Abstract
The title compound, C16H13NO3, crystallizes with two independent molecules (A and B) in the asymmetric unit. The dihedral angle between the mean planes of the 4-methylphenyl and 3-nitrophenyl groups is 4.0 (3)° in molecule A and 16.2 (7)° in molecule B. Intermolecular C—H⋯O hydrogen bonding involving the O atoms of the 3-nitrophenyl group of both independent molecules link the molecules into layers approximately parallel to the (110) plane. The layers are held together by π–π stacking interactions between the 4-methylphenyl ring of molecule A and the 3-nitrophenyl ring of molecule B of the adjacent layer, with the distance between the centroids of interacting rings being 3.6987 (7) Å.
Related literature
For related structures, see: Butcher, Jasinski, Narayana et al. (2007 ▶); Butcher, Jasinski, Yathirajan, Narayana et al. (2007 ▶); Butcher, Jasinski, Yathirajan, Veena et al. (2007 ▶); Rosli et al. (2007 ▶); Patil et al. (2007 ▶). For related literature, see: Dimmock et al. (1999 ▶); Go et al. (2005 ▶); Goto et al. (1991 ▶); Uchida et al. (1998 ▶); Tam et al. (1989 ▶).
Experimental
Crystal data
C16H13NO3
M r = 267.27
Triclinic,

a = 8.0951 (3) Å
b = 11.5088 (5) Å
c = 14.6970 (5) Å
α = 80.351 (3)°
β = 74.830 (3)°
γ = 84.416 (3)°
V = 1300.78 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 296 (2) K
0.41 × 0.35 × 0.28 mm
Data collection
Oxford Diffraction Gemini R CCD diffractometer
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2007 ▶) T min = 0.874, T max = 0.974
19776 measured reflections
8636 independent reflections
4667 reflections with I > 2σ(I)
R int = 0.027
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.127
S = 0.97
8636 reflections
363 parameters
H-atom parameters constrained
Δρmax = 0.31 e Å−3
Δρmin = −0.23 e Å−3
Data collection: CrysAlisPro (Oxford Diffraction, 2007 ▶); cell refinement: CrysAlisPro; data reduction: CrysAlisPro; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: SHELXTL (Bruker, 2000 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680706182X/ci2518sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680706182X/ci2518Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2A—H2A⋯O2B | 0.93 | 2.55 | 3.4644 (16) | 170 |
| C11A—H11A⋯O2B | 0.93 | 2.59 | 3.5156 (14) | 176 |
| C2B—H2B⋯O2Ai | 0.93 | 2.51 | 3.4311 (16) | 171 |
| C14A—H14A⋯O3Aii | 0.93 | 2.54 | 3.4455 (16) | 164 |
Symmetry codes: (i) 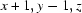 ; (ii)
; (ii)  .
.
Acknowledgments
KL thanks Mangalore University for use of their research facilities. RJB acknowledges the NSF MRI program (grant No. CHE-0619278) for funds to purchase the X-ray diffractometer.
supplementary crystallographic information
Comment
Chalcones can be easily obtained from the Claisen-Schmidt reaction of aromatic aldehydes and aromatic ketones. Chalcones have been reported to possess many useful properties including anti-inflammatory, antimicrobial, antifungal, antioxidant, cytotoxic, antitumour and anticancer activities (Dimmock et al. 1999; Go et al. 2005). They are also important intermediates in organic synthesis. Among several organic compounds reported to have NLO properties, chalcone derivatives are recognized material because of their excellent blue light transmittance and good crystallization ability. They provide necessary configuration to show NLO properties having two planar rings connected through a conjugated double bond (Goto et al. 1991; Uchida et al. 1998; Tam et al. 1989). The crystal structures of 1-(3-hydroxyphenyl)-3-(4-methylphenyl)prop-2-en-1-one (Butcher, Jasinski, Narayana et al., 2007), (2E)-1-(4-methylphenyl)-3-(4-nitrophenyl)prop-2-en-1-one (Butcher, Jasinski, Yathirajan, Veena et al., 2007), (E)-3-(4-fluorophenyl)-1-(4-methylphenyl)prop-2-en-1-one (Butcher, Jasinski, Yathirajan, Narayana et al. 2007), 3-(dimethylaminophenyl)-1-(3-nitrophenyl)prop-2-en-1-one (Rosli et al. 2007) and 3-(5-bromo-2-thienyl)-1-(4-nitrophenyl)prop-2-en-1-one (Patil et al. 2007) have been reported. We report here the crystal structure of a new chalcone, the title compound.
