Abstract
Purpose
The tracer 123I-2-([2-({dimethylamino}methyl)phenyl]thio)-5-iodophenylamine ([123I]ADAM) has been developed to image serotonin transporters (SERTs) with SPECT. Preclinical studies have shown that [123I]ADAM binds selectively to SERTs. Moreover, initial human studies have shown that [123I]ADAM binding could be blocked by selective serotonin reuptake inhibitors (SSRIs). However, in humans it has not been proven that [123I]ADAM binds selectively to SERTs.
Methods
We examined the in vivo availability of SERTs in 12 healthy young volunteers 5 h after bolus injection of [123I]ADAM. To evaluate the selectivity of binding, four participants were pretreated (double-blinded design) with placebo, four with paroxetine (20 mg) and four with the dopamine/norepinephrine blocker methylphenidate (20 mg). SPECT studies were performed on a brain-dedicated system (Neurofocus), and the SPECT images were coregistered with individual MR scans of the brain. ADAM binding in SERT-rich brain areas and cerebellar cortex (representing non-specific binding) was assessed by drawing regions of interest (ROIs) on the individual MR images. Specific to non-specific ratios were used as the outcome measure.
Results
We found that specific to non-specific ratios were statistically significantly lower in paroxetine-pretreated participants than in placebo- or methylphenidate-pretreated participants. No such difference was found between groups pretreated with placebo or methylphenidate.
Conclusion
Our preliminary findings suggest that [123I]ADAM binds selectively to SERTs in human brain.
Keywords: Serotonin transporter imaging, SPECT, [123I]ADAM, Human, Selectivity
Introduction
Serotonin transporters (SERTs) are located in the membrane of serotonergic neuron terminals and play an important role in the regulation of serotonin in the synaptic cleft. They are believed to be the primary target for antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) [1]. Disturbances in the expression of central SERTs are thought to play an important role in psychiatric disorders, such as major depression.
In vivo imaging of SERTs with PET or SPECT provides an important tool to study the availability of SERTs in living human brain. Recently, the diarylsulphide derivative [123I]-2-([2-({dimethylamino}methyl)phenyl]thio)-5-iodophenylamine ([123I]ADAM) has been developed to image SERTs with SPECT. Studies in small laboratory animals and non-human primates have shown that the in vivo binding of [123/125I]ADAM is selective for SERTs [2, 3]. In addition, preliminary studies in humans have shown that radiotracer binding in SERT-rich brain areas could be blocked by SSRIs [4, 5]. However, it has not been proven that [123I]ADAM binds selectively to SERTs in living human brain.
Therefore, the purpose of the current study was to evaluate whether [123I]ADAM binds selectively to SERTs in living human brain.
Materials and methods
Subjects
Twelve healthy male volunteers were included in the present study. Exclusion criteria were age below 18 or above 35 years, major mental or physical problems, use of psychopharmaceuticals such as SSRIs and use of hard drugs such as ecstasy, cocaine, amphetamine or heroin in the past. To exclude a clinical depression, all volunteers had to complete the Beck Depression Inventory (BDI), a validated self-report depression questionnaire. Written informed consent was obtained from all subjects and the study was approved by the local Medical Ethics Committee. In addition, we received permission from local competent authorities to administer radiation to young healthy subjects.
Pretreatment of participants
To evaluate the selectivity of [123I]ADAM binding to SERTs, four participants were pretreated with placebo (one tablet), four participants with the SSRI paroxetine (20 mg) and four with the dopamine/norepinephrine blocker methylphenidate (20 mg, immediate release). To guarantee accurate intake of the tablets, the intake was supervised by an independent person. Dosing was based on the following arguments: a single dose of each drug is well tolerated, and 20 mg paroxetine is able to block >70% of central SERTs, and one dose of 20 mg methylphenidate (immediate release) is able to occupy 55% of dopamine transporters in the striatum [6, 7]. The tablets were always administered orally 2.5 h prior to injection of [123I]ADAM, using a double-blind study approach. The participants were not allowed to eat after midnight, and they received the tablets always around 08.00 a.m. Two hours after intake of the tablet, the participants were allowed to eat.
[123I]ADAM SPECT experiments
Subjects were examined using SPECT with the ligand [123I]ADAM. Radiosynthesis of [123I]ADAM was performed at MAP Medical Technologies Oy (Tikkakoski, Finland). The 123I-labelled ADAM was prepared by iododestannylation of the corresponding trimethyltin precursor, with carrier-free 123I as NaI. The radiochemical purity of the solution was always higher than 95.0%. [123I]ADAM was injected intravenously as a bolus (140.6 ± 6.6 MBq = mean ± SD). Subjects received a potassium iodide solution to block thyroid uptake of free radioactive iodide.
