Abstract
Bisphenol-A (BPA) and methoxychlor (MXC), two endocrine disrupting chemicals (EDCs) with estrogenic and anti-androgenic effects, disrupt the reproductive system. BPA has profound effects on luteinizing hormone (LH) surge amplitude and MXC on LH surge timing in sheep. The neural mechanisms involved in differential disruption of the LH surge by these two EDCs remains to be elucidated. We tested the hypothesis that differential effects of BPA and MXC on LH surge system involved changes in hypothalamic gonadotropin releasing hormone (GnRH) and estrogen receptors (ESR), ESR1 and ESR2 mRNA expression. Pregnant sheep were given daily injections of cottonseed oil (controls), MXC or BPA (5 mg/kg/day) from day 30 to 90 of gestation (term 147 d). Offspring from these animals were euthanized as adults, during the late follicular phase following synchronization of estrus with prostaglandin F2α, just prior to the expected onset of preovulatory LH surge and changes in mRNA expression of hypothalamic GnRH, ESR1, and ESR2 quantified following in situ hybridization. GnRH mRNA expression was significantly lower in both groups of EDC-treated females compared to controls. ESR1 expression was increased in prenatal BPA- but not MXC-treated females in medial preoptic area relative to controls. In contrast ESR2 expression was reduced in the medial preoptic area of both EDC-treated groups. Differences in expression of ESR1/ESR2 receptors may contribute to the differential effects of BPA and MXC on the LH surge system. These findings provide support that prenatal exposure to EDCs alters the neural developmental trajectory leading to long-term reproductive consequences in the adult female.
Keywords: Bisphenol-A, Methoxychlor, Neuroendocrine, Female reproduction
Introduction
Endocrine disrupting chemicals (EDCs) are biologically active compounds that can both mimic and antagonize the effects of endogenous hormones. EDCs are found in pesticides, industrial chemicals, and naturally occurring plant hormones (Damstra et al., 2002; McLachlan et al., 2006; Hotchkiss et al., 2008) thus wildlife, domestic species, and humans regularly come in contact with these hormone-like compounds. In recent years there has been increasing societal concern regarding the adverse effects of these chemicals on health and environment. Particularly, developmental exposure to EDCs such as bisphenol-A (BPA) and methoxychlor (MXC) can disrupt reproductive processes, even when exposed to relatively low doses (ATSDR, 2002; Welshons et al., 2003; vom Saal and Hughes, 2005). Because sexual differentiation of the reproductive system, among others, depends upon precise exposure to steroid hormones at specific times during development, exposure to low doses of EDCs that can signal through steroid receptors during these hormone sensitive, critical periods of development can lead to long term deleterious effects on the adult organism.
BPA is widely used in the production of polycarbonate plastic and epoxy resins that are used to make food and drink packaging, paints, adhesives, drinking water pipe linings, and dental sealants (vom Saal and Hughes, 2005). MXC is a pesticide commonly used to treat fruits, vegetables, and animal feed (Reynolds et al., 1976; ATSDR, 2002). Both these EDCs have been shown to possess estrogenic and anti-androgenic properties (Staub et al., 2002; Kitamura et al., 2005). Adult female rats, exposed to MXC prenatally, are characterized by a decrease in sexually dimorphic exploratory and copulatory behaviors (Palanza et al., 2001; Suzuki et al., 2004), abnormal or extended estrous cycles, reduced follicle stimulating hormone (FSH) concentrations, reduced proestrus luteinizing hormone (LH) surge, decreased progesterone secretion, and reduced number of corpora lutea as adults (Chapin et al., 1997; Suzuki et al., 2004). Similarly, female rats and mice exposed to BPA during gestation or immediately after birth have an earlier onset of vaginal opening and first estrus (Howdeshell et al., 1999; Honma et al., 2002; Adewale et al., 2009), changes in estrogen receptor (ESR) density in the anterior pituitary (Khurana et al., 2000; Markey et al., 2002), defeminization of sexually dimorphic neuron populations (Kubo et al., 2003; Rubin et al., 2006), and changes in adult behaviors such as maternal care (Vandenberg et al., 2009).
In light of the vulnerability of developing systems, the exposure of developing organisms to exogenous and/or environmental hormones has become a significant public health concern. Human exposure to several EDCs is well documented and fetuses and infants may be exposed to EDCs via placental transfer, breast milk, or amniotic fluid (Schonfelder et al., 2002; Yamada et al., 2002; Ranjit et al., 2009; Vandenberg et al., 2009, 2010). Recent panels on EDC exposure and risk call for research that can provide an epidemiological model for human health and disease (vom Saal et al., 2007). Sheep are an ideal model for examining this critical issue at the reproductive level because 1) their ovarian cyclicity, steroid and gonadotropin releasing hormone (GnRH) secretion patterns are very similar to that of women, (McNeilly, 1991), 2) the distribution of ESR1 (also referred to as ERα) and ESR2 (ERβ) is known (Scott et al., 2000; Skinner and Dufourny, 2005), 3) the activation of GnRH neurons relative to the estradiol-induced LH surge has been well-characterized (Moenter et al., 1993), and 4) the critical period of sexual differentiation in sheep has been determined using native steroids (Robinson et al., 2002; Foster et al., 2007). Targeting critical periods established by exposure to native steroids, we have previously found that prenatal BPA treatment severely dampens the hormone induced LH surge, and prenatal MXC treatment delays LH surge onset in adult ewes (Savabieasfahani et al., 2006).
GnRH neurons in the hypothalamus trigger the release of LH from the pituitary, thus alterations to these critical neurons may underlie the significant impairments in LH secretion observed in animals prenatally exposed to EDC. Considering that a preovulatory estradiol rise is the trigger for initiation of GnRH/LH surge (Moenter et al., 1993) and both BPA and MXC can signal through estrogen receptors and initiate hormone dependent changes, we tested the hypothesis that exposure to BPA or MXC during critical periods of development alters steroid receptor expression in the medial preoptic area (mPOA) and GnRH expression, both key mediators of LH secretion. The mPOA was selected as the site for this investigation, as it contains GnRH and estrogen receptors (Herbison, 1995; Jansen et al., 1997), is sexually dimorphic (Scott et al., 2000), and is critical for generation of the LH surge in ewes (Caraty et al., 1998; Walsh and Clarke, 1998).