The title compound crystallizes with two independent molecules (A and B) in the asymmetric unit (Fig. 1). The dihedral angle between the mean planes of the 4-methylphenyl and 3-nitrophenyl groups is 4.0 (3)° in molecule A and 16.2 (7)° in molecule B. Crystal packing is stabilized by intermolecular C—H···O hydrogen bonding involving the O atoms on the 3-nitrophenyl group of both indpendent molecules. These hydrogen bonds (Table 1) link the molecules into a layer approximately parallel to the (1 1 0) plane (Fig. 2). Intermolecular π-π stacking interactions occur between 4-methylphenyl ring of molecule A at (x, y, z) and 3-nitrophenyl ring of molecule B of the adjacent layer at (1 - x, 1 - y, -z), with the distance between the centroids of interacting rings being 3.6987 (7) Å.
Experimental
A solution of 1-(3-nitrophenyl)ethanone (1.65 g, 0.01 mol) and 4-methylbenzaldehyde (1.20 g, 0.01 mol) in ethanol (25 ml) was stirred well and 10% NaOH solution (5 ml) was added. The reaction mixture was stirred for about 6 h and filtered. The product was crystallized from acetone (m.p. 414–416 K). Single crystals suitable for X-ray structure determination were grown by slow evaporation of an acetone solution of the title compound at room temperature. Analysis found: C 71.82, H 4.85, N 5.20%; C16H13NO3 requires: C 71.90, H 4.90, N 5.24%.
Refinement
All H atoms were placed in calculated positions (C—H = 0.93 or 0.96 Å) and refined using a riding model, with Uiso(H) = 1.16–1.21Ueq(C).
Figures
Fig. 1.
The asymmetric unit of the title compound, showing atom labeling. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Packing diagram of the title compound, viewed down the a axis. Dashed lines indicate intermolecular C—H···O hydrogen bonds.
Crystal data
| C16H13NO3 | Z = 4 |
| Mr = 267.27 | F000 = 560 |
| Triclinic, P1 | Dx = 1.365 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation λ = 0.71073 Å |
| a = 8.0951 (3) Å | Cell parameters from 6626 reflections |
| b = 11.5088 (5) Å | θ = 4.5–32.4º |
| c = 14.6970 (5) Å | µ = 0.10 mm−1 |
| α = 80.351 (3)º | T = 296 (2) K |
| β = 74.830 (3)º | Prism, pale yellow |
| γ = 84.416 (3)º | 0.41 × 0.35 × 0.28 mm |
| V = 1300.78 (9) Å3 |
Data collection
| Oxford Diffraction Gemini R CCD diffractometer | 8636 independent reflections |
| Radiation source: fine-focus sealed tube | 4667 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.027 |
| Detector resolution: 10.5081 pixels mm-1 | θmax = 32.5º |
| T = 296(2) K | θmin = 4.5º |
| φ and ω scans | h = −12→12 |
| Absorption correction: multi-scan(CrysAlis RED; Oxford Diffraction, 2007) | k = −15→17 |
| Tmin = 0.874, Tmax = 0.974 | l = −22→22 |
| 19776 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.047 | H-atom parameters constrained |
