Abstract
The title compound, C15H11N3OS2, was synthesized from benzoyl thiocyanate and 2-aminobenzothiazole in dry acetone. The thiourea group is in the thioamide form. The molecules are stabilized by two intermolecular C—H⋯S and C—H⋯O hydrogen bonds. Intramolecular N—H⋯O hydrogen bonding results in a pseudo-S(6) planar ring with dihedral angles of 11.23 and 11.91° with the benzothiazole ring system and the phenyl ring, respectively.
Related literature
For related literature see: Büyükgüngör et al. (2004 ▶); del Campo et al. (2002 ▶); Chen et al. (2003 ▶); D’hooghe et al. (2005 ▶); Koketsu & Ishihara (2006 ▶); Morales et al. (2000 ▶); Rodríguez-Fernández et al. (2005 ▶); Yamin & Hassan (2004 ▶); Yunus et al. (2007 ▶); Zeng et al. (2003 ▶).
Experimental
Crystal data
C15H11N3OS2
M r = 313.39
Monoclinic,

a = 12.4402 (8) Å
b = 5.8608 (4) Å
c = 19.7240 (13) Å
β = 90.223 (1)°
V = 1438.06 (16) Å3
Z = 4
Mo Kα radiation
μ = 0.37 mm−1
T = 293 (2) K
0.32 × 0.26 × 0.20 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 1999 ▶) T min = 0.933, T max = 1.000 (expected range = 0.866–0.928)
8487 measured reflections
3508 independent reflections
2724 reflections with I > 2σ(I)
R int = 0.019
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.102
S = 1.04
3508 reflections
190 parameters
H-atom parameters constrained
Δρmax = 0.31 e Å−3
Δρmin = −0.26 e Å−3
Data collection: SMART (Bruker, 1999 ▶); cell refinement: SAINT (Bruker, 1999 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: SHELXTL (Bruker, 1999 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053680706134X/bh2150sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680706134X/bh2150Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2B⋯O1 | 0.86 | 1.86 | 2.5878 (17) | 142 |
| C1—H1A⋯S2i | 0.93 | 2.84 | 3.4017 (17) | 120 |
| C5—H5A⋯O1ii | 0.93 | 2.57 | 3.470 (2) | 162 |
Symmetry codes: (i)  ; (ii)
; (ii) 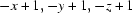 .
.
Acknowledgments
The authors gratefully acknowledge Allama Iqbal Open University, Islamabad, Pakistan, for providing research facilities.
supplementary crystallographic information
Comment
Thiourea and its derivatives have found extensive applications in the fields of medicine, agriculture and analytical chemistry. They are known to exhibit a wide variety of biological activities such as antiviral, antibacterial, antifungal, antitubercular, herbicidal and insecticidal (Koketsu & Ishihara, 2006). Thioureas are also widely used as precursors or intermediates towards the syntheisis of a variety of heterocyclic compounds (Zeng et al.2003; D'hooghe, et al. 2005). Among thiourea derivatives acylthiourea with potential donor atoms (O and S) have been found to display remarkably rich coordination chemistry. Such coordination compounds of thiourea have been studied for different biological systems (Rodríguez-Fernández et al. 2005). In recent years some attention has also been paid for potential use of acylthioureas as highly selective reagents for the enrichment and separation of metal cations (del Campo et al. 2002). The condensation of acyl / aroyl thiocyanates with primary amine affords 1,3-disubstituted thioureas in excellent yield in a single step. However, our attempt to synthesize thiourea derivative by treating benzoyl thiocyanate with 2-aminothiazole resulted in a fused 1,3,5-triazine instead of the expected thiourea product (Yunus et al. 2007). This observation prompted us to explore the outcome of the reaction with 2-aminothiazole derivatives such as 2-aminobenzothiazole. The reaction of the benzoyl thiocyanate with 2-aminobenzothiazole yielded the title compound (I) which is reported here.