Materials and Methods
Animals
All animal procedures were approved by the University of Michigan Committee for the Use and Care of Animals. Adult pregnant sheep were given daily subcutaneous injections of cottonseed oil (controls) or MXC or BPA (5 mg/kg/day in cotton seed oil) from day 30 to 90 of gestation. This represents a critical period for induction of reproductive disruptions in sheep as established from studies involving use of native steroids (reviewed in Padmanabhan et al., 2010). The offspring from these treated ewes were weaned at 8 weeks of age and allowed to develop in a natural social setting. The impact of these EDCs on birth weight, estrous cyclicity, and periovulatory changes have already been described (Savabieasfahani et al., 2006). For characterizing ESR and GnRH mRNA expression, control, prenatal BPA- and MXC-treated sheep were administered PGF2α twice 11 days apart to synchronize estrus during their second breeding season (~ 21 months of age). All animals were euthanized just prior to the expected onset of preovulatory luteinizing hormone surge (timed relative to controls) by injecting with an overdose of pentobarbital and removing the brains. The hypothalamus was dissected out and frozen in an isopentane bath cooled with dry ice. Brains were cut into series of 20 µm thickness on a freezing cryostat. Coronal sections were collected from the medial septum to just past the caudal suprachiasmatic nucleus. Tissue series was processed for in situ hybridization for the detection of GnRH, ESR1, or ESR2 respectively.
RNA probes
Probes for ovine GnRH and ESR2 were kind gifts from Dr. Heiko Jansen, Washington State University. The 339 bp ovine GnRH probe has a 95% homology to the GnRH sequence found in Ovis Aries (Genebank accession number UO2517.1) and Bos Taurus (Genebank accession number BC120472.1). The 239 bp ovine ESR2 probe has been characterized previously and has >80% homology to sequences in human, non-human primates, rat and mouse sequences (Jansen et al., 2001); Genbank accession number accession no. AF110817). The 336 bp ovine probe for ESR1 (a kind gift from Dr. Nancy Ing, College Station TX) is highly conserved and shared 91% and 83% homology to the sequence in humans and mice, respectively (Ing et al., 1996).
In situ hybridization
S35-labeled antisense and sense probes were prepared from these templates by using appropriate viral RNA polymerase depending on orientation of inserts. At the time of the in situ hybridization procedure, slides were thawed and immediately fixed in 4% paraformaldehyde (freshly prepared, in 0.1M phosphate buffer pH 7.3) for 1 hour, washed extensively in 2× SSC, acetylated in 0.1M triethanolamine/0.25% acetic anhydride for 10 min, and dehydrated through increasing concentrations of ethanol (50–100%). The S35-labeled probe was diluted in hybridization buffer (50% formamide, 10% dextran sulfate, 3× SSC, 50mM sodium phosphate buffer, pH 7.4, 1× Denhardt’s solution, 0.1 mg/ml yeast tRNA, and 10 mM DTT) to yield an approximate concentration of 1 × 106 cpm/80µl or higher for each probe. Each slide was covered with 80µl of diluted probe and then coverslipped. The slides were then placed in plastic trays lined with filter paper soaked with 50% formamide. The trays were sealed and incubated at 55° C overnight. Post-hybridization, coverslips were floated off in 2× SSC, and slides were rinsed an additional three times in 2× SSC at room temperature. Slides were then incubated in RNAse-A (200µg/ml) at 37° C (1hr). Slides were then rinsed in decreasing concentrations of SSC (2×, 1×, and 0.5×) at room temperature, and washed in a final stringency of 0.1× SSC at 65 °C for 1 hr; cooled to room temperature in 0.1× SSC, and dehydrated through increasing concentrations of alcohols (50–100%). Slides were exposed to x-ray film to obtain autoradiographs (Kodak Biomax-MR). GnRH processed slides underwent further steps and were dipped in emulsion (Ilford K5D), stored in light-tight boxes at 4° C, then developed (Kodak D-19). All slides (ESR and GnRH) were dehydrated in alcohols, counterstained with hemotoxylin, and coverslipped.
mRNA quantification
Autoradiographs of brain sections were captured digitally and used to analyze mRNA levels. For each section, the magnitude of the hybridization signal from the radioactive probes in single-labeled sections was determined using Scion Image (NIH) which automatically determined the signal above background. We define signal as the mean integrated density of pixels divided by the total number of pixels in the selected region. Then a template outline of the mPOA (ESR1, ESR2) was placed on each section or each individual GnRH neuron was outlined. In the selected area we determined the pixels with a mean gray value exceeding the background value by 2.0 SD (GnRH, ESR1) or 1.5 SD (ESR2). All photography and analysis was performed by an experimenter blind to the identity of each slide. Specificity of the probe was demonstrated by a lack of hybridization signal over background in tissue incubated with the sense probe (data not shown).
Statistical analysis
All cells containing GnRH mRNA (as identified by a density of silver grains) were identified in three hypothalamic sections (1 section of medial septum, 2 sections containing the mPOA). Then each individual cell was outlined and the total amount of GnRH expression within that area was determined (2.0 SD above background). This variable thus represents both the amount of GnRH mRNA and the size of the GnRH neuron. These values were averaged for each animal. For ESR1 and ESR2 an oval template was placed on the mPOA. The location of the mPOA was identified based on anatomical markers and further confirmed by a sheep brain atlas (Johnson et al. 2004) and published studies (Scott et al., 2000). For each animal we analyzed 3 mPOA sections; the values were averaged for each animal. The number of animals analyzed for GnRH was controls=5, MXC=4, and BPA=7 respectively. The number of animals analyzed for ESR1 was controls=5, MXC=6 and BPA=3, respectively. For ESR2 the number of animals analyzed was controls=5, MXC=5 and BPA=5, respectively. The average area covered by ESR1 and ESR2 mRNA, which was above background, was used to calculate the expression (integrated density of pixels/area measured). These data were analyzed with an ANOVA with treatment as the independent variable. When significant differences were detected (P<0.05), post hoc Tukey tests were used to compare groups. In addition to these standard statistical analyses, we also examined effect sizes (Cohen, 1992; Nakagawa and Cuthill, 2007). This power analysis allows comparison of the means between two time points with respect to the magnitude of difference, when sample size is small. Results are reported as a Cohen’s d value and 0.2, 0.5, and 0.8 can be considered as small, medium and large effect sizes, respectively (Cohen, 1992; Nakagawa and Cuthill, 2007).