| wR(F2) = 0.127 | w = 1/[σ2(Fo2) + (0.0653P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.97 | (Δ/σ)max = 0.001 |
| 8636 reflections | Δρmax = 0.31 e Å−3 |
| 363 parameters | Δρmin = −0.23 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | −0.10825 (12) | 0.78753 (8) | 0.47805 (6) | 0.0472 (2) | |
| O2A | −0.17664 (12) | 0.86596 (9) | 0.15922 (6) | 0.0496 (2) | |
| O3A | −0.03310 (14) | 0.76460 (9) | 0.05116 (7) | 0.0600 (3) | |
| N1A | −0.07106 (13) | 0.78738 (9) | 0.13266 (7) | 0.0367 (2) | |
| C1A | 0.05600 (14) | 0.67882 (10) | 0.35935 (8) | 0.0291 (2) | |
| C2A | 0.17987 (15) | 0.59163 (10) | 0.33036 (8) | 0.0342 (3) | |
| H2A | 0.2367 | 0.5488 | 0.3737 | 0.041* | |
| C3A | 0.22047 (15) | 0.56733 (11) | 0.23722 (9) | 0.0388 (3) | |
| H3A | 0.3027 | 0.5077 | 0.2191 | 0.047* | |
| C4A | 0.13914 (15) | 0.63148 (11) | 0.17161 (8) | 0.0360 (3) | |
| H4A | 0.1660 | 0.6165 | 0.1090 | 0.043* | |
| C5A | 0.01681 (14) | 0.71848 (10) | 0.20163 (8) | 0.0294 (2) | |
| C6A | −0.02749 (14) | 0.74350 (10) | 0.29349 (8) | 0.0302 (2) | |
| H6A | −0.1114 | 0.8023 | 0.3114 | 0.036* | |
| C7A | 0.00284 (14) | 0.70867 (10) | 0.45916 (8) | 0.0320 (2) | |
| C8A | 0.08663 (15) | 0.64361 (10) | 0.53138 (8) | 0.0341 (3) | |
| H8A | 0.1754 | 0.5877 | 0.5140 | 0.041* | |
| C9A | 0.03681 (15) | 0.66381 (10) | 0.62182 (8) | 0.0341 (3) | |
| H9A | −0.0554 | 0.7183 | 0.6359 | 0.041* | |
| C10A | 0.10965 (14) | 0.61071 (10) | 0.70091 (8) | 0.0303 (2) | |
| C11A | 0.24015 (15) | 0.52191 (10) | 0.69233 (8) | 0.0329 (3) | |
| H11A | 0.2816 | 0.4930 | 0.6347 | 0.039* | |
| C12A | 0.30868 (15) | 0.47626 (11) | 0.76861 (8) | 0.0354 (3) | |
| H12A | 0.3952 | 0.4166 | 0.7616 | 0.043* | |
| C13A | 0.25029 (15) | 0.51804 (11) | 0.85585 (8) | 0.0362 (3) | |
| C14A | 0.11819 (16) | 0.60523 (12) | 0.86462 (8) | 0.0400 (3) | |
| H14A | 0.0763 | 0.6336 | 0.9225 | 0.048* | |
| C15A | 0.04786 (16) | 0.65058 (11) | 0.78910 (8) | 0.0387 (3) | |
| H15A | −0.0415 | 0.7082 | 0.7970 | 0.046* | |
| C16A | 0.32905 (19) | 0.47144 (14) | 0.93769 (9) | 0.0519 (4) | |
| H16A | 0.3731 | 0.5354 | 0.9569 | 0.078* | |
| H16B | 0.4208 | 0.4144 | 0.9182 | 0.078* | |
| H16C | 0.2436 | 0.4348 | 0.9903 | 0.078* | |
| O1B | 0.39815 (12) | 0.28759 (8) | 0.17553 (7) | 0.0511 (2) | |
| O2B | 0.38505 (13) | 0.39995 (8) | 0.47951 (7) | 0.0530 (3) | |
| O3B | 0.47675 (14) | 0.27633 (10) | 0.58517 (7) | 0.0658 (3) | |
| N1B | 0.45688 (13) | 0.30623 (10) | 0.50460 (7) | 0.0416 (3) | |
| C1B | 0.55580 (14) | 0.17912 (10) | 0.27830 (8) | 0.0313 (2) | |
| C2B | 0.65291 (15) | 0.07736 (11) | 0.30205 (9) | 0.0371 (3) | |
| H2B | 0.6974 | 0.0269 | 0.2572 | 0.045* | |
| C3B | 0.68412 (16) | 0.05034 (11) | 0.39189 (9) | 0.0405 (3) | |
| H3B | 0.7478 | −0.0185 | 0.4071 | 0.049* | |
| C4B | 0.62101 (15) | 0.12525 (11) | 0.45862 (9) | 0.0391 (3) | |
