Abstract
In the molecule of the title compound, C16H14Cl2N2, the ethano-strapped 2,8-dichloro analogue of Tröger’s base, the dihedral angle between the two benzene rings is 87.01 (3)°.
Related literature
For related literature, see: Tröger (1887 ▶); Hamada & Mukai (1996 ▶); Ishida et al. (2005 ▶). For related structures, see: Spielman (1935 ▶); Larson & Wilcox (1986 ▶); Solano et al. (2005 ▶); Faroughi et al. (2006a
▶,b
▶); Faroughi, Try, Klepetko & Turner (2007 ▶); Faroughi, Try & Turner (2007 ▶).
Experimental
Crystal data
C16H14Cl2N2
M r = 305.19
Triclinic,
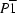
a = 6.8801 (11) Å
b = 10.1951 (17) Å
c = 10.2466 (16) Å
α = 85.320 (3)°
β = 84.956 (2)°
γ = 76.470 (3)°
V = 694.70 (19) Å3
Z = 2
Mo Kα radiation
μ = 0.46 mm−1
T = 150 (2) K
0.63 × 0.44 × 0.41 mm
Data collection
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.089
S = 1.06
3188 reflections
181 parameters
H-atom parameters constrained
Δρmax = 0.34 e Å−3
Δρmin = −0.27 e Å−3
Data collection: SMART (Siemens, 1995 ▶); cell refinement: SAINT (Siemens, 1995 ▶); data reduction: SAINT and XPREP (Siemens, 1995 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: Xtal3.6 (Hall et al., 1999 ▶), ORTEPII (Johnson, 1976 ▶) and WinGX (Farrugia, 1999 ▶); software used to prepare material for publication: WinGX.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536807062642/hk2402sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807062642/hk2402Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the Australian Research Council for a Discovery Project grant to ACT (grant No. DP0345180) and Macquarie University for the award of a Macquarie University Research Development grant.
supplementary crystallographic information
Comment
Tröger's base was first prepared in 1887 (Tröger, 1887) and its structure was elucidated until over 30 years latter (Spielman, 1935). The strucural assignment was confirmed by X-ray crystallography (Larson & Wilcox, 1986). Since then large number of related compounds have been reported and the dihedral angle, between the least-squares planes through the aromatic rings, has been measured across a range of simple dibenzo Tröger's base analogues and found to lie between 82° (Solano et al., 2005) and 108° (Faroughi et al., 2006b). A common structural feature in all of these compounds is the methano-strapped diazocine bridge. The conversion of methano-strapped compounds to ethano-strapped analogues of Tröger's base have been reported for 2,8-dimethyl- and 2,8-dimethoxy- (Hamada & Mukai, 1996) as well as 2,8-dibromo- (Ishida et al., 2005; Faroughi, Try, Klepetko & Turner, 2007) substitution patterns. We have previously reported that the dihedral angle in methano-strapped 2,8-dibromo Tröger's base is 94.5° (Faroughi et al., 2006a) whilst the corresponding angle in the ethano-strapped 2,8-dibromo analogue is 86.1° (Faroughi, Try, Klepetko & Turner, 2007). In the present case, the dihedral angle of ethano-strapped 2,8-dichloro Tröger's base (I), whose molecular structure is shown in Fig. 1, was also found to be reduced [87.01 (3)°] in comparison with the methano-strapped analogue, which has a dihedral angle of 95.6° (Faroughi, Try & Turner et al., 2007).
Experimental
The title compound was prepared according to the literature procedure (Hamada & Mukai, 1996) in 72% yield. Single crystals of (I) were produced from slow evaporation of a dichloromethane solution.
Refinement
H atoms were positioned geometrically, with C—H = 0.95 and 0.99 Å for aromatic and methylene H atoms, respectively, and constrained to ride on their parent atoms, with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
View of (I), showing the atomic numbering scheme. Displacement ellipsoids are shown at the 50% probability level.
Fig. 2.
Synthetic scheme for the synthesis of (I) showing the numbering system used in naming the compound.