The title compound crystallizes in the thioamide form. The conformation of the molecule with respect to the carbonyl and thiocarbonyl part is nearly planar as reflected by the torsion angles O1—C7—N1—C8, C7—N1—C8—S2 and C7—N1—C8—N2 of -0.42(°), -179.58(°) and -0.06(°) respectively. The benzoyl and benzothiazole groups are trans and cis, respectively, to the S atom across the thiourea C—N bonds (Figure 1 and Table 1). The C8—S2 and C7—O1 bonds show a typical double bond character with bond lengths of 1.6578 (15) and 1.2179 (19) Å respectively, closely related to other thiourea derivatives (Yamin & Hassan, 2004). All of the C—N bonds of thiourea fragment C7—N1, C8—N1, C8—N2 and C9—N2 are in the range 1.3933 (19) - 1.338 (2) Å, intermediate between those expected for single and double C—N bonds (1.47 and 1.27 Å respectively). Among these C—N bonds the C7—N1 is the longest indicating Csp2-Nsp2 single bond while C8—N2 is the shortest bond with more double bond character. This further demonstrate that there is π conjugation only along S2—C8—N2 system but not along O1—C7—N1 and C7—N1—C8 as found in 1-(3-methoxybenzoyl)-3,3-diethylthiourea (Morales et al., 2000). The bond lengths in the benzothizole ring system are normal and agree with the corresponding values found in 2-(benzothiazole-2-yliminomethyl)-6-methoxyphenol and N,N-(2-benzothia-zole)(2-pyridylmethyl)amine (Büyükgüngör et al. 2004; Chen et al. 2003). The bond length C9—N2 is very close to the value determined for the 2-(benzothiazole-2-yliminomethyl)-6-methoxyphenol suggesting the existence of a delocalized double bond in the benzothiazole moiety (Büyükgüngör et al. 2004). All bond lengths and angles confirm the sp2 hybridization for all C and N atoms of the benzothiazole ring with all C—C and C—N bond lengths intermediate between single and double bonds. The benzothiazole ring bonded to N2 is essentially planar and inclined at an angle -11.23(°) with respect to the plane of thiourea moiety. Similarly the phenyl ring bonded to C7 is at an angle of 11.91(°) with respect to the plane formed by the thiourea moiety.
The molecule is further stabilized by intermolecular and intramolecular hydrogen bonding. There are two types of intermolecular C1—H1A···S2i and C5—H5A···O1ii [Symmetry codes: (i) -x, -y + 1, -z + 1; (ii) -x + 1, -y + 1, -z + 1] hydrogen bonds. The intramolecular N2—H2B···O1 hydrogen bond is also observed. As a result, a pseudo six membered (O1—C7—N1—C8—N2···H2B) ring is formed.
Experimental
A mixture of ammonium thiocyanate (26 mmol) and benzoyl chloride (26 mmol) in dry acetone (60 ml) was stirred for 30 min. Then 2-aminobenzothiazole (26 mmol) was added and the reaction mixture was refluxed for 2 h. After cooling, the reaction mixture was poured in an acidified cold water. The resulting yellow solid was filtered and washed with cold acetone. The title compound (I) was obtained as suitable single crystals for X-Ray analysis after recrystallization of the solid from an 1:1 ethanol-dichloromethane mixture.
Refinement