| H4B | 0.6426 | 0.1084 | 0.5187 | 0.047* | |
| C5B | 0.52520 (14) | 0.22563 (10) | 0.43411 (8) | 0.0325 (3) | |
| C6B | 0.48975 (14) | 0.25382 (10) | 0.34598 (8) | 0.0329 (3) | |
| H6B | 0.4229 | 0.3216 | 0.3321 | 0.039* | |
| C7B | 0.51234 (15) | 0.21124 (11) | 0.18375 (8) | 0.0359 (3) | |
| C8B | 0.60755 (16) | 0.15048 (11) | 0.10342 (8) | 0.0377 (3) | |
| H8B | 0.7049 | 0.1024 | 0.1084 | 0.045* | |
| C9B | 0.55435 (15) | 0.16429 (10) | 0.02373 (8) | 0.0349 (3) | |
| H9B | 0.4563 | 0.2134 | 0.0230 | 0.042* | |
| C10B | 0.63097 (14) | 0.11180 (10) | −0.06276 (8) | 0.0315 (2) | |
| C11B | 0.76586 (15) | 0.02570 (11) | −0.06979 (9) | 0.0371 (3) | |
| H11B | 0.8095 | −0.0019 | −0.0171 | 0.045* | |
| C12B | 0.83539 (15) | −0.01901 (11) | −0.15411 (8) | 0.0377 (3) | |
| H12B | 0.9257 | −0.0760 | −0.1574 | 0.045* | |
| C13B | 0.77214 (16) | 0.02004 (11) | −0.23457 (9) | 0.0368 (3) | |
| C14B | 0.63582 (16) | 0.10371 (11) | −0.22680 (8) | 0.0372 (3) | |
| H14B | 0.5906 | 0.1302 | −0.2791 | 0.045* | |
| C15B | 0.56619 (15) | 0.14839 (11) | −0.14243 (8) | 0.0366 (3) | |
| H15B | 0.4742 | 0.2040 | −0.1388 | 0.044* | |
| C16B | 0.84893 (18) | −0.02696 (13) | −0.32704 (9) | 0.0475 (3) | |
| H16D | 0.8871 | 0.0373 | −0.3767 | 0.071* | |
| H16E | 0.7641 | −0.0671 | −0.3429 | 0.071* | |
| H16F | 0.9446 | −0.0811 | −0.3205 | 0.071* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0582 (6) | 0.0466 (6) | 0.0373 (5) | 0.0196 (4) | −0.0185 (4) | −0.0101 (4) |
| O2A | 0.0500 (5) | 0.0565 (6) | 0.0455 (5) | 0.0211 (5) | −0.0237 (4) | −0.0121 (5) |
| O3A | 0.0856 (8) | 0.0647 (7) | 0.0368 (5) | 0.0213 (5) | −0.0313 (5) | −0.0167 (5) |
| N1A | 0.0390 (6) | 0.0390 (6) | 0.0347 (5) | 0.0027 (5) | −0.0154 (4) | −0.0061 (4) |
| C1A | 0.0325 (6) | 0.0252 (6) | 0.0309 (6) | −0.0012 (4) | −0.0124 (4) | −0.0013 (4) |
| C2A | 0.0374 (6) | 0.0291 (6) | 0.0359 (6) | 0.0043 (5) | −0.0131 (5) | −0.0012 (5) |
| C3A | 0.0396 (7) | 0.0336 (7) | 0.0410 (7) | 0.0071 (5) | −0.0083 (5) | −0.0067 (5) |
| C4A | 0.0418 (7) | 0.0341 (7) | 0.0326 (6) | 0.0000 (5) | −0.0107 (5) | −0.0058 (5) |
| C5A | 0.0300 (5) | 0.0293 (6) | 0.0299 (5) | −0.0001 (4) | −0.0107 (4) | −0.0028 (5) |
| C6A | 0.0315 (6) | 0.0261 (6) | 0.0343 (6) | 0.0018 (4) | −0.0122 (4) | −0.0041 (5) |
| C7A | 0.0340 (6) | 0.0300 (6) | 0.0324 (6) | −0.0003 (5) | −0.0103 (5) | −0.0033 (5) |
| C8A | 0.0353 (6) | 0.0340 (7) | 0.0335 (6) | 0.0038 (5) | −0.0130 (5) | −0.0029 (5) |
| C9A | 0.0390 (6) | 0.0296 (6) | 0.0362 (6) | 0.0043 (5) | −0.0150 (5) | −0.0059 (5) |
| C10A | 0.0311 (6) | 0.0324 (6) | 0.0288 (5) | −0.0020 (5) | −0.0095 (4) | −0.0056 (5) |
| C11A | 0.0385 (6) | 0.0328 (6) | 0.0293 (6) | −0.0004 (5) | −0.0105 (5) | −0.0077 (5) |
| C12A | 0.0370 (6) | 0.0366 (7) | 0.0331 (6) | 0.0037 (5) | −0.0108 (5) | −0.0063 (5) |