Crystal data
| C16H14Cl2N2 | Z = 2 |
| Mr = 305.19 | F000 = 316 |
| Triclinic, P1 | Dx = 1.459 Mg m−3 |
| Hall symbol: -P 1 | Melting point = 454–455 K |
| a = 6.8801 (11) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 10.1951 (17) Å | Cell parameters from 925 reflections |
| c = 10.2466 (16) Å | θ = 3.5–27.9º |
| α = 85.320 (3)º | µ = 0.46 mm−1 |
| β = 84.956 (2)º | T = 150 (2) K |
| γ = 76.470 (3)º | Prism, colorless |
| V = 694.70 (19) Å3 | 0.63 × 0.44 × 0.41 mm |
Data collection
| Bruker SMART 1000 CCD diffractometer | 3188 independent reflections |
| Radiation source: fine-focus sealed tube | 2992 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.033 |
| T = 150(2) K | θmax = 28.3º |
| ω scans | θmin = 2.0º |
| Absorption correction: Gaussian(XPREP; Coppens et al., 1965; Siemens, 1995) | h = −8→9 |
| Tmin = 0.773, Tmax = 0.869 | k = −13→13 |
| 6876 measured reflections | l = −13→13 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.031 | H-atom parameters constrained |
| wR(F2) = 0.089 | w = 1/[σ2(Fo2) + (0.051P)2 + 0.1729P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.002 |
| 3188 reflections | Δρmax = 0.34 e Å−3 |
| 181 parameters | Δρmin = −0.27 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | −0.33151 (5) | 0.87520 (3) | 1.11085 (3) | 0.03317 (10) | |
| Cl2 | 0.19414 (5) | 1.02485 (3) | 0.31142 (3) | 0.03303 (10) | |
| N1 | 0.43135 (15) | 0.61940 (11) | 0.81861 (11) | 0.0288 (2) | |
| N2 | 0.19355 (15) | 0.52439 (10) | 0.65450 (10) | 0.0249 (2) | |
| C1 | 0.24125 (17) | 0.67773 (12) | 0.88354 (11) | 0.0256 (2) | |
| C2 | 0.23827 (19) | 0.77730 (13) | 0.96964 (13) | 0.0300 (3) | |
| H2 | 0.3590 | 0.8039 | 0.9806 | 0.036* | |
| C3 | 0.0642 (2) | 0.83825 (12) | 1.03940 (12) | 0.0297 (3) | |
| H3 | 0.0645 | 0.9057 | 1.0981 | 0.036* | |
| C4 | −0.11108 (18) | 0.79898 (12) | 1.02196 (11) | 0.0261 (2) | |
| C5 | −0.11437 (17) | 0.70205 (12) | 0.93531 (11) | 0.0242 (2) | |
| H5 | −0.2366 | 0.6777 | 0.9238 | 0.029* | |
| C6 | 0.06195 (17) | 0.64005 (11) | 0.86481 (11) | 0.0231 (2) | |
| C7 | 0.04879 (17) | 0.53679 (12) | 0.76854 (11) | 0.0247 (2) | |
| H7A | 0.0654 | 0.4472 | 0.8169 | 0.030* | |
| H7B | −0.0876 | 0.5605 | 0.7367 | 0.030* | |
| C8 | 0.19502 (17) | 0.64895 (11) | 0.57911 (11) | 0.0229 (2) | |
| C9 | 0.05736 (18) | 0.68840 (12) | 0.48295 (11) | 0.0250 (2) | |
