - |
| Gender | ||
| Male | 926 (64.8) | 722 (79.8) |
| Female | 500 (35.0) | 182 (20.1) |
| Transgender | 2 (0.1) | 1 (0.1) |
| HIV exposure | ||
| Heterosexual contact | 1138 (79.7) | 510 (56.4) |
| Homosexual contact | 64 (4.5) | 333 (36.8) |
| Other* | 226 (15.8) | 62 (6.9) |
| CDC classification | ||
| Category A | 548 (38.4) | 512 (56.6) |
| Category B | 219 (15.3) | 37 (4.1) |
| Category C | 661 (46.3) | 356 (39.3) |
| Hepatitis B | ||
| Negative | 386 (27.0) | 528 (58.3) |
| Positive | 50 (3.5) | 53 (5.9) |
| Not tested | 992 (69.5) | 324 (35.8) |
| Hepatitis C | ||
| Negative | 286 (20.0) | 544 (60.1) |
| Positive | 61 (4.3) | 35 (3.9) |
| Not tested | 1081 (75.7) | 326 (36.0) |
| First HAART regimen† | ||
| NNRTI‡ | 1296 (90.8) | 569 (62.9) |
| PI | 120 (8.4) | 276 (30.5) |
| NNRTI/PI | 10 (0.7) | 45 (5.0) |
| NRTI | 2 (0.1) | 15 (1.7) |
| Baseline HIV-1 viral load§ (copies/mL) | ||
| Median (range) | 125 399 (49–6531 200) | 97 600 (49–1100 000) |
| < 10 000 copies/mL | 41 (2.9) | 81 (9.0) |
| ≥ 10 000 | 201 (14.1) | 378 (41.8) |
| Unknown | 1186 (83.1) | 446 (49.3) |
| Baseline CD4 count∥ (cells/μL) | ||
| Median (range) | 91 (0–886) | 131 (0–922) |
| ≤ 100 cells/μL | 554 (38.8) | 260 (28.7) |
| 100–200 cells/μL | 287 (20.1) | 178 (19.7) |
| > 200 cells/μL | 193 (13.5) | 176 (19.4) |
| Unknown | 394 (27.6) | 291 (32.2) |
| Total | 1428 (61.2) | 905 (38.8) |
The HIV exposure category `Other' includes injecting drug users (IDUs), patients infected by blood products and unknown exposures.
HAART was a combination of three or more antiretrovirals (ARVs) with or without an NRTI backbone. NNRTI/PI included at least one PI and one NNRTI. PI included ritonavir-boosted regimens.
Of the NNRTI regimens, lamivudine/stavudine/nevirapine and lamivudine/zidovudine/efavirenz were the most frequently prescribed for low (58%)- and high (41%)-income countries, respectively.
Dichotomized for country income comparison.
`Unknown' category excluded for test of trend. CDC, Centers for Disease Control and Prevention; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Due to small numbers of injecting drug users (IDUs), patients infected by blood products or having unknown exposure, these modes of infection were collapsed into an `Other' exposures category. Transgender patients were included in the male category. For regression analyses, seven patients with unknown ages were excluded. Of eligible patients, 2326 (99.7%) were included in disease progression analyses while 1120 (48.2%) and 785 (33.7%) patients contributed data to multivariate linear and logistic regressions, respectively.
As shown in Table 1, low-income sites contributed 61% of eligible patients. For country income comparisons at baseline, HIV RNA results were dichotomized as `Unknown' (low, 83.1%; high, 49.3%) or `Available'. Patients with unknown CD4 cell counts were excluded from trend tests. Significant differences existed for all patient covariates. Patients from high-income countries had significantly higher proportions of male patients (low, 64.8%; high, 79.8%; P<0.0001), HIV exposure reported as homosexual contact (low, 4.5%; high, 36.8%; P<0.0001) and patients older than 40 years (low, 29.4%; high, 41.0%; P<0.0001). Patients from low-income countries demonstrated poorer baseline health status in that more patients had CD4 counts of 100 cells/μL or less (low, 38.8%; high, 28.7%; P<0.0001) and fewer were asymptomatic (CDC A) (low, 38.4%; high, 56.6%; P<0.0001). Low-income country patients were also less likely to have been tested for coinfection with hepatitis B (low, 69.5%; high, 35.8%; P<0.0001) or hepatitis C (low, 75.7%; high, 36.0%; P<0.0001). Higher proportions of patients in low-income countries did not have access to VL testing prior to being prescribed a first-line regimen (low, 83.1%; high, 49.3%; P<0.0001) and although the most frequently prescribed HAART regimens were based on nonnucleoside reverse transcriptase inhibitors, more patients in high-income countries were prescribed a protease inhibitor (PI)-based regimen (low, 8.4%; high, 30.5%; P<0.0001). High-income country sites reported that patients were monitored virologically at least annually and CD4 tested at least three times per year (Table 2).