Results
GnRH
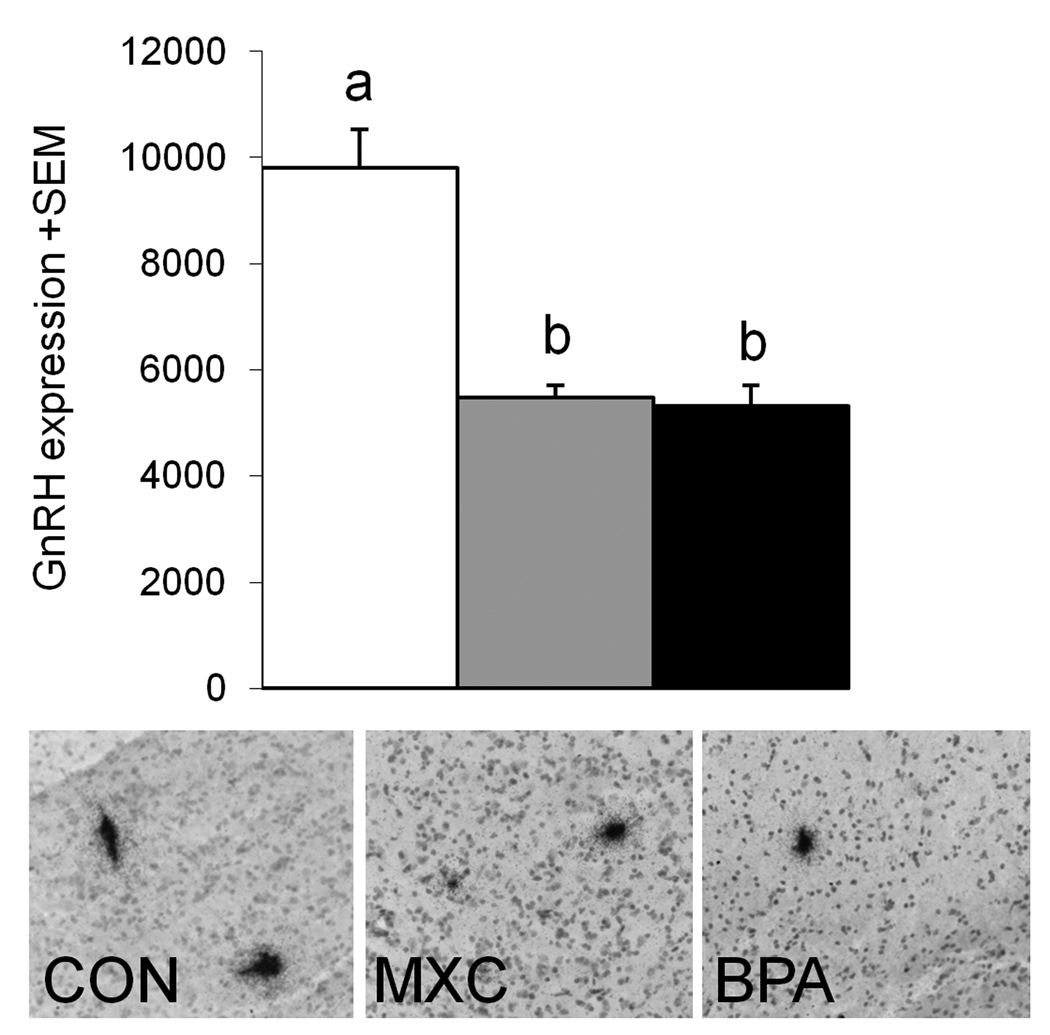
Labeled hybridized GnRH neurons were identified by dense silver grain clusters located in the septal regions, organum vasculosum of the lamina terminalis, and the preoptic area (Fig. 1). Groups differed significantly with respect to degree of GnRH expression (P=0.001) (Fig. 1). Post hoc analysis found prenatal BPA and MXC-treated animals had significantly less expression than that found in control females (P<0.05).
Figure 1.
Top: Mean GnRH expression in control (white bar), prenatal MXC (grey bar) and prenatal BPA treated ewes (black bar). A total of 7–15 GnRH neurons were identified in each animal and the expression level (mean integrated density/total number of pixels) was averaged for each animal and each group. Bars with different letters differ significantly with post-hoc Tukey tests (P<0.05). Bottom: Photomicrographs of silver grain clusters over GnRH cell body in the mPOA of a control, prenatal MXC- and BPA- treated animal. Tissue was hybridized with an antisense probe for ovine GnRH. Right: GnRH expression in control animals and those prenatally exposed to MXC or BPA.