| C13A | 0.0380 (6) | 0.0414 (7) | 0.0311 (6) | −0.0010 (5) | −0.0136 (5) | −0.0038 (5) |
| C14A | 0.0452 (7) | 0.0480 (8) | 0.0290 (6) | 0.0035 (6) | −0.0105 (5) | −0.0130 (5) |
| C15A | 0.0419 (7) | 0.0389 (7) | 0.0383 (6) | 0.0082 (5) | −0.0147 (5) | −0.0130 (5) |
| C16A | 0.0619 (9) | 0.0624 (10) | 0.0353 (7) | 0.0088 (7) | −0.0240 (6) | −0.0064 (6) |
| O1B | 0.0595 (6) | 0.0492 (6) | 0.0496 (5) | 0.0225 (5) | −0.0259 (4) | −0.0164 (4) |
| O2B | 0.0731 (7) | 0.0400 (6) | 0.0460 (5) | 0.0174 (5) | −0.0179 (5) | −0.0135 (4) |
| O3B | 0.0876 (8) | 0.0755 (8) | 0.0382 (5) | 0.0277 (6) | −0.0280 (5) | −0.0186 (5) |
| N1B | 0.0448 (6) | 0.0441 (7) | 0.0365 (6) | 0.0055 (5) | −0.0113 (5) | −0.0105 (5) |
| C1B | 0.0278 (5) | 0.0314 (6) | 0.0338 (6) | 0.0008 (5) | −0.0060 (4) | −0.0062 (5) |
| C2B | 0.0344 (6) | 0.0346 (7) | 0.0401 (6) | 0.0040 (5) | −0.0053 (5) | −0.0092 (5) |
| C3B | 0.0380 (7) | 0.0359 (7) | 0.0436 (7) | 0.0085 (5) | −0.0098 (5) | −0.0015 (5) |
| C4B | 0.0391 (7) | 0.0395 (7) | 0.0371 (6) | 0.0024 (5) | −0.0122 (5) | 0.0006 (5) |
| C5B | 0.0311 (6) | 0.0334 (6) | 0.0319 (6) | 0.0005 (5) | −0.0062 (5) | −0.0059 (5) |
| C6B | 0.0318 (6) | 0.0294 (6) | 0.0381 (6) | 0.0015 (5) | −0.0114 (5) | −0.0043 (5) |
| C7B | 0.0368 (6) | 0.0331 (7) | 0.0396 (7) | 0.0023 (5) | −0.0125 (5) | −0.0086 (5) |
| C8B | 0.0389 (6) | 0.0377 (7) | 0.0381 (6) | 0.0051 (5) | −0.0130 (5) | −0.0082 (5) |
| C9B | 0.0348 (6) | 0.0308 (6) | 0.0376 (6) | 0.0000 (5) | −0.0090 (5) | −0.0018 (5) |
| C10B | 0.0332 (6) | 0.0298 (6) | 0.0315 (6) | −0.0042 (5) | −0.0096 (4) | −0.0009 (5) |
| C11B | 0.0422 (7) | 0.0348 (7) | 0.0360 (6) | −0.0011 (5) | −0.0172 (5) | 0.0010 (5) |
| C12B | 0.0351 (6) | 0.0354 (7) | 0.0408 (7) | 0.0018 (5) | −0.0087 (5) | −0.0042 (5) |
| C13B | 0.0403 (7) | 0.0345 (7) | 0.0368 (6) | −0.0098 (5) | −0.0097 (5) | −0.0035 (5) |
| C14B | 0.0399 (7) | 0.0398 (7) | 0.0344 (6) | −0.0048 (5) | −0.0156 (5) | −0.0010 (5) |
| C15B | 0.0369 (6) | 0.0327 (7) | 0.0424 (7) | 0.0003 (5) | −0.0164 (5) | −0.0029 (5) |
| C16B | 0.0509 (8) | 0.0494 (8) | 0.0419 (7) | −0.0040 (6) | −0.0095 (6) | −0.0084 (6) |
Geometric parameters (Å, °)
| O1A—C7A | 1.2266 (14) | O2B—N1B | 1.2224 (14) |
| O2A—N1A | 1.2222 (13) | O3B—N1B | 1.2242 (14) |
| O3A—N1A | 1.2227 (13) | N1B—O2B | 1.2224 (14) |
| N1A—C5A | 1.4698 (15) | N1B—C5B | 1.4704 (15) |
| C1A—C2A | 1.3849 (16) | C1B—C6B | 1.3904 (16) |
| C1A—C6A | 1.3967 (16) | C1B—C2B | 1.3924 (17) |
| C1A—C7A | 1.5050 (15) | C1B—C7B | 1.5000 (17) |
| C2A—C3A | 1.3910 (16) | C2B—C3B | 1.3876 (17) |
| C2A—H2A | 0.93 | C2B—H2B | 0.93 |
| C3A—C4A | 1.3811 (17) | C3B—C4B | 1.3790 (18) |
| C3A—H3A | 0.93 | C3B—H3B | 0.93 |
| C4A—C5A | 1.3800 (17) | C4B—C5B | 1.3778 (17) |
| C4A—H4A | 0.93 | C4B—H4B | 0.93 |
| C5A—C6A | 1.3751 (15) | C5B—C6B | 1.3788 (16) |