SPECT study
SPECT studies were always acquired 5 h after injection of the radiotracer [8]. They were performed using a 12-detector single-slice brain-dedicated scanner (Neurofocus 810, which is an upgrade of the Strichmann Medical Equipment) with a full-width at half-maximum (FWHM) resolution of approximately 6.5 mm throughout the 20-cm field of view (http://www.neurophysics.com). In this study, the energy window was set at 135–190 keV. Attenuation correction of all images was performed as earlier described (see references in [8]). Images were reconstructed in 3-D mode.
MRI study
For anatomical reference, in each individual T1-weighted 3-D MRI was performed using a 3-T Philips Intera scanner (Philips Healthcare, Best, The Netherlands) with a standard head coil.
Image analysis
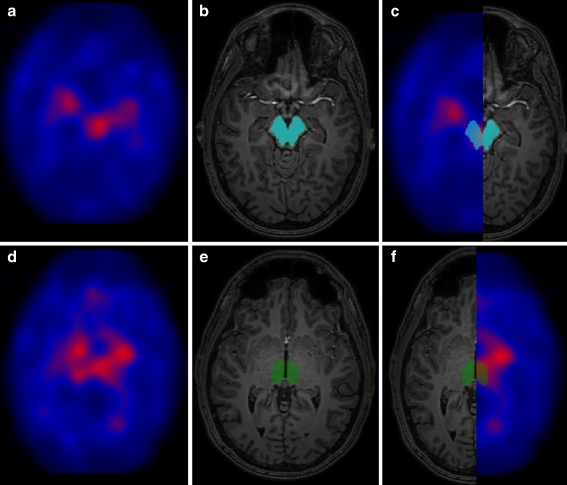
For the analysis of SERT availability, regions of interest (ROIs) were drawn manually on each individual T1-weighted 3-D MRI scan for the midbrain and thalamus, which are SERT-rich regions, and for the cerebellar cortex (Fig. 1), which was assumed to represent non-displaceable activity and therefore could be used as reference region. ROIs were drawn on the MRI scan by making use of self-developed software [9]. The volumes of the ROIs were not statistically significantly different between groups. By means of the same program, we matched the SPECT scans of the subjects with their individual T1-weighted 3-D MRI scans and extracted SERT availability of the respective ROIs. Specific to non-specific binding ratios for SERT were calculated: (activity in ROI minus activity in cerebellar cortex)/activity in cerebellar cortex.
Fig. 1.
a Transverse SPECT image obtained 5 h after injection of approximately 140 MBq [123I]ADAM at the level of the midbrain. b Transverse T1-weighted MRI image of the same subject with ROI drawn in the midbrain. c Coregistered SPECT and T1-weighted MRI image with ROI drawn in the midbrain. d Transverse SPECT image (5 h post-injection) at the level of the thalamus. e Transverse T1-weighted MRI image of the same subject with ROI drawn in the thalamus. f Coregistered SPECT and T1-weighted MRI image with ROI drawn in the thalamus. The SPECT images are colour encoded for low (black) to high activity (white)
Statistical analysis
A Mann-Whitney U test was used to detect differences in specific to non-specific ratios of [123I]ADAM binding between groups. In the statistical analysis, which was two-tailed, p < 0.05 was considered significant.
Results
Subjects
The mean age of the male subjects was 22.7 ± 2.7 years (mean ± SD; range: 19–29 years). The mean BDI scores were 0.4 ± 0.8 (range: 0–2). As BDI scores between 0 and 9 are regarded as normal, none of the subjects was suspected of having clinical depression.
SPECT measures
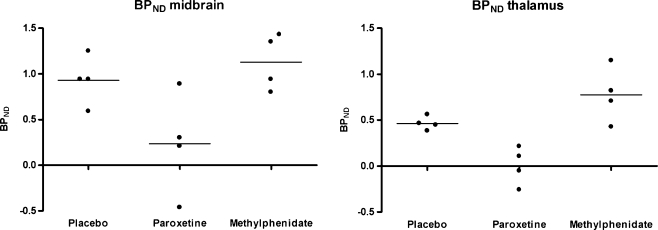
The specific to non-specific ratios in the midbrain were statistically significantly lower in the group that was pretreated with paroxetine (mean ± SD: 0.24 ± 0.55) than in the placebo- (0.93 ± 0.27) or methylphenidate-pretreated group (1.13 ± 0.31; p = 0.042 and 0.043, respectively; Fig. 2). These ratios in the midbrain were not statistically significantly different between the placebo- and methylphenidate-pretreated groups (p = 0.375).
Fig. 2.