Table 2.
Country income by site frequency of diagnostic testing
| CD4 assay (per year) | Viral load assay (per year) | n (%) | |
|---|---|---|---|
| Low income | ≥3 | ≥3 | 171 (12.0) |
| <1 | 190 (13.3) | ||
| <3 | 1–2 | 271 (19.0) | |
| <1 | 796 (55.7) | ||
| High income | ≥3 | ≥3 | 530 (58.6) |
| 1–2 | 375 (41.4) |
Progression to AIDS or death
The 2326 patients included in disease progression analyses (Table 3) contributed 5872.4 person-years of retrospective and prospective follow-up (median 2.4; interquartile range 1.2–3.7 person-years). During this time, there were a total of 393 events (347 AIDS diagnoses and 46 deaths) giving an event rate of 6.7 per 100 person-years. Significant univariate patient parameter associations were maintained after adjustment and formed the base patient model. Patients coinfected with hepatitis C [hazard ratio (HR)=1.8; P=0.011] and with a pre-HAART diagnosis of CDC category C illness (HR=1.4; P=0.003) had a higher level of disease progression. Female gender (HR=0.8; P=0.040) and a baseline CD4 count >100 cells/μL were shown to have a protective effect (100–200 cells/μL, HR=0.5; >200 cells/μL, HR=0.4; P<0.001). In univariate analyses, patients at low-income sites and with sites reporting less frequent VL testing had a poorer prognosis. After adjustment for the patient model, only less-than-annual frequency of VL testing was significantly associated with higher rates of disease progression (HR=1.4; P=0.032). Although there was a higher risk of disease progression for RNA testing one to two times per year compared with at least three times per year, the increase in risk was not significantly different.
Table 3.
Factors associated with progression to Centers for Disease Control and Prevention (CDC) class C event or death after initiating highly active antiretroviral therapy (HAART)
| Univariate analysis |
Multivariate analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Follow-up (years) | Number of events | Rate per 100 person-years | HR | P | HR (95% CI) | P | |
| Patient covariates | ||||||||
| Age (years) at entry | ||||||||
| <30 | 455 | 1036.6 | 69 | 6.7 | 0.626 | 0.743 | ||
| 30–40 | 1080 | 2727.9 | 198 | 7.3 | 1.17 | 0.272 | 1.12 (0.9–1.5) | 0.413 |
| >40 | 791 | 2107.9 | 126 | 6.0 | 0.98 | 0.885 | 0.99 (0.7–1.3) | 0.939 |
| Gender | ||||||||
| Male | 1646 | 4134.0 | 303 | 7.3 | ||||
| Female | 680 | 1738.5 | 90 | 5.2 | 0.71 | 0.004 | 0.78 (0.6–1.0) | 0.040 |
| HIV exposure | ||||||||
| Heterosexual contact | 1644 | 4349.4 | 292 | 6.7 | 0.167 | |||
| Homosexual contact | 397 | 995.8 | 53 | 5.3 | 0.76 | 0.070 | 0.76 (0.6–1.0) | 0.084 |
| Other | 285 | 527.3 | 48 | 9.1 | 1.12 | 0.483 | 1.09 (0.8–1.5) | 0.635 |
| CDC classification | ||||||||
| Category A | 1055 | 2891.6 | 136 | 4.7 | 0.000 | 0.002 | ||
| Category B | 255 | 511.3 | 34 | 6.6 | 1.21 | 0.328 | 1.06 (0.7–1.6) | 0.759 |
| Category C | 1016 | 2469.5 | 223 | 9.0 | 1.84 | 0.000 | 1.42 (1.1–1.8) | 0.003 |
| Hepatitis B | ||||||||
| Negative | 914 | 2228.2 | 151 | 6.8 | ||||
| Positive | 103 | 239.7 | 18 | 7.5 | 1.08 | 0.751 | 1.02 (0.6–1.7) | 0.935 |
| Not tested | 1309 | 3404.5 | 224 | 6.6 | 0.99 | 0.944 | 0.87 (0.6–1.2) | 0.364 |
| Hepatitis C | ||||||||