H atoms were placed in idealized positions and refined using a riding model approximation. Bond lengths were fixed to 0.86 (amine NH) or 0.93 Å (aromatic CH). Isotropic displacement parameters were fixed to Uiso(H) = 1.2Ueq(carrier atom).
Figures
Fig. 1.
The molecular structure of (I) with atomic numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C15H11N3OS2 | F000 = 648 |
| Mr = 313.39 | Dx = 1.447 Mg m−3 |
| Monoclinic, P2/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2yc | Cell parameters from 8487 reflections |
| a = 12.4402 (8) Å | θ = 2.6–28.3º |
| b = 5.8608 (4) Å | µ = 0.37 mm−1 |
| c = 19.7240 (13) Å | T = 293 (2) K |
| β = 90.223 (1)º | Block, pale-yellow |
| V = 1438.06 (16) Å3 | 0.32 × 0.26 × 0.20 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD diffractometer | 3508 independent reflections |
| Radiation source: fine-focus sealed tube | 2724 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.019 |
| T = 293(2) K | θmax = 28.3º |
| φ and ω scans | θmin = 2.6º |
| Absorption correction: multi-scan(SADABS; Bruker, 1999) | h = −16→16 |
| Tmin = 0.933, Tmax = 1.000 | k = −7→7 |
| 8487 measured reflections | l = −19→26 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.035 | H-atom parameters constrained |
| wR(F2) = 0.102 | w = 1/[σ2(Fo2) + (0.0541P)2 + 0.2538P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 3508 reflections | Δρmax = 0.31 e Å−3 |
| 190 parameters | Δρmin = −0.26 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.21573 (13) | 0.0594 (3) | 0.53895 (8) | 0.0416 (4) | |
| H1A | 0.1480 | 0.0792 | 0.5197 | 0.050* | |
| C2 | 0.23408 (16) | −0.1206 (3) | 0.58247 (9) | 0.0515 (4) | |
| H2A | 0.1785 | −0.2203 | 0.5932 | 0.062* | |
| C3 | 0.33485 (18) | −0.1525 (4) | 0.61000 (10) | 0.0602 (5) | |
| H3A | 0.3473 | −0.2749 | 0.6390 | 0.072* | |
| C4 | 0.41698 (17) | −0.0051 (4) | 0.59497 (11) | 0.0646 (5) | |
| H4A | 0.4848 | −0.0280 | 0.6137 | 0.078* | |
| C5 | 0.39901 (14) | 0.1779 (3) | 0.55189 (9) | 0.0529 (5) | |
| H5A | 0.4547 | 0.2781 | 0.5419 | 0.063* | |
| C6 | 0.29787 (12) | 0.2116 (3) | 0.52370 (8) | 0.0378 (3) | |
| C7 | 0.28504 (12) | 0.4117 (3) | 0.47818 (8) | 0.0380 (3) | |
| C8 | 0.14589 (12) | 0.6519 (3) | 0.42051 (8) | 0.0372 (3) | |
| C9 | 0.22056 (12) | 0.9776 (3) | 0.35659 (7) | 0.0354 (3) | |
| C10 | 0.29090 (13) | 1.2734 (3) | 0.30503 (8) | 0.0388 (3) | |
| C11 | 0.36820 (14) | 1.4337 (3) | 0.28664 (9) | 0.0472 (4) | |
| H11A | 0.4382 | 1.4225 | 0.3030 | 0.057* | |
| C12 | 0.33920 (16) | 1.6082 (3) | 0.24397 (9) | 0.0517 (4) | |
| H12A | 0.3902 | 1.7159 | 0.2313 | 0.062* | |
| C13 | 0.23408 (17) | 1.6266 (3) | 0.21919 (9) | 0.0551 (5) | |
| H13A | 0.2164 | 1.7450 | 0.1898 | 0.066* | |
| C14 | 0.15669 (15) | 1.4723 (3) | 0.23769 (9) | 0.0514 (4) | |
| H14A | 0.0867 | 1.4855 | 0.2214 | 0.062* | |
| C15 | 0.18525 (13) | 1.2952 (3) | 0.28139 (8) | 0.0412 (4) | |
| N1 | 0.18011 (10) | 0.4716 (2) | 0.46078 (7) | 0.0395 (3) | |
| H1B | 0.1303 | 0.3861 | 0.4770 | 0.047* | |
| N2 | 0.22510 (10) | 0.7819 (2) | 0.39602 (7) | 0.0385 (3) | |