| C6A—H6A | 0.93 | C6B—H6B | 0.93 |
| C7A—C8A | 1.4692 (16) | C7B—C8B | 1.4739 (16) |
| C8A—C9A | 1.3372 (16) | C8B—C9B | 1.3307 (16) |
| C8A—H8A | 0.93 | C8B—H8B | 0.93 |
| C9A—C10A | 1.4571 (16) | C9B—C10B | 1.4620 (16) |
| C9A—H9A | 0.93 | C9B—H9B | 0.93 |
| C10A—C11A | 1.3928 (16) | C10B—C15B | 1.3916 (16) |
| C10A—C15A | 1.3985 (15) | C10B—C11B | 1.3963 (17) |
| C11A—C12A | 1.3827 (16) | C11B—C12B | 1.3819 (17) |
| C11A—H11A | 0.93 | C11B—H11B | 0.93 |
| C12A—C13A | 1.3943 (16) | C12B—C13B | 1.3988 (17) |
| C12A—H12A | 0.93 | C12B—H12B | 0.93 |
| C13A—C14A | 1.3890 (18) | C13B—C14B | 1.3865 (18) |
| C13A—C16A | 1.5031 (17) | C13B—C16B | 1.5050 (17) |
| C14A—C15A | 1.3810 (17) | C14B—C15B | 1.3825 (17) |
| C14A—H14A | 0.93 | C14B—H14B | 0.93 |
| C15A—H15A | 0.93 | C15B—H15B | 0.93 |
| C16A—H16A | 0.96 | C16B—H16D | 0.96 |
| C16A—H16B | 0.96 | C16B—H16E | 0.96 |
| C16A—H16C | 0.96 | C16B—H16F | 0.96 |
| O1B—C7B | 1.2272 (15) | ||
| O2A—N1A—O3A | 123.18 (11) | O2B—N1B—O3B | 123.47 (11) |
| O2A—N1A—C5A | 118.36 (9) | O2B—N1B—O3B | 123.47 (11) |
| O3A—N1A—C5A | 118.45 (10) | O2B—N1B—C5B | 118.42 (10) |
| C2A—C1A—C6A | 119.17 (10) | O2B—N1B—C5B | 118.42 (10) |
| C2A—C1A—C7A | 123.95 (10) | O3B—N1B—C5B | 118.11 (11) |
| C6A—C1A—C7A | 116.88 (10) | C6B—C1B—C2B | 119.13 (11) |
| C1A—C2A—C3A | 120.87 (11) | C6B—C1B—C7B | 117.43 (11) |
| C1A—C2A—H2A | 119.6 | C2B—C1B—C7B | 123.40 (10) |
| C3A—C2A—H2A | 119.6 | C3B—C2B—C1B | 120.77 (11) |
| C4A—C3A—C2A | 120.24 (12) | C3B—C2B—H2B | 119.6 |
| C4A—C3A—H3A | 119.9 | C1B—C2B—H2B | 119.6 |
| C2A—C3A—H3A | 119.9 | C4B—C3B—C2B | 120.20 (12) |
| C5A—C4A—C3A | 118.08 (11) | C4B—C3B—H3B | 119.9 |
| C5A—C4A—H4A | 121.0 | C2B—C3B—H3B | 119.9 |
| C3A—C4A—H4A | 121.0 | C5B—C4B—C3B | 118.39 (12) |
| C6A—C5A—C4A | 122.98 (11) | C5B—C4B—H4B | 120.8 |
| C6A—C5A—N1A | 118.30 (10) | C3B—C4B—H4B | 120.8 |
| C4A—C5A—N1A | 118.72 (10) | C4B—C5B—C6B | 122.72 (11) |
| C5A—C6A—C1A | 118.66 (11) | C4B—C5B—N1B | 118.99 (11) |
| C5A—C6A—H6A | 120.7 | C6B—C5B—N1B | 118.29 (11) |
| C1A—C6A—H6A | 120.7 | C5B—C6B—C1B | 118.78 (11) |
| O1A—C7A—C8A | 121.56 (10) | C5B—C6B—H6B | 120.6 |
| O1A—C7A—C1A | 119.20 (10) | C1B—C6B—H6B | 120.6 |
| C8A—C7A—C1A | 119.24 (10) | O1B—C7B—C8B | 121.84 (11) |
| C9A—C8A—C7A | 120.81 (11) | O1B—C7B—C1B | 118.90 (11) |
| C9A—C8A—H8A | 119.6 | C8B—C7B—C1B | 119.26 (11) |
| C7A—C8A—H8A | 119.6 | C9B—C8B—C7B | 120.09 (12) |
| C8A—C9A—C10A | 127.80 (11) | C9B—C8B—H8B | 120.0 |
| C8A—C9A—H9A | 116.1 | C7B—C8B—H8B | 120.0 |
| C10A—C9A—H9A | 116.1 | C8B—C9B—C10B | 128.03 (12) |
| C11A—C10A—C15A | 118.21 (10) | C8B—C9B—H9B | 116.0 |
| C11A—C10A—C9A | 122.66 (10) | C10B—C9B—H9B | 116.0 |
| C15A—C10A—C9A | 119.13 (11) | C15B—C10B—C11B | 117.82 (11) |
| C12A—C11A—C10A | 120.69 (10) | C15B—C10B—C9B | 118.74 (11) |