ESR1 and ESR2
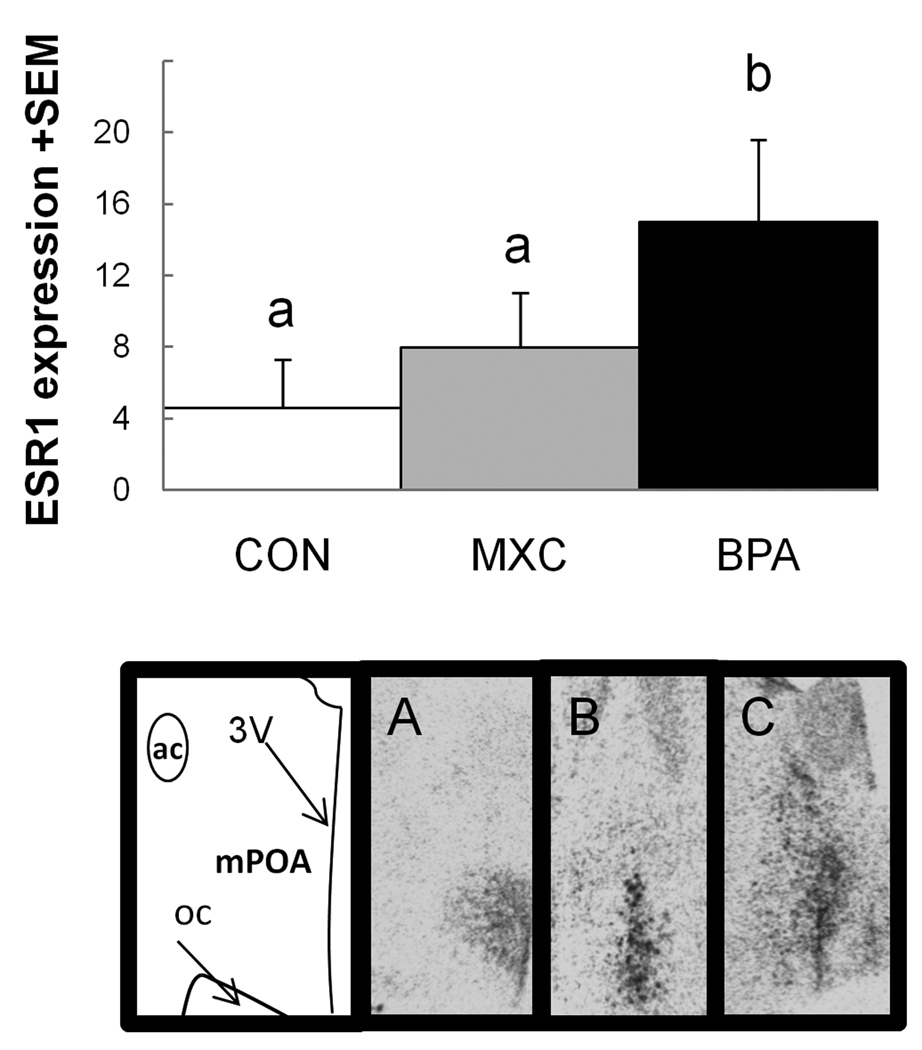
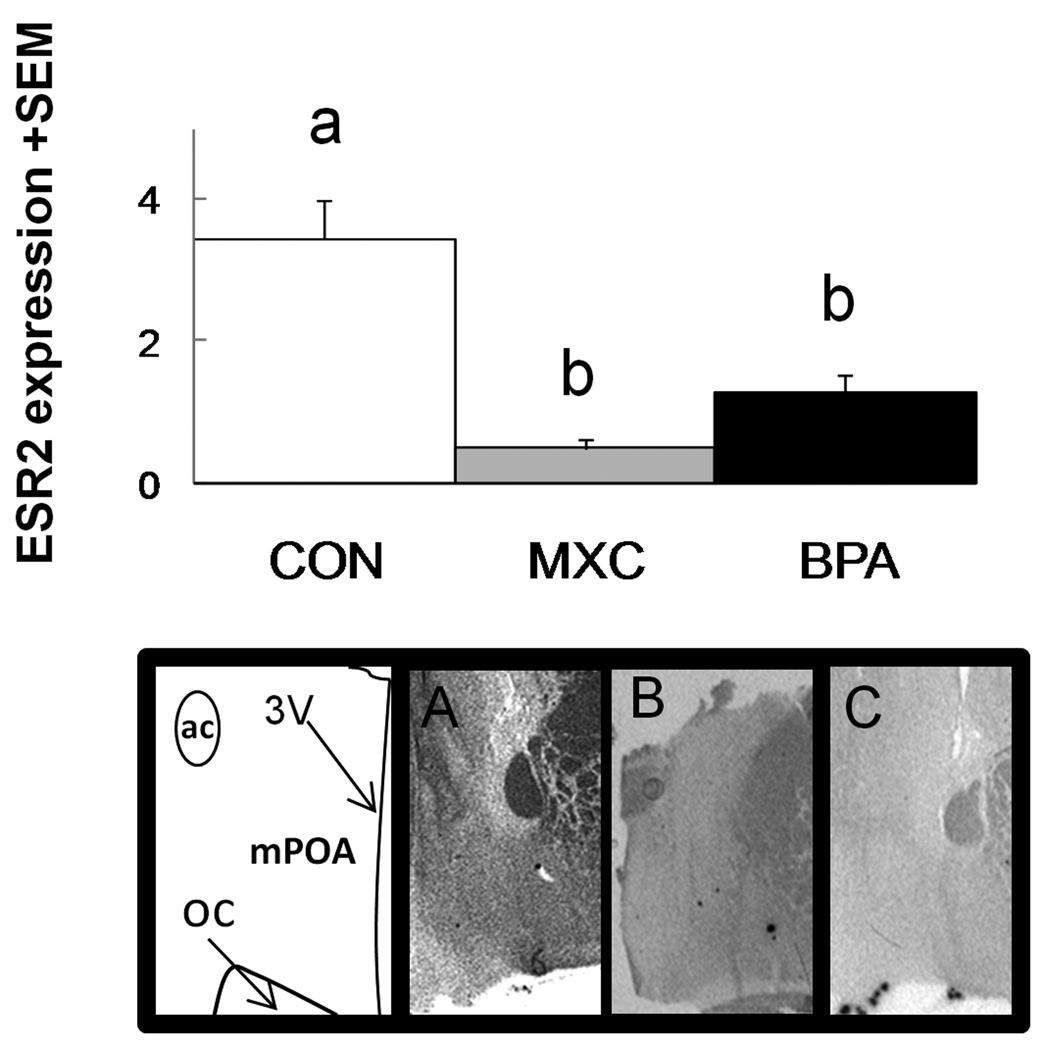
Within the hypothalamus, ESR mRNA positive regions were characterized by dark clusters of hybridization signal (Fig. 2). The distribution and density of ESR1 and ESR2 hybridization signal was similar to that previously reported for sheep (Scott et al., 2000). Effect size analysis found a robust difference between control animals and prenatal BPA-treated animals (d=1.47) and between prenatal MXC- and BPA-treated animals (d=0.9). Specifically, BPA animals had a relatively larger amount of ESR1 signal in the mPOA than the other two groups. There was a significant difference between groups with respect to signal of ESR2 in the mPOA (P<0.001, Fig. 3). Both groups of treated females (prenatal BPA and prenatal MXC) had reduced expression than did controls females (P<0.001 for both comparisons).