| Negative | 830 | 2018.9 | 125 | 6.2 | ||||
| Positive | 96 | 206.2 | 24 | 11.6 | 1.79 | 0.009 | 1.76 (1.1–2.7) | 0.011 |
| Not tested | 1400 | 3647.3 | 244 | 6.7 | 1.12 | 0.324 | 1.11 (0.9–1.4) | 0.334 |
| First HAART regimen | ||||||||
| NNRTI | 1860 | 4647.7 | 325 | 7.0 | 0.056 | 0.071 | ||
| PI | 394 | 1021.9 | 50 | 4.9 | 0.70 | 0.021 | 0.73 (0.5–1.0) | 0.041 |
| NNRTI/PI | 55 | 153.2 | 14 | 9.1 | 1.40 | 0.219 | 1.43 (0.8–2.4) | 0.190 |
| NRTI | 17 | 49.7 | 4 | 8.0 | 1.21 | 0.704 | 1.44 (0.5–3.9) | 0.466 |
| Baseline HIV-1 viral load (copies/mL) | ||||||||
| < 10 000 | 122 | 369.6 | 14 | 3.8 | ||||
| ≥ 10 000 | 577 | 1608.2 | 86 | 5.3 | 1.36 | 0.287 | 1.19 (0.7–2.1) | 0.555 |
| Unknown | 1627 | 3894.6 | 293 | 7.5 | 1.78 | 0.035 | 1.46 (0.8–2.5) | 0.180 |
| Baseline CD4 count (cells/mL) | ||||||||
| ≤100 | 813 | 1902.8 | 185 | 9.7 | 0.000 | 0.000 | ||
| 100–200 | 462 | 1184.3 | 47 | 4.0 | 0.42 | 0.000 | 0.48 (0.3–0.7) | 0.000 |
| > 200 | 367 | 964.4 | 31 | 3.2 | 0.34 | 0.000 | 0.41 (0.3–0.6) | 0.000 |
| Unknown | 684 | 1820.9 | 130 | 7.1 | 0.77 | 0.022 | 0.81 (0.6–1.0) | 0.079 |
| Resource covariates | ||||||||
| World Bank income | ||||||||
| Low income | 1421 | 3211.3 | 251 | 7.8 | ||||
| High income | 905 | 2661.1 | 142 | 5.3 | 0.78 | 0.017 | 0.80 (0.6–1.0) | 0.066 |
| Frequency of RNA testing | ||||||||
| ≥3 times per year | 701 | 1986.8 | 98 | 4.9 | 0.002 | 0.033 | ||
| 1–2 times per year | 646 | 1934.5 | 123 | 6.4 | 1.35 | 0.028 | 1.22 (0.9–1.6) | 0.155 |
| < 1 time per year | 979 | 1951.1 | 172 | 8.8 | 1.49 | 0.002 | 1.35 (1.0–1.8) | 0.032 |
| Frequency of CD4 testing | ||||||||
| ≥ 3 times per year | 1265 | 3560.2 | 229 | 6.4 | ||||
| < 3 times per year | 1061 | 2312.2 | 164 | 7.1 | 0.95 | 0.638 | 0.83 (0.7–1.0) | 0.097 |
| Total | 2326 | 5872.4 | 393 | 6.7 | ||||
P-values for age (years) at entry, CDC classification, HIV exposure, first HAART regimen, baseline CD4 cell count and frequency of RNA testing are for tests for trend or homogeneity, as appropriate, evaluated by excluding classifications representing unavailable information.
CI, confidence interval; HR, hazard ratio; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The first HAART regimen, after adjustment, was not found to be associated with disease progression for our patients. The overall (trend or heterogeneity) P-value must be significant before category effects can be interpreted as contributing. Dichotomizing the first HAART regimen to PI use Yes/No did not change final model interpretations.
Change in CD4 cell count at 12 months following HAART
For immunologic analyses, 1120 patients had CD4 counts available at baseline and at 12 months following HAART initiation with a mean increase of 161 cells/μL over the period (Table 4). Unadjusted estimates for age at enrolment, HIV exposure, HAART regimen, baseline HIV RNA and CD4 cell counts were associated with the outcome. After patient covariate adjustment, smaller increases in CD4 counts were associated with age older than 40 years (P=0.001), HIV exposure (P=0.043) and baseline CD4 counts >200 cells/μL (P=0.020). Univariate estimates for country income effects and VL testing frequency were associated with 12-month change in CD4 cell count.
Table 4.