| H2B | 0.2889 | 0.7372 | 0.4064 | 0.046* | |
| N3 | 0.30872 (10) | 1.0881 (2) | 0.34739 (7) | 0.0397 (3) | |
| O1 | 0.36174 (9) | 0.5195 (2) | 0.45724 (6) | 0.0482 (3) | |
| S1 | 0.10503 (3) | 1.08096 (8) | 0.31634 (2) | 0.04440 (13) | |
| S2 | 0.01565 (3) | 0.69188 (9) | 0.40697 (2) | 0.05128 (15) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0415 (8) | 0.0417 (9) | 0.0415 (8) | 0.0026 (7) | 0.0017 (7) | −0.0018 (7) |
| C2 | 0.0636 (11) | 0.0424 (10) | 0.0486 (10) | −0.0033 (8) | 0.0075 (8) | 0.0042 (8) |
| C3 | 0.0734 (13) | 0.0535 (11) | 0.0536 (11) | 0.0096 (10) | −0.0028 (10) | 0.0161 (9) |
| C4 | 0.0549 (11) | 0.0708 (13) | 0.0680 (13) | 0.0098 (10) | −0.0134 (9) | 0.0184 (11) |
| C5 | 0.0423 (9) | 0.0610 (12) | 0.0553 (11) | 0.0008 (8) | −0.0066 (8) | 0.0146 (9) |
| C6 | 0.0389 (8) | 0.0385 (8) | 0.0362 (8) | 0.0038 (6) | 0.0000 (6) | 0.0004 (6) |
| C7 | 0.0358 (7) | 0.0394 (8) | 0.0388 (8) | 0.0009 (6) | −0.0025 (6) | 0.0003 (7) |
| C8 | 0.0360 (8) | 0.0391 (8) | 0.0366 (8) | 0.0027 (6) | −0.0012 (6) | −0.0027 (6) |
| C9 | 0.0359 (7) | 0.0363 (8) | 0.0339 (7) | 0.0054 (6) | −0.0023 (6) | −0.0005 (6) |
| C10 | 0.0422 (8) | 0.0398 (8) | 0.0343 (8) | 0.0048 (7) | 0.0034 (6) | 0.0000 (6) |
| C11 | 0.0471 (9) | 0.0494 (10) | 0.0451 (9) | −0.0003 (8) | 0.0056 (7) | 0.0020 (8) |
| C12 | 0.0631 (11) | 0.0460 (10) | 0.0460 (10) | −0.0016 (8) | 0.0126 (8) | 0.0040 (8) |
| C13 | 0.0740 (13) | 0.0474 (10) | 0.0441 (10) | 0.0154 (9) | 0.0092 (9) | 0.0106 (8) |
| C14 | 0.0511 (10) | 0.0554 (11) | 0.0476 (10) | 0.0141 (8) | 0.0005 (8) | 0.0088 (8) |
| C15 | 0.0442 (9) | 0.0414 (9) | 0.0381 (8) | 0.0061 (7) | 0.0022 (7) | 0.0018 (7) |
| N1 | 0.0324 (6) | 0.0398 (7) | 0.0461 (7) | −0.0007 (5) | −0.0018 (5) | 0.0073 (6) |
| N2 | 0.0323 (6) | 0.0392 (7) | 0.0441 (7) | 0.0031 (5) | −0.0024 (5) | 0.0046 (6) |
| N3 | 0.0380 (7) | 0.0413 (7) | 0.0399 (7) | 0.0021 (6) | −0.0018 (5) | 0.0030 (6) |
| O1 | 0.0351 (6) | 0.0515 (7) | 0.0581 (7) | −0.0035 (5) | −0.0043 (5) | 0.0143 (6) |
| S1 | 0.0367 (2) | 0.0473 (3) | 0.0491 (2) | 0.00442 (17) | −0.00595 (17) | 0.00779 (18) |
| S2 | 0.0328 (2) | 0.0624 (3) | 0.0586 (3) | 0.00376 (19) | −0.00173 (18) | 0.0142 (2) |
Geometric parameters (Å, °)
| C1—C2 | 1.379 (2) | C9—N3 | 1.287 (2) |
| C1—C6 | 1.390 (2) | C9—N2 | 1.387 (2) |
| C1—H1A | 0.9300 | C9—S1 | 1.7476 (15) |
| C2—C3 | 1.377 (3) | C10—N3 | 1.387 (2) |
| C2—H2A | 0.9300 | C10—C11 | 1.393 (2) |
| C3—C4 | 1.371 (3) | C10—C15 | 1.399 (2) |
| C3—H3A | 0.9300 | C11—C12 | 1.372 (2) |
| C4—C5 | 1.386 (3) | C11—H11A | 0.9300 |
| C4—H4A | 0.9300 | C12—C13 | 1.398 (3) |
| C5—C6 | 1.388 (2) | C12—H12A | 0.9300 |
| C5—H5A | 0.9300 | C13—C14 | 1.371 (3) |
| C6—C7 | 1.486 (2) | C13—H13A | 0.9300 |
| C7—O1 | 1.2179 (19) | C14—C15 | 1.395 (2) |
| C7—N1 | 1.3933 (19) | C14—H14A | 0.9300 |
| C8—N2 | 1.338 (2) | C15—S1 | 1.7471 (17) |
| C8—N1 | 1.388 (2) | N1—H1B | 0.8600 |
| C8—S2 | 1.6578 (15) | N2—H2B | 0.8600 |
| C2—C1—C6 | 120.37 (16) | N3—C10—C11 | 125.14 (15) |
| C2—C1—H1A | 119.8 | N3—C10—C15 | 114.84 (14) |