Individual and mean specific to non-specific [123I]ADAM binding ratios (BPND) in the midbrain and thalamus per pretreatment group. BP ND binding potential non-displaceable
The specific to non-specific ratios in the thalamus area were statistically significantly lower in the paroxetine-pretreated group (mean ± SD: 0.00 ± 0.21) than in the placebo- (0.46 ± 0.07) or methylphenidate-pretreated groups (0.76 ± 0.30; p = 0.021 and 0.021, respectively; Fig. 2). These ratios in the thalamus were not significantly different between the placebo- and the methylphenidate-pretreated groups (p = 0.149).
Discussion
To the best of our knowledge, the present preliminary study is the first to show that [123I]ADAM binds selectively to SERTs in living human brain. We were able to show this selectivity, since healthy male controls pretreated with an SSRI (paroxetine) had significantly lower [123I]ADAM binding to SERTs than participants pretreated with placebo or the dopamine/norepinephrine blocker methylphenidate, while [123I]ADAM binding did not significantly differ between the placebo- and methylphenidate-pretreated participants. This was true both for [123I]ADAM binding in the SERT-rich thalamus as well as in the SERT-rich midbrain. This result validates the use of [123I]ADAM as a selective tracer for the SERT in human studies and is complementary to results obtained in ex vivo rat studies and in vivo experiments in non-human primates [2, 3].
In this study, we found high specific binding of [123I]ADAM in the thalamus and midbrain. This finding is in line with a previous PET study using selective tracers for the SERT [10] and human necropsy studies [11].
Strengths of our study are its design (placebo-controlled, double-blind), coregistration of the SPECT images with individual MR images and use of the cerebellar grey matter cortex as a reference region. Indeed, one has to keep in mind that the cerebellar vermis is not devoid of SERTs [12]. Besides, several limitations of the current study should be discussed. First, only young men were included. However, it is likely that our present findings could be generalized to older men and are gender independent. Second, a small group was studied. This small sample size limits the statistical power of our results, and therefore our results should be interpreted as preliminary and are in need of replication. Third, with our study protocol only [123I]ADAM binding to SERTs can be measured accurately in SERT-rich brain areas. We can therefore not prove that binding of ADAM is also selective for SERT in brain areas expressing low densities of SERTs.
In the present study, single SPECT scans were acquired during transient equilibrium 5 h after bolus injection of the tracer. This time point was based on the results of a previous study, performed in young adults, in which the mean ratio of specific to non-specific [123I]ADAM binding was highest in SERT-rich areas at 5 h post-injection [8]. A recent study [5], however, showed that the ratio method, when based on time frames from 200–240 min or 240–280 min after injection of [123I]ADAM, slightly, but statistically significantly overestimated specific binding in humans, and particularly in brain regions expressing high densities of SERTs (by 10% on average). This overestimation is consistent with theoretic predictions that the ratio of specific binding to non-specific binding during transient equilibrium overestimates the ratio at true equilibrium. Consequently, we can not exclude that particularly in the placebo- and methylphenidate-pretreated groups the specific binding ratios were slightly overestimated (on average 10%). However, given the large difference in binding ratios between the paroxetine-pretreated group and the placebo- and methylphenidate-pretreated groups (see Fig. 2), it is likely that when [123I]ADAM binding to SERT will be measured at true equilibrium similar results can be found.
[123I]ADAM binding was higher after methylphenidate pretreatment than after placebo pretreatment, particularly in the thalamus. Although this difference was not statistically significant, and should be described at best as a possible trend, it may be of interest to investigate in further studies whether acute intervention with methylphenidate might influence the expression of SERTs, since direct interactions between the central dopaminergic and serotonergic neurotransmission systems are well known [13]. Previous animal studies, using ADAM as a SPECT or PET tracer, however, did not show statistically significant effects of pretreatment with methylphenidate on ADAM binding [2, 3, 14]. For example, a SPECT study performed in non-primates by Ma and co-workers [3] found no statistically significant effects of pretreatment with methylphenidate (1 mg/kg body weight) on specific uptake ratios of [123I]ADAM in the SERT-rich midbrain and thalamus, and these ratios were even lower than ratios obtained in the control condition at most time points of the SPECT measurements.
In this study, a single dose of 20 mg paroxetine was used to evaluate binding to SERTs in healthy controls. In the present study this dose induced an occupancy of >70% of SERTs in the midbrain and thalamus (specific ratios in the paroxetine-pretreated group compared to those obtained in the placebo-pretreated group). A SERT PET study by Meyer and co-workers [7] showed that treatment with 20 mg/day of paroxetine for a 4-week period in depressed patients showed a mean proportion of SERT occupied by paroxetine of 83%. In addition, they showed that SERT occupancy increased in a non-linear relationship with serum levels of paroxetine. After intake of one dose of oral paroxetine by healthy subjects, plasma levels are highly variable between subjects, with a mean plasma level of approximately 8 µg/l 3 h after oral intake of the tablet [15]. Interestingly, the reported relationship of paroxetine SERT occupancy with serum levels as described by Meyer and co-workers [7] predicted that a plasma paroxetine level of 8 µg/l will induce an occupancy of approximately 70% of SERTs, which is in agreement with our present finding, although a limitation of the present study was that we did not measure plasma levels of paroxetine.