Factors associated with 12-month change in CD4 cell counts from initiation of highly active antiretroviral therapy (HAART)
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| n | Change in CD4 (cells/mL) | Coefficient* | P | Coefficient (95% CI) | P | |
| Patient covariates | ||||||
| Age at entry | ||||||
| <30 years | 205 | 178.4 | 0.000 | 0.000 | ||
| 30–40 years | 531 | 176.0 | −2.44 | 0.848 | −2.69 (−27.8, 22.4) | 0.833 |
| >40 years | 384 | 131.4 | −46.98 | 0.001 | −47.22 (−73.6, −20.8) | 0.001 |
| Gender | ||||||
| Male | 796 | 164.8 | ||||
| Female | 324 | 152.1 | −12.76 | 0.217 | −15.40 (−36.3, 5.6) | 0.151 |
| HIV exposure | ||||||
| Heterosexual contact | 852 | 162.9 | 0.033 | 0.043 | ||
| Homosexual contact | 159 | 175.4 | 12.41 | 0.358 | 19.11 (−8.1, 46.3) | 0.169 |
| Other | 109 | 126.3 | −36.67 | 0.021 | −29.37 (−60.5, 1.7) | 0.064 |
| CDC classification | ||||||
| Category A | 522 | 156.0 | 0.163 | 0.343 | ||
| Category B | 140 | 150.6 | −5.39 | 0.718 | −4.20 (−33.8, 25.4) | 0.780 |
| Category C | 458 | 170.2 | 14.20 | 0.157 | 10.40 (−11.1, 31.8) | 0.343 |
| Hepatitis B | ||||||
| Negative | 462 | 143.2 | ||||
| Positive | 65 | 167.6 | 24.41 | 0.238 | 16.70 (−23.5, 57) | 0.415 |
| Not tested | 593 | 174.4 | 31.20 | 0.001 | 27.70 (8.8, 46.6) | 0.004 |
| Hepatitis C | ||||||
| Negative | 450 | 148.8 | ||||
| Positive | 52 | 115.1 | −33.76 | 0.140 | −27.10 (−73.3, 19.1) | 0.251 |
| Not tested | 618 | 174.0 | 25.14 | 0.010 | 27.00 (7.9, 46.0) | 0.006 |
| First HAART regimen | ||||||
| NNRTI | 944 | 165.1 | 0.040 | 0.057 | ||
| PI | 132 | 153.1 | −12.00 | 0.409 | −14.40 (−44.1, 15.4) | 0.344 |
| NNRTI/PI | 32 | 114.1 | −51.02 | 0.070 | −36.80 (−91.6, 18.0) | 0.188 |
| NRTI | 12 | 65.3 | −99.83 | 0.028 | −104.90 (−193.2, −16.5) | 0.020 |
| Baseline HIV-1 viral load | ||||||
| < 10 000 copies/mL | 87 | 113.9 | ||||
| ≥ 10 000 copies/mL | 417 | 150.6 | 36.71 | 0.046 | 34.20 (−1.5, 70.0) | 0.060 |
| Unknown | 616 | 174.9 | 61.06 | 0.001 | 55.70 (20.2, 91.2) | 0.002 |
| Baseline CD4 count | ||||||
| ≤ 100 cells/mL | 567 | 163.4 | 0.082 | 0.003 | ||
| 100–200 cells/mL | 308 | 176.4 | 13.02 | 0.239 | 16.60 (−4.9, 38.1) | 0.130 |
| > 200 cells/mL | 245 | 136.6 | −26.81 | 0.025 | −29.45 (−53.4, −5.5) | 0.020 |
| Resource covariates | ||||||
| World Bank income | ||||||
| Low income | 613 | 174.9 | ||||
| High income | 507 | 144.5 | −30.46 | 0.001 | −32.05 (−51.7, −12.4) | 0.001 |
| Frequency of RNA testing | ||||||
| ≥ 3 times per year | 328 | 139.0 | 0.000 | 0.000 | ||
| 1–2 times per year | 307 | 148.2 | 9.22 | 0.455 | 19.38 (−5.8, 44.6) | 0.130 |
| < 1 time per year | 485 | 184.3 | 45.26 | 0.000 | 53.60 (29.5, 77.7) | 0.000 |
| Frequency of CD4 testing | ||||||
| ≥ 3 times per year | 671 | 154.5 | ||||
| <3 times per year | 449 | 171.0 | 16.48 | 0.085 | 17.60 (−2.3, 37.5) | 0.080 |
| Total | 1120 | 161.1 | ||||
P-values for age (years) at entry, Centers for Disease Control and Prevention (CDC) classification, HIV exposure, first HAART regimen, baseline CD4 cell count and frequency of RNA testing are for tests for trend or homogeneity, as appropriate, evaluated by excluding classifications representing unavailable information.
The difference compared with the reference category of each variable in the univariate analysis.
CI, confidence interval; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
After adjustment for the base patient model, less than annual VL testing frequency was significantly associated with higher mean 12-month increases in CD4 cell count (P<0.001). To investigate if this result was associated with patients who were experiencing acute CD4 pre-therapy decline, an unadjusted Kruskal-Wallis test was performed on the 25% of patients who had CD4 cell counts 6 (± 3) months pre-HAART. Patients from sites with less than annual VL testing had steeper pre-therapy median CD4 decline compared with patients from the most resourced sites (CD4 count decline less than once per year, −50 cells/μL; one to two times per year, −49 cells/μL; at least three times per year, −18 cells/μL; P<0.008). Higher mean CD4 increases were also noted for patients from low-income sites (P<0.001).