| C6—C1—H1A | 119.8 | C11—C10—C15 | 120.01 (15) |
| C3—C2—C1 | 119.85 (18) | C12—C11—C10 | 118.83 (17) |
| C3—C2—H2A | 120.1 | C12—C11—H11A | 120.6 |
| C1—C2—H2A | 120.1 | C10—C11—H11A | 120.6 |
| C4—C3—C2 | 120.47 (18) | C11—C12—C13 | 121.01 (18) |
| C4—C3—H3A | 119.8 | C11—C12—H12A | 119.5 |
| C2—C3—H3A | 119.8 | C13—C12—H12A | 119.5 |
| C3—C4—C5 | 120.11 (18) | C14—C13—C12 | 120.85 (17) |
| C3—C4—H4A | 119.9 | C14—C13—H13A | 119.6 |
| C5—C4—H4A | 119.9 | C12—C13—H13A | 119.6 |
| C4—C5—C6 | 119.95 (18) | C13—C14—C15 | 118.55 (17) |
| C4—C5—H5A | 120.0 | C13—C14—H14A | 120.7 |
| C6—C5—H5A | 120.0 | C15—C14—H14A | 120.7 |
| C5—C6—C1 | 119.24 (15) | C14—C15—C10 | 120.72 (16) |
| C5—C6—C7 | 116.71 (15) | C14—C15—S1 | 129.37 (14) |
| C1—C6—C7 | 124.06 (14) | C10—C15—S1 | 109.89 (12) |
| O1—C7—N1 | 121.35 (14) | C8—N1—C7 | 128.15 (13) |
| O1—C7—C6 | 122.16 (14) | C8—N1—H1B | 115.9 |
| N1—C7—C6 | 116.49 (14) | C7—N1—H1B | 115.9 |
| N2—C8—N1 | 114.57 (13) | C8—N2—C9 | 130.20 (13) |
| N2—C8—S2 | 125.60 (12) | C8—N2—H2B | 114.9 |
| N1—C8—S2 | 119.83 (12) | C9—N2—H2B | 114.9 |
| N3—C9—N2 | 117.53 (13) | C9—N3—C10 | 110.14 (13) |
| N3—C9—S1 | 117.47 (12) | C15—S1—C9 | 87.62 (7) |
| N2—C9—S1 | 124.97 (12) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2B···O1 | 0.86 | 1.86 | 2.5878 (17) | 142 |
| C1—H1A···S2i | 0.93 | 2.84 | 3.4017 (17) | 120 |
| C5—H5A···O1ii | 0.93 | 2.57 | 3.470 (2) | 162 |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2150).
References
- Bruker (1999). SMART (Version 5.0), SAINT (Version 6.02a), SADABS (Version 2.03) and SHELXTL (Version 5.1). Bruker AXS Inc., Madison, Wisconsin, USA.
- Büyükgüngör, O., Çalışkan, N., Davran, C. & Batı, H. (2004). Acta Cryst. E60, o1414–o1416.
- Campo, R. del, Criado, J. J., García, E., Hermosa, M. R., Jiménez-Sánchez, A., Manzano, J. L., Monte, E., Rodríguez-Fernández, E. & Sanz, F. (2002). J. Inorg. Biochem.89, 74–82. [DOI] [PubMed]
- Chen, Z.-F., Tang, Y.-Z., Shi, S.-M., Wang, X.-W., Liang, H. & Yu, K.-B. (2003). Acta Cryst. E59, o1461–o1463.
- D’hooghe, M., Waterinckx, A. & De Kimpe, N. (2005). J. Org. Chem.70, 227–232. [DOI] [PubMed]
- Koketsu, M. & Ishihara, H. (2006). Curr. Org. Synth.3, 439–455.
- Morales, A. D., Novoa de Armas, H., Blaton, N. M., Peeters, O. M., De Ranter, C. J., Márquez, H. & Pomés Hernández, R. (2000). Acta Cryst. C56, 503–504. [DOI] [PubMed]
- Rodríguez-Fernández, E., Manzano, J. L., Benito, J. J., Hermosa, R., Monte, E. & Criado, J. J. (2005). J. Inorg. Biochem.99, 1558–1572. [DOI] [PubMed]
- Sheldrick, G. M. (1997). SHELXS97 and SHELXL97 University of Göttingen, Germany.
- Yamin, B. M. & Hassan, I. N. (2004). Acta Cryst. E60, o2513–o2514.
- Yunus, U., Tahir, M. K., Bhatti, M. H., Ali, S. & Helliwell, M. (2007). Acta Cryst. E63, o3690.
- Zeng, R.-S., Zou, J.-P., Zhi, S.-J. & Shen, Q. (2003). Org. Lett.5, 1657–1659. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053680706134X/bh2150sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680706134X/bh2150Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



