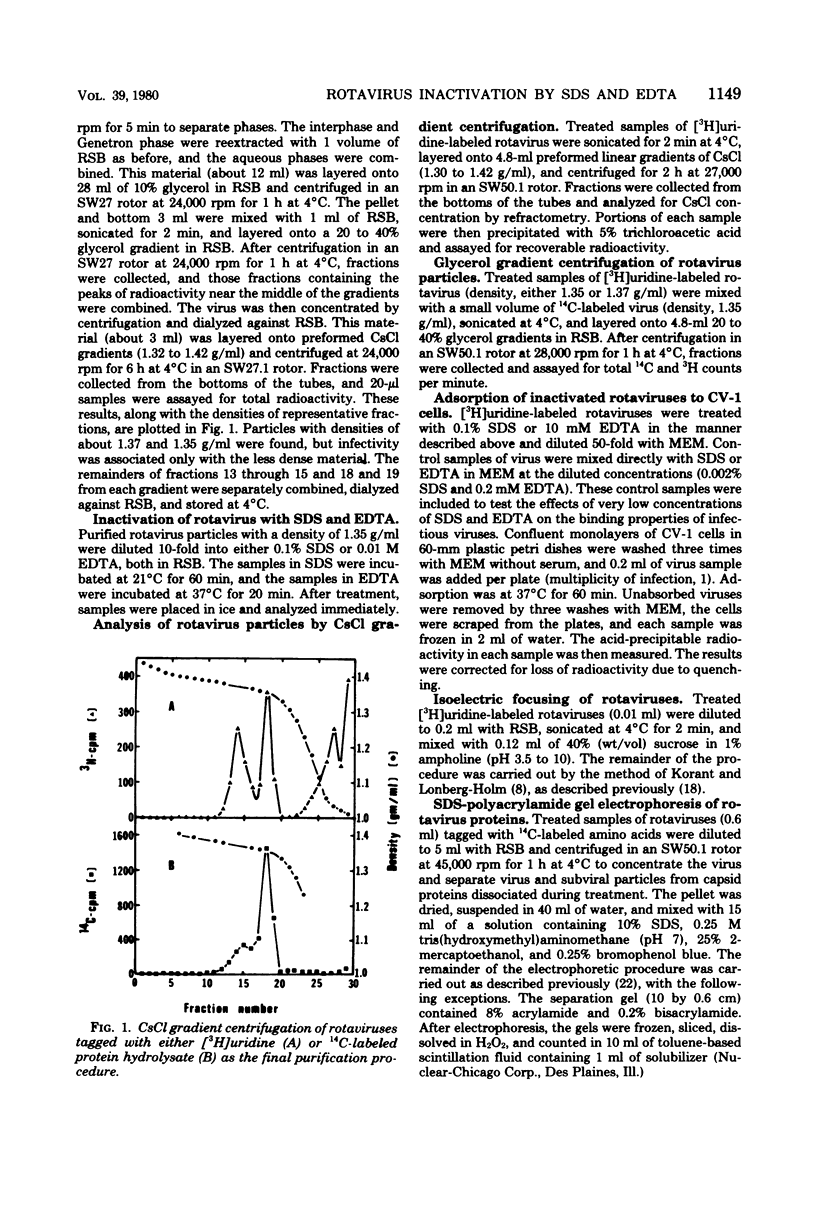
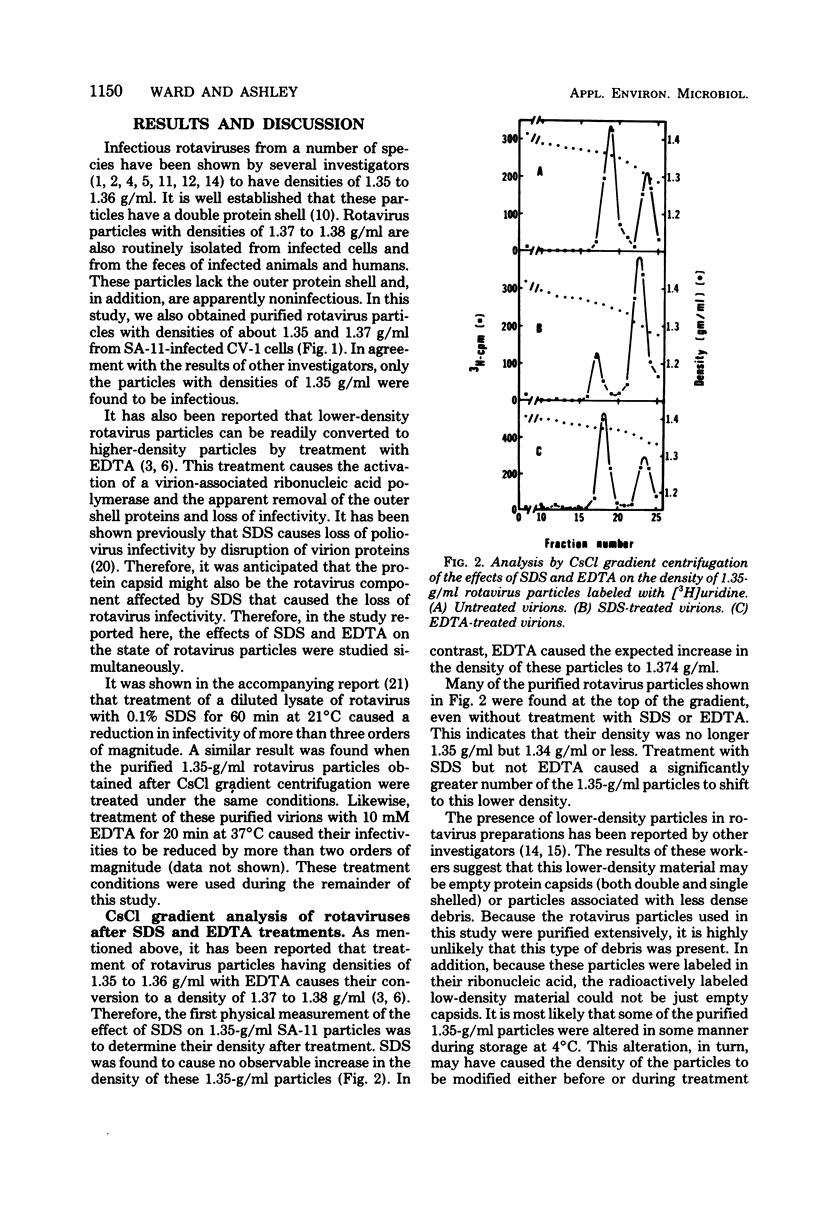
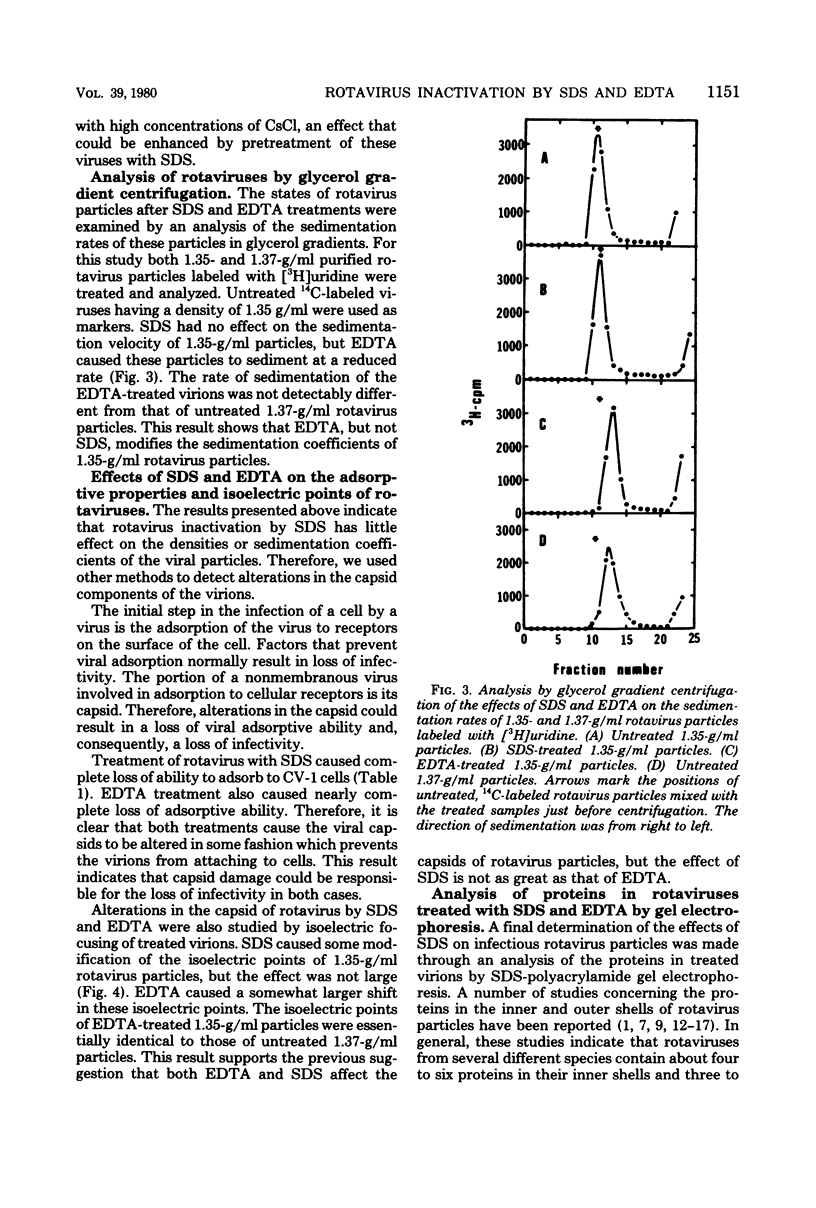
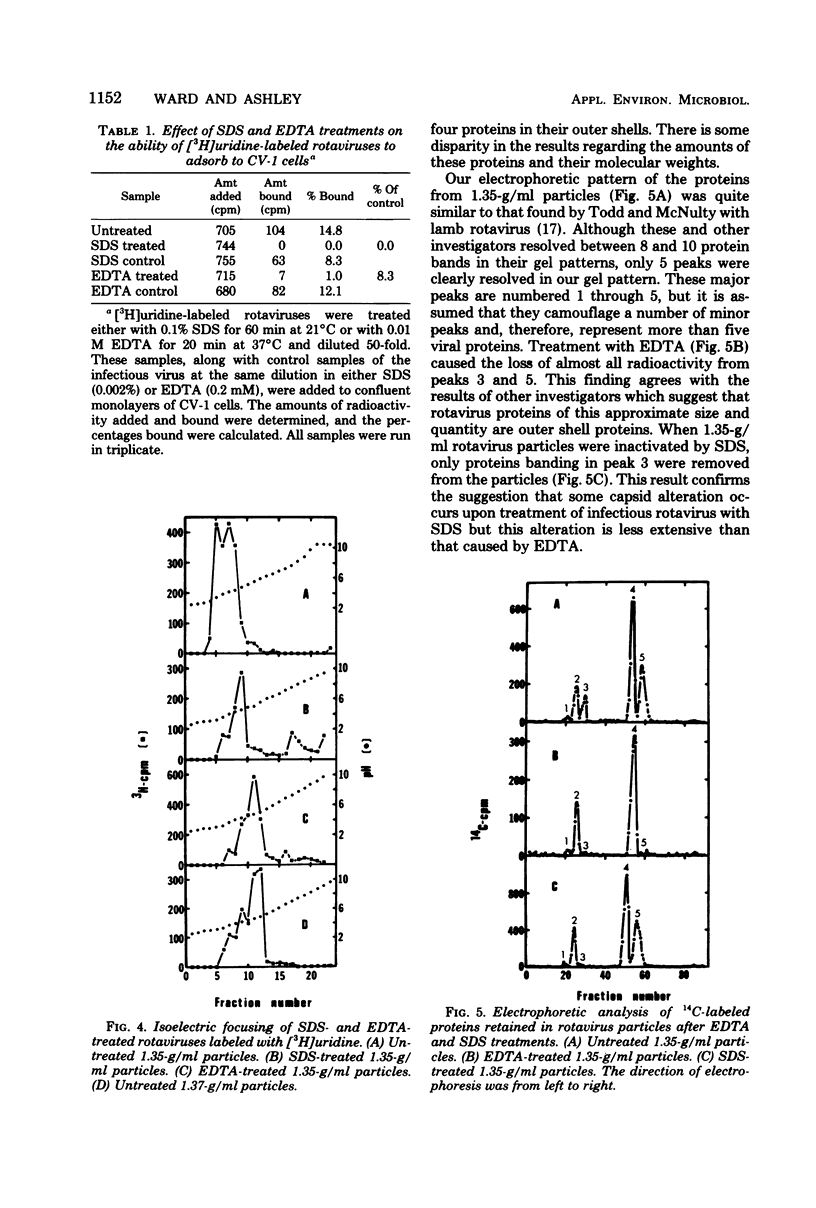
Abstract
This report describes a comparative study on the effects of the anionic detergent sodium dodecyl sulfate and the chelating agent ethylenediaminetetraacetate on purified rotavirus SA-11 particles. Both chemicals readily inactivated rotavirus at quite low concentrations and under very mild conditions. In addition, both agents modified the viral capsid and prevented the adsorption of inactivated virions to cells. Capsid damage by ethylenediaminetetraacetate caused a shift in the densities of rotavirions from about 1.35 to about 1.37 g/ml and a reduction in their sedimentation coefficients. Sodium dodecyl sulfate, on the other hand, did not detectably alter either of these physical properties of rotavirions. Both agents caused some alteration of the isoelectric points of the virions. Finally, analysis of rotavirus proteins showed that ethylenediaminetetraacetate caused the loss of two protein peaks from the electrophoretic pattern of virions but sodium dodecyl sulfate caused the loss of only one of these same protein peaks.