| C12A—C11A—H11A | 119.7 | C11B—C10B—C9B | 123.44 (10) |
| C10A—C11A—H11A | 119.7 | C12B—C11B—C10B | 120.84 (11) |
| C11A—C12A—C13A | 121.17 (12) | C12B—C11B—H11B | 119.6 |
| C11A—C12A—H12A | 119.4 | C10B—C11B—H11B | 119.6 |
| C13A—C12A—H12A | 119.4 | C11B—C12B—C13B | 121.04 (12) |
| C14A—C13A—C12A | 117.97 (11) | C11B—C12B—H12B | 119.5 |
| C14A—C13A—C16A | 120.87 (11) | C13B—C12B—H12B | 119.5 |
| C12A—C13A—C16A | 121.16 (12) | C14B—C13B—C12B | 118.00 (11) |
| C15A—C14A—C13A | 121.26 (11) | C14B—C13B—C16B | 120.67 (11) |
| C15A—C14A—H14A | 119.4 | C12B—C13B—C16B | 121.33 (12) |
| C13A—C14A—H14A | 119.4 | C15B—C14B—C13B | 120.95 (11) |
| C14A—C15A—C10A | 120.66 (12) | C15B—C14B—H14B | 119.5 |
| C14A—C15A—H15A | 119.7 | C13B—C14B—H14B | 119.5 |
| C10A—C15A—H15A | 119.7 | C14B—C15B—C10B | 121.32 (12) |
| C13A—C16A—H16A | 109.5 | C14B—C15B—H15B | 119.3 |
| C13A—C16A—H16B | 109.5 | C10B—C15B—H15B | 119.3 |
| H16A—C16A—H16B | 109.5 | C13B—C16B—H16D | 109.5 |
| C13A—C16A—H16C | 109.5 | C13B—C16B—H16E | 109.5 |
| H16A—C16A—H16C | 109.5 | H16D—C16B—H16E | 109.5 |
| H16B—C16A—H16C | 109.5 | C13B—C16B—H16F | 109.5 |
| O2B—O2B—N1B | 0(10) | H16D—C16B—H16F | 109.5 |
| O2B—N1B—O2B | 0.00 (11) | H16E—C16B—H16F | 109.5 |
| C6A—C1A—C2A—C3A | −0.59 (16) | C6B—C1B—C2B—C3B | −0.10 (16) |
| C7A—C1A—C2A—C3A | 178.84 (10) | C7B—C1B—C2B—C3B | −177.82 (10) |
| C1A—C2A—C3A—C4A | 1.01 (17) | C1B—C2B—C3B—C4B | −0.93 (17) |
| C2A—C3A—C4A—C5A | −0.60 (17) | C2B—C3B—C4B—C5B | 0.89 (17) |
| C3A—C4A—C5A—C6A | −0.22 (17) | C3B—C4B—C5B—C6B | 0.17 (17) |
| C3A—C4A—C5A—N1A | −179.90 (10) | C3B—C4B—C5B—N1B | 179.91 (10) |
| O2A—N1A—C5A—C6A | 1.82 (15) | O2B—N1B—C5B—C4B | 173.67 (11) |
| O3A—N1A—C5A—C6A | −179.32 (10) | O2B—N1B—C5B—C4B | 173.67 (11) |
| O2A—N1A—C5A—C4A | −178.48 (10) | O3B—N1B—C5B—C4B | −5.53 (16) |
| O3A—N1A—C5A—C4A | 0.38 (15) | O2B—N1B—C5B—C6B | −6.57 (16) |
| C4A—C5A—C6A—C1A | 0.62 (16) | O2B—N1B—C5B—C6B | −6.57 (16) |
| N1A—C5A—C6A—C1A | −179.69 (9) | O3B—N1B—C5B—C6B | 174.22 (11) |
| C2A—C1A—C6A—C5A | −0.20 (15) | C4B—C5B—C6B—C1B | −1.18 (17) |
| C7A—C1A—C6A—C5A | −179.68 (9) | N1B—C5B—C6B—C1B | 179.07 (9) |
| C2A—C1A—C7A—O1A | 179.98 (11) | C2B—C1B—C6B—C5B | 1.12 (15) |
| C6A—C1A—C7A—O1A | −0.57 (15) | C7B—C1B—C6B—C5B | 178.98 (10) |
| C2A—C1A—C7A—C8A | 0.68 (16) | C6B—C1B—C7B—O1B | −13.01 (16) |
| C6A—C1A—C7A—C8A | −179.87 (9) | C2B—C1B—C7B—O1B | 164.75 (11) |
| O1A—C7A—C8A—C9A | 4.01 (17) | C6B—C1B—C7B—C8B | 167.00 (10) |
| C1A—C7A—C8A—C9A | −176.71 (10) | C2B—C1B—C7B—C8B | −15.23 (16) |
| C7A—C8A—C9A—C10A | −177.55 (10) | O1B—C7B—C8B—C9B | −10.23 (18) |
| C8A—C9A—C10A—C11A | −4.54 (18) | C1B—C7B—C8B—C9B | 169.75 (10) |
| C8A—C9A—C10A—C15A | 174.93 (11) | C7B—C8B—C9B—C10B | −179.97 (10) |
| C15A—C10A—C11A—C12A | −1.23 (16) | C8B—C9B—C10B—C15B | −172.74 (11) |