| H9 | −0.0354 | 0.6346 | 0.4732 | 0.030* | |
| C10 | 0.05307 (18) | 0.80449 (12) | 0.40135 (11) | 0.0267 (2) | |
| H10 | −0.0423 | 0.8310 | 0.3370 | 0.032* | |
| C11 | 0.19158 (18) | 0.88103 (12) | 0.41608 (11) | 0.0256 (2) | |
| C12 | 0.32586 (18) | 0.84588 (12) | 0.51236 (12) | 0.0265 (2) | |
| H12 | 0.4170 | 0.9009 | 0.5220 | 0.032* | |
| C13 | 0.32901 (17) | 0.73018 (12) | 0.59568 (11) | 0.0250 (2) | |
| C14 | 0.48177 (18) | 0.69776 (14) | 0.69929 (13) | 0.0317 (3) | |
| H14A | 0.6103 | 0.6474 | 0.6582 | 0.038* | |
| H14B | 0.5042 | 0.7841 | 0.7254 | 0.038* | |
| C15 | 0.47175 (18) | 0.47337 (13) | 0.80467 (13) | 0.0324 (3) | |
| H15A | 0.4103 | 0.4303 | 0.8826 | 0.039* | |
| H15B | 0.6183 | 0.4358 | 0.8017 | 0.039* | |
| C16 | 0.39027 (19) | 0.43836 (13) | 0.68131 (13) | 0.0319 (3) | |
| H16A | 0.4863 | 0.4477 | 0.6052 | 0.038* | |
| H16B | 0.3797 | 0.3428 | 0.6914 | 0.038* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.03691 (18) | 0.03002 (17) | 0.03158 (17) | −0.00488 (12) | −0.00344 (12) | −0.00303 (12) |
| Cl2 | 0.0492 (2) | 0.02418 (16) | 0.02644 (16) | −0.01157 (13) | −0.00413 (12) | 0.00531 (11) |
| N1 | 0.0231 (5) | 0.0311 (5) | 0.0321 (5) | −0.0078 (4) | −0.0082 (4) | 0.0091 (4) |
| N2 | 0.0270 (5) | 0.0203 (5) | 0.0266 (5) | −0.0053 (4) | −0.0019 (4) | 0.0024 (4) |
| C1 | 0.0256 (5) | 0.0257 (6) | 0.0264 (5) | −0.0078 (4) | −0.0101 (4) | 0.0080 (4) |
| C2 | 0.0314 (6) | 0.0291 (6) | 0.0333 (6) | −0.0125 (5) | −0.0160 (5) | 0.0065 (5) |
| C3 | 0.0403 (7) | 0.0237 (6) | 0.0279 (6) | −0.0100 (5) | −0.0145 (5) | 0.0035 (4) |
| C4 | 0.0316 (6) | 0.0228 (5) | 0.0231 (5) | −0.0051 (4) | −0.0069 (4) | 0.0042 (4) |
| C5 | 0.0269 (5) | 0.0242 (5) | 0.0232 (5) | −0.0094 (4) | −0.0072 (4) | 0.0047 (4) |
| C6 | 0.0264 (5) | 0.0219 (5) | 0.0223 (5) | −0.0085 (4) | −0.0075 (4) | 0.0055 (4) |
| C7 | 0.0261 (5) | 0.0239 (5) | 0.0258 (5) | −0.0097 (4) | −0.0039 (4) | 0.0020 (4) |
| C8 | 0.0253 (5) | 0.0198 (5) | 0.0231 (5) | −0.0048 (4) | −0.0006 (4) | 0.0001 (4) |
| C9 | 0.0291 (5) | 0.0244 (6) | 0.0231 (5) | −0.0089 (4) | −0.0026 (4) | −0.0028 (4) |
| C10 | 0.0326 (6) | 0.0266 (6) | 0.0213 (5) | −0.0064 (5) | −0.0058 (4) | −0.0006 (4) |
| C11 | 0.0333 (6) | 0.0203 (5) | 0.0222 (5) | −0.0060 (4) | 0.0001 (4) | 0.0011 (4) |
| C12 | 0.0277 (5) | 0.0252 (6) | 0.0277 (6) | −0.0096 (4) | −0.0012 (4) | 0.0009 (4) |