HIV RNA at 12 months following HAART
Due to the heterogeneity of virology assays and associated dynamic ranges across sites, we defined the lower limit of detection (LLD) as 400 copies/mL. Analyses included 785 patients who had an HIV RNA result available at 12 months and 83% of patients were virologically suppressed below the LLD. In univariate analyses (Table 5), hepatitis C coinfection, baseline CD4 cell count and HIV exposure were associated with virologic suppression. After adjustment, patients reporting IDU, receipt of blood products or `Other', undefined exposure were significantly disadvantaged [odds ratio (OR)=0.28; P<0.001] while female patients had a higher odd of being suppressed (OR=1.69; P=0.040). Therefore, HIV exposure and gender formed the base patient model.
Table 5.
Factors associated with undetectable HIV at 12 months in patients initiating highly active antiretroviral therapy (HAART)
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| n | Number HIV viral loads undetectable (%) | OR | P | OR (95% CI) | P | |
| Patient covariates | ||||||
| Age at entry | ||||||
| <30 years | 153 | 125 (81.7) | 0.384 | 0.133 | ||
| 30–40 years | 334 | 274 (82.0) | 1.02 | 0.929 | 1.17 (0.7–2.0) | 0.564 |
| >40 years | 298 | 252 (84.6) | 1.23 | 0.437 | 1.48 (0.9–2.6) | 0.155 |
| Gender | ||||||
| Male | 596 | 487 (81.7) | ||||
| Female | 189 | 164 (86.8) | 1.47 | 0.109 | 1.69 (1.0–2.8) | 0.038 |
| HIV exposure | ||||||
| Heterosexual contact | 496 | 417 (84.1) | 0.000 | 0.000 | ||
| Homosexual contact | 226 | 197 (87.2) | 1.29 | 0.280 | 1.51 (0.9–2.4) | 0.090 |
| Other | 63 | 37 (58.7) | 0.27 | 0.000 | 0.28 (0.2–0.5) | 0.000 |
| CDC classification | ||||||
| Category A | 428 | 365 (85.3) | 0.074 | 0.252 | ||
| Category B | 36 | 28 (77.8) | 0.60 | 0.234 | 0.67 (0.3–1.6) | 0.354 |
| Category C | 321 | 258 (80.4) | 0.71 | 0.077 | 0.79 (0.5–1.2) | 0.253 |
| Hepatitis B | ||||||
| Negative | 382 | 326 (85.3) | ||||
| Positive | 37 | 32 (86.5) | 1.10 | 0.851 | 1.07 (0.4–2.9) | 0.899 |
| Not tested | 366 | 293 (80.1) | 0.69 | 0.057 | 0.68 (0.5–1.0) | 0.056 |
| Hepatitis C | ||||||
| Negative | 384 | 330 (85.9) | ||||
| Positive | 36 | 24 (66.7) | 0.33 | 0.004 | 0.62 (0.3–1.4) | 0.269 |
| Not tested | 365 | 297 (81.4) | 0.72 | 0.092 | 0.75 (0.5–1.1) | 0.168 |
| First HAART regimen | ||||||
| NNRTI | 544 | 452 (83.1) | 0.277 | 0.151 | ||
| PI | 197 | 166 (84.3) | 1.09 | 0.704 | 0.91 (0.6–1.5) | 0.697 |
| NNRTI/PI | 34 | 27 (79.4) | 0.79 | 0.582 | 0.72 (0.3–1.7) | 0.464 |
| NRTI | 10 | 6 (60.0) | 0.31 | 0.070 | 0.23 (0.1–0.8) | 0.027 |
| Baseline HIV-1 viral load | ||||||
| < 10 000 copies/mL | 69 | 53 (76.8) | ||||
| ≥ 10 000 copies/mL | 334 | 277 (82.9) | 1.47 | 0.231 | 1.37 (0.7–2.6) | 0.338 |
| Unknown | 382 | 321 (84.0) | 1.59 | 0.145 | 1.54 (0.8–2.9) | 0.190 |
| Baseline CD4 count | ||||||
| ≤ 100 cells/μL | 242 | 190 (78.5) | 0.029 | 0.058 | ||
| 100–200 cells/μL | 152 | 125 (82.2) | 1.27 | 0.369 | 1.22 (0.7–2.1) | 0.467 |
| >200 cells/μL | 156 | 136 (87.2) | 1.86 | 0.030 | 1.73 (1–3.1.0) | 0.067 |
| Unknown | 235 | 200 (85.1) | 1.56 | 0.064 | 1.47 (0.9–2.4) | 0.127 |
| Resource covariates | ||||||
| World Bank income | ||||||
| Low income | 204 | 161 (78.9) | ||||
| High income | 581 | 490 (84.3) | 1.44 | 0.078 | 1.19 (0.8–1.8) | 0.449 |
| Frequency of RNA testing | ||||||
| ≥ 3 times per year | 403 | 349 (86.6) | 0.000 | 0.000 | ||
| 1–2 times per year | 297 | 251 (84.5) | 0.84 | 0.435 | 0.91 (0.6–1.5) | 0.709 |
| < 1 time per year | 85 | 51 (60.0) | 0.23 | 0.000 | 0.30 (0.2–0.5) | 0.000 |
| Frequency of CD4 testing | ||||||
| ≥ 3 times per year | 636 | 538 (84.6) | ||||
| <3 times per year | 149 | 113 (75.8) | 0.57 | 0.011 | 0.68 (0.4–1.1) | 0.108 |
| Total | 785 | 651 (82.9) | ||||
P-values for age (years) at entry, Centers for Disease Control and Prevention (CDC) classification, HIV exposure, first HAART regimen, baseline CD4 cell count and frequency of RNA testing are for tests for trend or homogeneity, as appropriate, evaluated by excluding classifications representing unavailable information.