Figure 2.
Top: Mean ESR1 signal (mean integrated density of pixels/total number of pixels) in the mPOA of control ewes and those prenatally exposed to MXC or BPA. Effect size analysis found a robust difference between control and BPA treated animals (d=1.47) and between BPA and MXC animals (d=0.9). Bars with different letters over them have robust differences with Cohen’s d effect size analysis. Bottom: Representative images of ESR1 hybridization signal in the medial preoptic area (mPOA) of a (A) control, (B) MXC, and (C) BPA ewe. ac= anterior commissure, 3V= 3rd ventricle, oc= optic chiasm.
Figure 3.
Top: mean ESR2 mRNA signal (mean integrated density of pixels/by the total number of pixels) in the mPOA of control, BPA, and MXC ewes. Bars with different letters differ significantly with post-hoc Tukey tests (P<0.05). Bottom: representative images of ESR2 hybridization signal in the medial preoptic area (mPOA) of a (A) control, (B) MXC, and (C) BPA ewe. ac= anterior commissure, 3V= 3rd ventricle, oc= optic chiasm.
Discussion
Developing mammals undergo “critical periods” of sexual differentiation when they are exquisitely sensitive to the actions of steroid hormones. Previously we have found that sheep prenatally exposed to BPA and MXC have disrupted LH surges, albeit differentially (Savabieasfahani et al., 2006). Here using adult animals from the same cohort and euthanized during the presumptive late follicular phase of the estrous cycle, we identify potential neural mechanisms underlying these differential LH surge defects. Prenatal treatment with both EDCs significantly decreased the amount of GnRH mRNA signal per cell (Fig. 1), amount of ESR2 in the mPOA relative to control animals (Fig. 3), and a selective increase in ESR1 expression in prenatal BPA- but not MXC-treated females compared to controls (Fig.2, effect size analysis). Differential effects of prenatal BPA and MXC on ESR1 and similar effects on ESR2 suggest that the relative ratio of ESR2 and ESR1 may contribute to the differential effects on LH surge.
The mechanisms by which BPA and MXC permanently alter steroid sensitive neural and reproductive structures have not been completely characterized. Both compounds bind to ESR1 and ESR2 leading to transcriptional changes (Kuiper et al., 1998; Wetherill et al., 2007). The action of these compounds is complex and cannot solely be considered as estrogen agonists or antagonists. In vitro studies in human cell lines indicate MXC is a somewhat weak ESR2 antagonist and a strong ESR1 agonist (Kuiper et al., 1998; Lemaire et al., 2006). In addition to binding to ESRs, BPA can also act on androgen sensitive systems and thyroid cells (reviewed in Kuiper et al., 1998). These compounds also bind to non-classical membrane bound ESRs and utilize estrogen independent mechanisms (Ropero et al., 2006). Nanomolar concentrations of BPA applied to human cell cultures caused a rapid release of Ca2+. The same results occurred in a cell line that did not express either form of ESR indicating an estrogen receptor independent mechanism (Walsh et al., 2005). Similarly wild type and ESR1 knockout mice given an injection of MXC had a dose dependent increase in mRNA for estrogen responsive uterine genes (Ghosh et al., 1999). These effects were not blocked when the animals were treated with the ESR antagonist ICI 182,780 indicating that this was not an estrogenic or ESR2 induced mechanism. These agonist and antagonist effects may explain the complex outcomes resulting from BPA and MXC exposure.
The EDC doses selected for use in this study were based upon the lowest observed effect level reported by the Environmental Protection Agency’s report on environmental endocrine disruptors (EPA, 1997). The BPA dosage used in this study resulted in maternal plasma levels that were 2–3 fold higher than the upper level of human internal exposure (Schonfelder et al., 2002; Welshons et al., 2003; Savabieasfahani et al., 2006; Padmanabhan et al., 2008; Vandenberg et al., 2009, 2010). The dose of MXC resulted in lipid levels that were several-fold higher than that reported for human populations (Botella et al., 2004). These differences do not invalidate the data as it is likely that there are sex and species differences in the rates of uptake, responsiveness and metabolism of EDCs. For example, a sex difference has been detected in the serum BPA levels of fasted humans, which is likely due to differences in metabolism (Takeuchi and Tsutsumi, 2002). Additionally, MXC and other organochlorines bio-accumulate in fat (Botella et al., 2004; Quintana et al., 2004; Savabieasfahani et al., 2006) and thus human exposure to this chemical may be greater than just the documented plasma levels.
Our results contribute to the increasing body of literature, across a variety of research models, indicating prenatal exposure to BPA or MXC produces alterations in sexually dimorphic brain regions, cell populations, and pathways critical for reproduction (Navarro et al., 2009). In female rats, administration of BPA from postnatal day (PND) 1 to 7 was found to increase ESR1 mRNA transcripts in the mPOA at PND 8 and 21 (Monje et al., 2007). Additionally, administration of BPA for 5 days following birth increased the amount of ESR1 in the medial basal hypothalamus relative to oil treated controls when animals were sampled at 30 days of age (Khurana et al., 2000). Other studies in male mice exposed to BPA in utero also showed an earlier increase in ESR1 in the mPOA at 5 and 13 weeks of age (Kawai et al., 2007). In contrast, there are reports that early treatment of mice or rats with BPA did not alter or decreased the number of neurons expressing ESR1 (Patisaul et al., 2006; Monje et al., 2009), expression level of ESR1 (total RNA) (Takagi et al., 2005), or ESR2 (total RNA) (Khurana et al., 2000; Kawai et al., 2007) in the preoptic area. These differences in outcome measures may be a function of differences in the timing of treatment relative to when differentiation of the various components of the reproductive system is occurring. Other studies have documented age and sex-dependent changes in ESR expression (Ramos et al., 2003; Kawai et al., 2007). Furthermore, effects also appear to differ depending upon whether exposure involved low or high BPA doses (Rubin et al., 2006; Monje et al., 2007). Studies addressing the effects of MXC on sexually dimorphic brain regions are far fewer. In utero MXC treatment reduced ESR2 expression in the MPOA in 10-day old pups (Takagi et al., 2005) similar to our findings in adult sheep.