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridger J. C., Woode G. N. Characterization of two particle types of calf rotavirus. J Gen Virol. 1976 May;31(2):245–250. doi: 10.1099/0022-1317-31-2-245. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Elias M. M. Separation and infectivity of two particle types of human rotavirus. J Gen Virol. 1977 Oct;37(1):191–194. doi: 10.1099/0022-1317-37-1-191. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Notter M. F., Menegus M. A., Steinhoff M. C. RNA polymerase associated with human rotaviruses in diarrhea stools. J Virol. 1978 May;26(2):544–546. doi: 10.1128/jvi.26.2.544-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantile enteritis viruses: morphogenesis and morphology. J Virol. 1975 Oct;16(4):937–943. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Theodore T. S. Polypeptides of simian rotavirus (SA-11) determined by a continuous polyacrylamide gel electrophoresis method. J Gen Virol. 1979 May;43(2):463–466. doi: 10.1099/0022-1317-43-2-463. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K. Zonal electrophoresis and isoelectric focusing of proteins and virus particles in density gradients of small volume. Anal Biochem. 1974 May;59(1):75–82. doi: 10.1016/0003-2697(74)90011-6. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- McNulty M. S., Curran W. L., McFerran J. B. The morphogenesis of a cytopathic bovine rotavirus in Madin-Darby bovine kidney cells. J Gen Virol. 1976 Dec;33(3):503–508. doi: 10.1099/0022-1317-33-3-503. [DOI] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Palmer E. L., Martin M. L. Biochemical characterization of infantile gastroenteritis virus (IGV). J Gen Virol. 1977 Mar;34(3):485–497. doi: 10.1099/0022-1317-34-3-485. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Biochemical studies on a reovirus-like agent (rotovirus) from lambs. J Virol. 1977 Mar;21(3):1215–1218. doi: 10.1128/jvi.21.3.1215-1218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Effects of wastewater sludge and its detergents on the stability of rotavirus. Appl Environ Microbiol. 1980 Jun;39(6):1154–1158. doi: 10.1128/aem.39.6.1154-1158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Identification of detergents as components of wastewater sludge that modify the thermal stability of reovirus and enteroviruses. Appl Environ Microbiol. 1978 Dec;36(6):889–897. doi: 10.1128/aem.36.6.889-897.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. pH modification of the effects of detergents on the stability of enteric viruses. Appl Environ Microbiol. 1979 Aug;38(2):314–322. doi: 10.1128/aem.38.2.314-322.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L. Mechanism of poliovirus inactivation by ammonia. J Virol. 1978 May;26(2):299–305. doi: 10.1128/jvi.26.2.299-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Stevens J. G. Effect of cytosine arabinoside on viral-specific protein synthesis in cells infected with herpes simplex virus. J Virol. 1975 Jan;15(1):71–80. doi: 10.1128/jvi.15.1.71-80.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