CI, confidence interval; OR, odds ratio; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Unadjusted estimates of the frequency of VL and CD4 testing were associated with 12-month virologic suppression. After adjustment for the base patient model, only the frequency of VL testing remained significant. Patients at sites reporting less than annual VL testing had lower odds of being virologically suppressed at 12 months than those at sites reporting VL testing frequencies of three times per year or more (OR=0.30, P<0.001).
Discussion
In our cohort of predominantly ARV-naïve patients, a previous diagnosis of an AIDS-defining illness, lower pre-HAART CD4 cell counts and HIV/HCV coinfection were predictive of higher rates of HIV disease progression, consistent with other studies [19–23]. Smaller increases in CD4 cell count were associated with older age and higher baseline CD4 cell counts, similar to prognostic factors reported elsewhere [24,25]. Patients reporting IDU, receipt of blood products or undefined exposure experienced less immunologic and virologic benefit. Female patients in our cohort were more likely to be virologically suppressed and had a lower risk of disease progression. As the modified World Bank high/low criterion may not be a sensitive measure of an individual site's resourcing, we also categorized sites according to routine frequencies of VL and CD4 testing.
In the patient outcomes we assessed, site-reported VL testing was an important determinant. Our results showed an increased risk of disease progression for patients at sites reporting less than annual VL testing. This is possibly attributable to lower pretreatment CD4 cell count nadirs and diminished lymphocyte proliferative capacity from delayed initiation of HAART [26]. The magnitude of the increase in risk was similar to that seen in patients having a pre-therapy diagnosis of severely symptomatic HIV disease. Larger CD4 increases post-HAART were found in patients from sites with low levels of resourcing. Although group summary responses do not reflect individual variation, immunologically suppressed patients generally experience more rapid increases in CD4 cell count during the first 12 months post-HAART [27,28]. This is consistent with persons initiating HAART in advanced stages of HIV infection and experiencing acute pre-therapy CD4 decline [29]. Steeper pre-therapy CD4 decline was noted in our patients from sites with less than annual VL testing, in an unadjusted analysis based on limited data.
Patients from sites with lower levels of resourcing showed most rapid preliminary CD4 increases and higher rates of disease progression, however, both findings are consistent with patients having a higher disease burden. Less than annual reported VL testing was associated with reduced odds of virologic suppression. We believe that this reflects sites with low capacity identifying patients at high risk of failure for VL testing. Use of VL diagnostics to confirm treatment failure rather than to monitor treatment efficacy implies that the technology is not being used for treatment management and this could impact negatively on long-term patient outcomes.
Only those patients with diagnostic results contribute data for virologic and immunologic analysis, therefore, missing baseline CD4 cell counts or HIV RNA data could have introduced bias into our model estimates. As we are unable to test for any potential bias, this should be taken into account when interpreting the results of analyses. Patients being VL tested may be retained on failing regimens when second-line therapies are not available. Alternatively, clinicians may not expend scarce resources on diagnostically monitoring patients who are failing clinically and for whom no viable treatment options exist. Consequently, we may be either under- or overestimating the proportion of patients who were virologically suppressed. We did not distinguish between AIDS-related and non-AIDS-related deaths, possibly leading to an overestimation of the number of patients having clinical progression. Patient socio-economic and adherence to therapy data were unavailable.
Timely access to CD4 and VL results is crucial for monitoring the efficacy of ARV treatment. These staging data are frequently unavailable in resource-limited settings, and their lack compromises the generalizability of published results and trends. Our analyses included 70% of TAHOD enrollees in disease progression analyses, and 75% (80%) of sites reported that TAHOD patients' access to VL (CD4) testing did not differ to that routinely available in their respective countries. Consequently, our estimates of diagnostic resource allocation should be fairly representative of the Asia-Pacific region. However, TAHOD sites are self-selected and patients may differ from other HIV-infected patients within a specific country. Still, our findings highlight challenges for less resourced sites in the region and potential negative effects on patient outcomes.