Although the effects of BPA and MXC on GnRH mRNA expression level were similar (Fig. 1), they differed in the downstream manifestation of LH surge dynamics (Savabieasfahani et al., 2006). Compared to control ewes, females exposed to BPA prenatally had a reduced LH surge amplitude whereas MXC exposed ewes had a delayed but similar amplitude LH surge. Other studies in female rats and prepubertal sheep have found that BPA treatment during development (prenatal and/or early postnatal exposure) reduces LH release (Rubin et al., 2001; Evans et al., 2004; Fernandez et al., 2009). These differences may reflect changes in dynamics of GnRH synthesis/release stemming from BPA/MXC exposure. In rats, GnRH transcript levels are positively correlated with the amount of LH released (Wang et al., 1995) and blockade of LH surge with pentobarbital injections is associated with a decreased number of GnRH mRNA expressing neurons (Park et al., 1990). Since GnRH is the primary mediator of LH release from the pituitary, reduced GnRH expression seen in the present study in the sheep model is consistent with reduced LH surge amplitude seen in BPA-treated sheep (Savabieasfahani et al., 2006). The effects of these EDCs may also be mediated upstream of GnRH neurons; this is suggested by findings that BPA treatment of female rats during puberty, another critical period of development, decreases mRNA expression level of Kisspeptin, a mediator of GnRH release (Navarro et al., 2009). Paradoxically, rodent studies have found no differences in the ability of GnRH neuron to respond to the positive feedback effects of steroid hormones (Patisaul et al., 2007; Adewale et al., 2009). As such the effects of BPA may extend far beyond the hypothalamo-pituitary axis and involve changes in level of estradiol signal from the ovary or pituitary responses to GnRH (Adewale et al., 2009; Patisaul and Adewale, 2009). Studies addressing effects of MXC on GnRH expression in vivo is limited. MXC treatment reduces GnRH gene transcription and mRNA levels in the immortal GnRH cell line (GT1-7 cells) (Gore, 2002).
In sheep relatively small amounts of GnRH is needed to induce an LH surge (Bowen et al., 1998), thus, the EDC associated reduction in GnRH mRNA described here may have very little impact on the amount of LH released. Conceivably, prenatal EDC exposure may alter the sensitivity to GnRH by reducing the amount of GnRH receptors available or inducing changes in members of GnRH signaling pathways. Sheep that were prenatally exposed to maternal diet containing a mixture of EDCs had a reduced amount of GnRH receptor mRNA in the pituitary (Bellingham et al., 2010). Young rats (13 days old) prenatally exposed to BPA release less LH in response to a GnRH stimulus while manifesting an increase in GnRH pulse frequency (Fernandez-Galaz et al., 2000). Estradiol increases and GnRH decreases the number of GnRH receptors and amount of GnRH receptor mRNA in the sheep pituitary, respectively (Adams et al., 1996; Turzillo et al., 1998; Turzillo and Nett, 1999; Rispoli and Nett, 2005). Therefore the estrogenic effects of BPA may also be elicited at various levels of the neuroendocrine axis.
The differences in the LH surge dynamics in prenatal BPA and MXC-treated sheep (Savabieasfahani et al., 2006) may be related to changes in relative expression of ESR1 and ESR2 described in this study. Transgenic studies provide evidence in support of ESR1 but not ESR2 as essential for generation of the surge (Wintermantel et al., 2006). It is conceivable, however, that the altered ratio of ESR1 and ESR2 may account for the observed differences in LH surge attributes of BPA and MXC treated female sheep (Savabieasfahani et al., 2006). ESR1 and ESR2 form both homodimers and heterodimers, each with differential affinities for estrogens and activation of gene transcription (Matthews and Gustafsson, 2003; Gougelet et al., 2007). When ESR2 is co-expressed with ESR1 in human cell lines, it represses the transcriptional activity of ESR1, and reduces its’ sensitivity to its ligand (Hall and McDonnell, 1999). In some instances ESR2 opposes the actions of ESR1 (Matthews and Gustafsson, 2003). The tissue and gene specific activity of ESR1, ESR2, and their homo/heterodimers remain to be clarified. In light of the current data, however, the altered balance between the ESR isoforms seen in MXC and BPA animals relative to controls may be partially responsible for the altered LH surge characteristics.
Conclusions
This research provides further evidence that EDCs that have estrogenic properties can program fetal development, leading to long-term health consequences in the adult organism. From a broader perspective, these data indicate that the underlying neural physiology associated with female reproductive function is compromised by EDC exposure. Perturbations in the neuroendocrine axis may have dire consequences and lead to earlier onset and /or early cessation of reproductive cycles, altered mating and social behaviors, and impaired fertility (Howdeshell et al., 1999; Honma et al., 2002; Porrini et al., 2005; Seta et al., 2005; Savabieasfahani et al., 2006; Fernandez et al., 2009). These findings also highlight the usefulness of sheep, an estrogen sensitive animal model, in testing the impact of other EDCs that signal through estrogen receptors. From a translational perspective human fetuses are getting exposed to EDCs (Schonfelder et al., 2002; Welshons et al., 2003; Padmanabhan et al., 2008; Ranjit et al., 2009). Overall, our findings support and emphasize the concept that fetal exposure to EDCs, at levels approaching human exposure levels, can have a significant impact on the physiology and health of adult animals (vom Saal and Hughes, 2005; Richter et al., 2007; Gore, 2008).