In conclusion, in this preliminary double-blind, placebo-controlled study we show that in vivo [123I]ADAM binding to SERT is selective in young healthy men.
Acknowledgements
This study was supported by a grant from the Dutch Brain Foundation (Hersenstichting Nederland; grantnr. 11F03{2}32), Den Haag, The Netherlands.
We would like to thank Berthe van Eck-Smit for coding the pretreatment medication and administering the medication to the subjects.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Ruhé HG, Booij J, van Weert HC, Reitsma JB, Franssen EJ, Michel MC, et al. Evidence why paroxetine dose escalation is not effective in major depressive disorder: a randomized controlled trial with assessment of serotonin transporter occupancy. Neuropsychopharmacology. 2009;34:999–1010. doi: 10.1038/npp.2008.148. [DOI] [PubMed] [Google Scholar]
- 2.Choi SR, Hou C, Oya S, Mu M, Kung MP, Siciliano M, et al. Selective in vitro and in vivo binding of [(125)I]ADAM to serotonin transporters in rat brain. Synapse. 2000;38:403–412. doi: 10.1002/1098-2396(20001215)38:4<403::AID-SYN5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Ma KH, Lee JK, Huang SY, Yeh CB, Shen YC, Shen LH, et al. Simultaneous [99mTc]TRODAT-1 and [123I]ADAM brain SPECT in nonhuman primates. Mol Imaging Biol. 2009;11:253–262. doi: 10.1007/s11307-009-0197-0. [DOI] [PubMed] [Google Scholar]
- 4.Klein N, Sacher J, Geiss-Granadia T, Mossaheb N, Altarbaschi T, Lanzenberger R, et al. Higher serotonin transporter occupancy after multiple dose administration of escitalopram compared to citalopram: an [123I]ADAM SPECT study. Psychopharmacology (Berl) 2007;191:333–339. doi: 10.1007/s00213-006-0666-y. [DOI] [PubMed] [Google Scholar]
- 5.Frokjaer VG, Pinborg LH, Madsen J, de Nijs R, Svarer C, Wagner A, et al. Evaluation of the serotonin transporter ligand 123I-ADAM for SPECT studies on humans. J Nucl Med. 2008;49:247–254. doi: 10.2967/jnumed.107.046102. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 7.Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 8.Booij J, de Win MM. Brain kinetics of the new selective serotonin transporter tracer [(123)I]ADAM in healthy young adults. Nucl Med Biol. 2006;33:185–191. doi: 10.1016/j.nucmedbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.van Herk M, de Jaeger K, de Munck J, Hoogeman M, Meinders J, Ploeger L. A delineation system for N modalities-software aspects (extended abstract). In: 13th ICCR, Heidelberg, Germany, 2000, pp 73–75
- 10.Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, et al. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 2004;45:682–694. [PubMed] [Google Scholar]
- 11.Kish SJ, Furukawa Y, Chang LJ, Tong J, Ginovart N, Wilson A, et al. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region? Nucl Med Biol. 2005;32:123–128. doi: 10.1016/j.nucmedbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, et al. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Fleckenstein AE, Haughey HM, Metzger RR, Kokoshka JM, Riddle EL, Hanson JE, et al. Differential effects of psychostimulants and related agents on dopaminergic and serotonergic transporter function. Eur J Pharmacol. 1999;382:45–49. doi: 10.1016/S0014-2999(99)00588-9. [DOI] [PubMed] [Google Scholar]
- 14.Shiue GG, Choi SR, Fang P, Hou C, Acton PD, Cardi C, et al. N, N-dimethyl-2-(2-amino-4–18F-fluorophenylthio)-benzylamine (4–18F-ADAM): an improved PET radioligand for serotonin transporters. J Nucl Med. 2003;44:1890–1897. [PubMed] [Google Scholar]
- 15.Segura M, Ortuño J, Farré M, Pacifici R, Pichini S, Joglar J, et al. Quantitative determination of paroxetine and its 4-hydroxy-3-methoxy metabolite in plasma by high-performance liquid chromatography/electrospray ion trap mass spectrometry: application to pharmacokinetic studies. Rapid Commun Mass Spectrom. 2003;17:1455–1461. doi: 10.1002/rcm.1067. [DOI] [PubMed] [Google Scholar]