The United Nations General Assembly report for the sixty-second session stated that 3 million people from low-income and middle-income countries had access to ARVs in 2007 and that coverage had increased to approximately 30% of those in need [30]. Despite the importance of surrogate laboratory markers in evaluating ARV treatment efficacy, estimates of the availability of diagnostic testing lagged behind treatment access at between 3 and 6% [13]. While recent modelling of HIV infection suggests modest benefits to patient survival from VL monitoring [31], our results show that low levels of site VL testing are associated with poorer treatment outcomes. Further, lack of VL testing increases the risk of patients being maintained on failing regimens and developing highly resistant HIV which may be transmitted to other individuals [32,33].
The World Health Organization has recommended supporting and extending diagnostic technologies to monitor the response to antiretroviral therapy (ART) and to aid in the prevention of emergence and transmission of HIVDR [34,35]. To assess the extent of HIVDR in the Asia-Pacific, the TREAT Asia network has developed the TREAT Asia Studies to Evaluate Resistance (TASER) programme [36]. The programme includes a monitoring protocol (TASER-M), a surveillance protocol (TASER-S) and a laboratory component, the TREAT Asia Quality Assurance Scheme (TAQAS). Patients eligible for TASER-M are those initiating first-line ART or switching to second-line ART. Objectives are to assess the prevalence and incidence of emerging HIVDR and to produce evidence-based recommendations to inform treatment guidelines. The objective of TASER-S is to evaluate the prevalence and changes in prevalence of HIVDR in treatment-naïve, recently infected HIV-positive individuals. TAQAS is a laboratory network building capacity for the genetic analysis of clinical specimens and participating laboratories provide genotypic results for the TASER protocols.
In summary, less-than-annual site-reported VL testing was associated with less favourable patient outcomes, in particular, a 35% increased risk of AIDS and death. Outcomes for patients at sites reporting VL testing one to two times annually did not differ substantially from those of patients at sites reporting more frequent monitoring. Our findings emphasize the need to partner the expanded international access to ARVs with appropriate levels of VL diagnostic testing and to address the critical lack of second- and third-line treatment regimens in resource-limited settings.
Acknowledgements
The TREAT Asia HIV Observational Database is part of the Asia Pacific HIV Observational Database and is an initiative of TREAT Asia, a programme of amfAR, The Foundation for AIDS Research, with support from the National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes of Health (NIH) as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (grant no. U01AI069907), and from the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Appendix A
The TREAT Asia HIV Observational Database
V. Saphonn*, C.V. Mean and K. Vohith, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
F.J. Zhang*, H.X. Zhao and N. Han, Beijing Ditan Hospital, Beijing, China;
P.C.K. Li*† and M.P. Lee, Queen Elizabeth Hospital, Hong Kong, China;
N. Kumarasamy* and S. Saghayam, YRG Centre for AIDS Research and Education, Chennai, India;
S. Pujari* and K. Joshi, Institute of Infectious Diseases, Pune, India;
T.P. Merati* and F. Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
S. Oka* and M. Honda, International Medical Centre of Japan, Tokyo, Japan;
J.Y. Choi* and S.H. Han, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
C.K.C. Lee* and R. David, Hospital Sungai Buloh, Kuala Lumpur, Malaysia;
A. Kamarulzaman* and A. Kajindran, University of Malaya, Kuala Lumpur, Malaysia;
G. Tau*, Port Moresby General Hospital, Port Moresby, Papua New Guinea;
R. Ditangco* and R. Capistrano, Research Institute for Tropical Medicine, Manila, Philippines;
Y.M.A. Chen*, W.W. Wong and Y.W. Yang, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan;
P.L. Lim*, O.T. Ng and E. Foo, Tan Tock Seng Hospital, Singapore;
P. Phanuphak*, and M. Khongphattanayothing, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S. Sungkanuparph* and B. Piyavong, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
T. Sirisanthana*‡ and W. Kotarathititum, Research Institute for Health Sciences, Chiang Mai, Thailand;
J. Chuah*, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia;
A. Sohn*, J. Smith*, K. Frost and B. Nakornsri, TREAT Asia/amfAR, The Foundation for AIDS Research, NY, USA;
D.A. Cooper, M.G. Law*, R. Oyomopito and J. Zhou*, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia.
*TAHOD Steering Committee member; †Current Steering Committee chair; ‡co-chair.
Footnotes
Potential conflicts of interest: PL Lim is an investigator on Tibotec study TMC 114-C211 (Artemis). There are no conflicts of interest to report for any of the other authors.
Role of the funding source: The funding source played no role in the study design, data collection, analysis, data interpretation or writing of the report.
References
- 1.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS AIDS epidemic update: special report on HIV/AIDS. 2006 Available at www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2006/default.asp.