| C9A—C10A—C11A—C12A | 178.24 (10) | C8B—C9B—C10B—C11B | 7.71 (18) |
| C10A—C11A—C12A—C13A | −0.41 (17) | C15B—C10B—C11B—C12B | 1.75 (16) |
| C11A—C12A—C13A—C14A | 1.45 (17) | C9B—C10B—C11B—C12B | −178.70 (10) |
| C11A—C12A—C13A—C16A | −177.81 (11) | C10B—C11B—C12B—C13B | −0.39 (17) |
| C12A—C13A—C14A—C15A | −0.83 (18) | C11B—C12B—C13B—C14B | −0.96 (16) |
| C16A—C13A—C14A—C15A | 178.43 (12) | C11B—C12B—C13B—C16B | 179.09 (10) |
| C13A—C14A—C15A—C10A | −0.82 (19) | C12B—C13B—C14B—C15B | 0.92 (17) |
| C11A—C10A—C15A—C14A | 1.84 (17) | C16B—C13B—C14B—C15B | −179.13 (10) |
| C9A—C10A—C15A—C14A | −177.65 (11) | C13B—C14B—C15B—C10B | 0.48 (17) |
| O2B—O2B—N1B—O3B | 0.00 (5) | C11B—C10B—C15B—C14B | −1.80 (16) |
| O2B—O2B—N1B—C5B | 0.00 (8) | C9B—C10B—C15B—C14B | 178.63 (10) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2A—H2A···O2B | 0.93 | 2.55 | 3.4644 (16) | 170 |
| C11A—H11A···O2B | 0.93 | 2.59 | 3.5156 (14) | 176 |
| C2B—H2B···O2Ai | 0.93 | 2.51 | 3.4311 (16) | 171 |
| C14A—H14A···O3Aii | 0.93 | 2.54 | 3.4455 (16) | 164 |
Symmetry codes: (i) x+1, y−1, z; (ii) x, y, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI2518).
References
- Bruker (2000). SHELXTL Version 6.10. Bruker AXS Inc., Madison, Wisconsin, USA.
- Butcher, R. J., Jasinski, J. P., Narayana, B., Lakshmana, K. & Yathirajan, H. S. (2007). Acta Cryst. E63, o3660. [DOI] [PMC free article] [PubMed]
- Butcher, R. J., Jasinski, J. P., Yathirajan, H. S., Narayana, B. & Veena, K. (2007). Acta Cryst. E63, o3833.
- Butcher, R. J., Jasinski, J. P., Yathirajan, H. S., Veena, K. & Narayana, B. (2007). Acta Cryst. E63, o3680.
- Dimmock, J. R., Elias, D. W., Beazely, M. A. & Kandepu, N. M. (1999). Curr. Med. Chem.6, 1125–1149. [PubMed]
- Go, M. L., Wu, X. & Liu, X. L. (2005). Curr. Med. Chem.12, 483–499.
- Goto, Y., Hayashi, A., Kimura, Y. & Nakayama, M. (1991). J. Cryst. Growth, 108, 688–698.
- Oxford Diffraction (2007). CrysAlisPro (Version 171.31.8) and CrysAlis RED (Version 1.171.31.8). Oxford Diffraction Ltd, Abingdon, Oxfordshire, England.
- Patil, P. S., Rosli, M. M., Fun, H.-K., Razak, I. A. & Dharmaprakash, S. M. (2007). Acta Cryst. E63, o785–o786.
- Rosli, M. M., Patil, P. S., Fun, H.-K., Razak, I. A. & Dharmaprakash, S. M. (2007). Acta Cryst. E63, o2692.
- Sheldrick, G. M. (1997). SHELXS97 and SHELXL97 University of Göttingen, Germany.
- Tam, W., Gurein, B., Calabrese, J. C. & Stevenson, S. H. (1989). Chem. Phys. Lett.154, 93–96.
- Uchida, J., Kozawa, K., Sakai, T., Aoki, M., Yoguchi, H., Abdeuyim, A. & Wadanabe, Y. (1998). Mol. Cryst. Liq. Cryst.315, 135–140.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680706182X/ci2518sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680706182X/ci2518Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