Acknowledgements
The authors would like to thank the large number of researchers who helped develop the model, collected and analyzed hormone data, and provided technical support. These include Douglas Doop and Gary McCalla who helped with the breeding/lambing and maintenance of the sheep used in this study. We thank Mozhan Savabieasfahani, Jim Lee, Carol Herkimer, Mohan Manikkam and Teresa Steckler for assistance with prenatal treatment and tissue harvest and Dr. Robert Thompson and Jessica Koch for assistance with in situ hybridization. M. Mahoney was supported by the EHS Toxicology T32 training grant (5T32ES007062). This study was supported by USPHS grants R01 ES016541 to VP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have nothing to disclose.
References
- Adams BM, Sakurai H, Adams TE. Concentrations of gonadotropin-releasing hormone (GnRH) receptor messenger ribonucleic acid in pituitary tissue of orchidectomized sheep: effect of estradiol and GnRH. Biol Reprod. 1996;54:407–412. doi: 10.1095/biolreprod54.2.407. [DOI] [PubMed] [Google Scholar]
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81:690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Methoxychlor. Atlanta: Agency for Toxic Substances and Disease Registry; 2002. [PubMed] [Google Scholar]
- Bellingham M, Fowler PA, Amezaga MR, Whitelaw CM, Rhind SM, Cotinot C, Mandon-Pepin B, Sharpe RM, Evans NP. Foetal Hypothalamic and Pituitary Expression of GnRH and Galanin Systems is Disturbed by Exposure to Sewage Sludge Chemicals via Maternal Ingestion. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Dahl GE, Evans NP, Thrun LA, Wang Y, Brown MB, Karsch FJ. Importance of the gonadotropin-releasing hormone (GnRH) surge for induction of the preovulatory luteinizing hormone surge of the ewe: dose-response relationship and excess of GnRH. Endocrinology. 1998;139:588–595. doi: 10.1210/endo.139.2.5719. [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139:1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–157. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- Cohen J. A Power Primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Damstra T, Barlow S, Bergman A, Kavlock R, Van der Kraak G International Programme on Chemical Safety (IPCS) Global Assessment of the State-of-the-Science of Endocrine Disruptors. 2002 [Google Scholar]
- EPA. Special report on environmental endocrine disruption: an effects assessment and analysis. Washington DC: US Environmental Protection Agency; 1997. [Google Scholar]
- Evans NP, North T, Dye S, Sweeney T. Differential effects of the endocrine-disrupting compounds bisphenol-A and octylphenol on gonadotropin secretion, in prepubertal ewe lambs. Domest Anim Endocrinol. 2004;26:61–73. doi: 10.1016/j.domaniend.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Martinez Munoz R, Villanua M, Garcia-Segura LM ICHBB and SBN Joint Meeting. Diurnal oscillations in glial fibrillary acidic protein (GFAP) in a perichiasmatic area and its relationship to the LH surge in the female rat; 2000. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117:757–762. doi: 10.1289/ehp.0800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. Soc Reprod Fertil Suppl. 2007;64:83–107. doi: 10.5661/rdr-vi-83. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Taylor JA, Green JA, Lubahn DB. Methoxychlor stimulates estrogen-responsive messenger ribonucleic acids in mouse uterus through a non-estrogen receptor (non-ER) alpha and non-ER beta mechanism. Endocrinology. 1999;140:3526–3533. doi: 10.1210/endo.140.8.6877. [DOI] [PubMed] [Google Scholar]
- Gore AC. Organochlorine pesticides directly regulate gonadotropin-releasing hormone gene expression and biosynthesis in the GT1-7 hypothalamic cell line. Mol Cell Endocrinol. 2002;192:157–170. doi: 10.1016/s0303-7207(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougelet A, Mueller SO, Korach KS, Renoir JM. Oestrogen receptors pathways to oestrogen responsive elements: the transactivation function-1 acts as the keystone of oestrogen receptor (ER)beta-mediated transcriptional repression of ERalpha. J Steroid Biochem Mol Biol. 2007;104:110–122. doi: 10.1016/j.jsbmb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Neurochemical identity of neurones expressing oestrogen and androgen receptors in sheep hypothalamus. J Reprod Fertil Suppl. 1995;49:271–283. [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after "Wingspread"--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Ing NH, Spencer TE, Bazer FW. Estrogen enhances endometrial estrogen receptor gene expression by a posttranscriptional mechanism in the ovariectomized ewe. Biol Reprod. 1996;54:591–599. doi: 10.1095/biolreprod54.3.591. [DOI] [PubMed] [Google Scholar]
- Jansen HT, Hileman SM, Lubbers LS, Kuehl DE, Jackson GL, Lehman MN. Identification and distribution of neuroendocrine gonadotropin- releasing hormone neurons in the ewe. Biol Reprod. 1997;56:655–662. doi: 10.1095/biolreprod56.3.655. [DOI] [PubMed] [Google Scholar]
- Jansen HT, West C, Lehman MN, Padmanabhan V. Ovarian estrogen receptor-beta (ERbeta) regulation: I. Changes in ERbeta messenger RNA expression prior to ovulation in the ewe. Biol Reprod. 2001;65:866–872. doi: 10.1095/biolreprod65.3.866. [DOI] [PubMed] [Google Scholar]
- Johnson JI, Sudheimer KD, Davis KK, Kerndt G, Winn BM. The Sheep Brain Atlas. 2004 [Google Scholar]
- Kawai K, Murakami S, Senba E, Yamanaka T, Fujiwara Y, Arimura C, Nozaki T, Takii M, Kubo C. Changes in estrogen receptors alpha and beta expression in the brain of mice exposed prenatally to bisphenol A. Regul Toxicol Pharmacol. 2007;47:166–170. doi: 10.1016/j.yrtph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006;79:1160–1169. doi: 10.1016/j.lfs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Markey CM, Rubin BS, Soto AM, Sonnenschein C. Endocrine disruptors: from Wingspread to environmental developmental biology. J Steroid Biochem Mol Biol. 2002;83:235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab. 2006;20:63–75. doi: 10.1016/j.beem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- McNeilly AS. The ovarian follicle and fertility. J Steroid Biochem Mol Biol. 1991;40:29–33. doi: 10.1016/0960-0760(91)90164-z. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133:896–903. doi: 10.1210/endo.133.2.8344224. [DOI] [PubMed] [Google Scholar]
- Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5'-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. [DOI] [PubMed] [Google Scholar]
- Monje L, Varayoud J, Munoz-de-Toro M, Luque EH, Ramos JG. Neonatal exposure to bisphenol A alters estrogen-dependent mechanisms governing sexual behavior in the adult female rat. Reprod Toxicol. 2009;28:435–442. doi: 10.1016/j.reprotox.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Sanchez-Garrido MA, Castellano JM, Roa J, Garcia-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology. 2009;150:2359–2367. doi: 10.1210/en.2008-0580. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl. 2010 doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Parmigiani S, vom Saal FS. Effects of prenatal exposure to low doses of diethylstilbestrol, o,p'DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm Behav. 2001;40:252–265. doi: 10.1006/hbeh.2001.1697. [DOI] [PubMed] [Google Scholar]
- Park OK, Gugneja S, Mayo KE. Gonadotropin-releasing hormone gene expression during the rat estrous cycle: effects of pentobarbital and ovarian steroids. Endocrinology. 1990;127:365–372. doi: 10.1210/endo-127-1-365. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:1–10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Seta DD, Farabollini F, Giannelli G, Dessi-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65:261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Quintana PJ, Delfino RJ, Korrick S, Ziogas A, Kutz FW, Jones EL, Laden F, Garshick E. Adipose tissue levels of organochlorine pesticides and polychlorinated biphenyls and risk of non-Hodgkin's lymphoma. Environ Health Perspect. 2004;112:854–861. doi: 10.1289/ehp.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JG, Varayoud J, Kass L, Rodriguez H, Costabel L, Munoz-De-Toro M, Luque EH. Bisphenol a induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. 2003;144:3206–3215. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2009 doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PJ, Lindahl IL, Cecil HC, Bitman J. A comparison of DDT and methoxychlor accumulation and depletion in sheep. Bull Environ Contam Toxicol. 1976;16:240–247. doi: 10.1007/BF01685234. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispoli LA, Nett TM. Pituitary gonadotropin-releasing hormone (GnRH) receptor: structure, distribution and regulation of expression. Anim Reprod Sci. 2005;88:57–74. doi: 10.1016/j.anireprosci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Taylor JA, Foster DL, Padmanabhan V. In utero programming of sexually differentiated gonadotrophin releasing hormone (GnRH) secretion. Domest Anim Endocrinol. 2002;23:43–52. doi: 10.1016/s0739-7240(02)00144-3. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Alonso-Magdalena P, Ripoll C, Fuentes E, Nadal A. Rapid endocrine disruption: environmental estrogen actions triggered outside the nucleus. J Steroid Biochem Mol Biol. 2006;102:163–169. doi: 10.1016/j.jsbmb.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CJ, Tilbrook AJ, Simmons DM, Rawson JA, Chu S, Fuller PJ, Ing NH, Clarke IJ. The distribution of cells containing estrogen receptor-alpha (ERalpha) and ERbeta messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinology. 2000;141:2951–2962. doi: 10.1210/endo.141.8.7622. [DOI] [PubMed] [Google Scholar]
- Seta DD, Minder I, Dessi-Fulgheri F, Farabollini F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull. 2005;65:255–260. doi: 10.1016/j.brainresbull.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroendocrinol. 2005;17:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- Staub C, Hardy VB, Chapin RE, Harris MW, Johnson L. The hidden effect of estrogenic/antiandrogenic methoxychlor on spermatogenesis. Toxicol Appl Pharmacol. 2002;180:129–135. doi: 10.1006/taap.2002.9369. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Lee HC, Chiba S, Yonezawa T, Nishihara M. Effects of methoxychlor exposure during perinatal period on reproductive function after maturation in rats. J Reprod Dev. 2004;50:455–461. doi: 10.1262/jrd.50.455. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shibutani M, Lee KY, Masutomi N, Fujita H, Inoue K, Mitsumori K, Hirose M. Impact of maternal dietary exposure to endocrine-acting chemicals on progesterone receptor expression in microdissected hypothalamic medial preoptic areas of rat offspring. Toxicol Appl Pharmacol. 2005;208:127–136. doi: 10.1016/j.taap.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- Turzillo AM, Nett TM. Regulation of GnRH receptor gene expression in sheep and cattle. J Reprod Fertil Suppl. 1999;54:75–86. [PubMed] [Google Scholar]
- Turzillo AM, Nolan TE, Nett TM. Regulation of gonadotropin-releasing hormone (GnRH) receptor gene expression in sheep: interaction of GnRH and estradiol. Endocrinology. 1998;139:4890–4894. doi: 10.1210/endo.139.12.6344. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chauhoud I, Heindell JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010 Mar 23;2010 doi: 10.1289/ehp.0901716. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Jr, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DE, Dockery P, Doolan CM. Estrogen receptor independent rapid non-genomic effects of environmental estrogens on [Ca2+]i in human breast cancer cells. Mol Cell Endocrinol. 2005;230:23–30. doi: 10.1016/j.mce.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Walsh JP, Clarke IJ. Blockade of the oestrogen-induced luteinizing hormone surge in ovariectomized ewes by a highly selective opioid mu-receptor agonist: evidence for site of action. Neuroendocrinology. 1998;67:164–170. doi: 10.1159/000054311. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Hoffman GE, Smith MS. Increased GnRH mRNA in the GnRH neurons expressing cFos during the proestrous LH surge. Endocrinology. 1995;136:3673–3676. doi: 10.1210/endo.136.8.7628409. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16:735–739. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]