- 4.Egger M, Boulle A, Schechter M, Miotti P. Antiretroviral therapy in resource-poor settings: scaling up inequalities? Int J Epidemiol. 2005;34:509–512. doi: 10.1093/ije/dyi110. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sow PS, Otieno LF, Bissagnene E, et al. Implementation of an antiretroviral access program for HIV-1-infected individuals in resource-limited settings: clinical results from 4 African countries. J Acquir Immune Defic Syndr. 2007;44:262–267. doi: 10.1097/QAI.0b013e31802bf109. [DOI] [PubMed] [Google Scholar]
- 7.Hughes MD. Evaluating surrogate endpoints. Controlled Clin Trials. 2002;23:703–707. doi: 10.1016/s0197-2456(02)00264-7. [DOI] [PubMed] [Google Scholar]
- 8.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4 + lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society – USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 10.Hughes MD, Johnson VA, Hirsch MS, et al. Monitoring plasma HIV-1 RNA levels in addition to CD4 + lymphocyte count improves assessment of antiretroviral therapeutic response. ACTG 241 protocol virology substudy team. Ann Intern Med. 1997;126:929–938. doi: 10.7326/0003-4819-126-12-199706150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Panel on antiretroviral guidelines for adults and adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2008. [accessed 21 April 2008]. pp. 1–128. Available at www.aidsinfo.nih.gov. [Google Scholar]
- 12.European AIDS Clinical Society (EACS) [accessed 21 April 2008];Guidelines for the clinical management and treatment of HIV infected adults in Europe. doi: 10.1111/j.1468-1293.2007.00533.x. Available at www.europeanaidsclinicalsociety.org. [DOI] [PubMed]
- 13.Cohen GM. Access to diagnostics in support of HIV/AIDS and tuberculosis treatment in developing countries. AIDS. 2007;21(Suppl. 4):S81–S87. doi: 10.1097/01.aids.0000279710.47298.5c. [DOI] [PubMed] [Google Scholar]
- 14.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 15.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings – the case of Côte d'Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 16.Fiscus SA, Cheng B, Crowe SM, et al. HIV-1 viral load assays for resource-limited settings. PLoS Med. 2006;3:e417. doi: 10.1371/journal.pmed.0030417. doi: 10.1371/journal.pmed.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 18.World Bank [accessed July 2007];World Bank list of economies. Available at http://go.worldbank.org/K2CKM78CC0.
- 19.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 20.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 21.Braitstein P, Zala C, Yip B, et al. Immunologic response to antiretroviral therapy in hepatitis C virus-coinfected adults in a population-based HIV/AIDS treatment program. J Infect Dis. 2006;193:259–268. doi: 10.1086/498908. [DOI] [PubMed] [Google Scholar]
- 22.Klein MB, Lalonde RG, Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;33:365–372. doi: 10.1097/00126334-200307010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 24.Bosch RJ, Bennett K, Collier AC, Zackin RB. Pretreatment factors associated with 3-year (144-week) virologic and immunologic responses to potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:268–277. doi: 10.1097/QAI.0b013e31802c7e20. [DOI] [PubMed] [Google Scholar]
- 25.Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–413. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 26.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4 + T-cell count and numbers of CD28 + CD4 + T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–2023. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Connick E, Kuritzkes DR, et al. Multiple CD4 + cell kinetic patterns and their relationships with baseline factors and virological responses in HIV type 1 patients receiving highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:1231–1240. doi: 10.1089/088922201750461285. [DOI] [PubMed] [Google Scholar]
- 28.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederman MM, Valdez H. Immune restoration with antiretroviral therapies: implications for clinical management. JAMA. 2000;284:223–228. doi: 10.1001/jama.284.2.223. [DOI] [PubMed] [Google Scholar]
- 30.United Nations General Assembly [accessed 18 May 2008];Declaration of Commitment on HIV/AIDS and Political Declaration on HIV/AIDS: midway to the Millennium Development Goals. 2008 Available at www.un.org/ga/president/62/issues/hiv/A-62-780.pdf.
- 31.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371:1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 32.Cozzi-Lepri A, Phillips AN, Ruiz L, et al. Evolution of drug resistance in HIV-infected patients remaining on a virologically failing combination antiretroviral therapy regimen. AIDS. 2007;21:721–732. doi: 10.1097/QAD.0b013e3280141fdf. [DOI] [PubMed] [Google Scholar]
- 33.Kumarasamy N, Madhavan V, Venkatesh KK, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis. 2009;49:306–309. doi: 10.1086/600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organisation [accessed 2 July 2008];Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. 2008 Available at www.who.int/hiv/pub/towards_universal_access_report_2008.pdf.
- 35.World Health Organisation [accessed 23 January 2009];Technical Working Group for the development of an HIV/AIDS diagnostic support toolkit. 2002 Available at www.who.int/bct.
- 36.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl. 2):1–13. [PubMed] [Google Scholar]