| C13 | 0.0235 (5) | 0.0254 (6) | 0.0262 (5) | −0.0064 (4) | −0.0033 (4) | 0.0019 (4) |
| C14 | 0.0240 (5) | 0.0366 (7) | 0.0358 (7) | −0.0120 (5) | −0.0081 (5) | 0.0104 (5) |
| C15 | 0.0252 (6) | 0.0308 (6) | 0.0376 (7) | −0.0019 (5) | −0.0051 (5) | 0.0099 (5) |
| C16 | 0.0311 (6) | 0.0236 (6) | 0.0367 (6) | −0.0012 (5) | 0.0003 (5) | 0.0053 (5) |
Geometric parameters (Å, °)
| Cl1—C4 | 1.7477 (13) | C7—H7B | 0.9900 |
| Cl2—C11 | 1.7471 (12) | C8—C9 | 1.3968 (16) |
| N1—C1 | 1.4334 (16) | C8—C13 | 1.4043 (16) |
| N1—C15 | 1.4657 (17) | C9—C10 | 1.3882 (17) |
| N1—C14 | 1.4659 (16) | C9—H9 | 0.9500 |
| N2—C8 | 1.4332 (14) | C10—C11 | 1.3901 (17) |
| N2—C7 | 1.4608 (15) | C10—H10 | 0.9500 |
| N2—C16 | 1.4653 (16) | C11—C12 | 1.3795 (17) |
| C1—C2 | 1.3937 (18) | C12—C13 | 1.3956 (16) |
| C1—C6 | 1.4077 (15) | C12—H12 | 0.9500 |
| C2—C3 | 1.3802 (19) | C13—C14 | 1.5223 (16) |
| C2—H2 | 0.9500 | C14—H14A | 0.9900 |
| C3—C4 | 1.3865 (17) | C14—H14B | 0.9900 |
| C3—H3 | 0.9500 | C15—C16 | 1.5269 (19) |
| C4—C5 | 1.3866 (17) | C15—H15A | 0.9900 |
| C5—C6 | 1.3980 (17) | C15—H15B | 0.9900 |
| C5—H5 | 0.9500 | C16—H16A | 0.9900 |
| C6—C7 | 1.5230 (16) | C16—H16B | 0.9900 |
| C7—H7A | 0.9900 | ||
| C1—N1—C15 | 115.51 (10) | C10—C9—C8 | 121.47 (11) |
| C1—N1—C14 | 113.66 (10) | C10—C9—H9 | 119.3 |
| C15—N1—C14 | 114.24 (11) | C8—C9—H9 | 119.3 |
| C8—N2—C7 | 114.43 (9) | C9—C10—C11 | 118.43 (11) |
| C8—N2—C16 | 115.99 (9) | C9—C10—H10 | 120.8 |
| C7—N2—C16 | 113.67 (9) | C11—C10—H10 | 120.8 |
| C2—C1—C6 | 119.43 (11) | C12—C11—C10 | 121.14 (11) |
| C2—C1—N1 | 116.75 (10) | C12—C11—Cl2 | 119.53 (9) |
| C6—C1—N1 | 123.82 (11) | C10—C11—Cl2 | 119.33 (9) |
| C3—C2—C1 | 121.62 (11) | C11—C12—C13 | 120.58 (11) |
| C3—C2—H2 | 119.2 | C11—C12—H12 | 119.7 |
| C1—C2—H2 | 119.2 | C13—C12—H12 | 119.7 |
| C2—C3—C4 | 118.57 (11) | C12—C13—C8 | 119.04 (10) |
| C2—C3—H3 | 120.7 | C12—C13—C14 | 117.56 (10) |
| C4—C3—H3 | 120.7 | C8—C13—C14 | 123.39 (10) |
| C3—C4—C5 | 121.36 (12) | N1—C14—C13 | 116.80 (10) |
| C3—C4—Cl1 | 118.71 (10) | N1—C14—H14A | 108.1 |
| C5—C4—Cl1 | 119.92 (9) | C13—C14—H14A | 108.1 |
| C4—C5—C6 | 120.10 (10) | N1—C14—H14B | 108.1 |
| C4—C5—H5 | 120.0 | C13—C14—H14B | 108.1 |
| C6—C5—H5 | 120.0 | H14A—C14—H14B | 107.3 |
| C5—C6—C1 | 118.91 (11) | N1—C15—C16 | 112.65 (10) |
| C5—C6—C7 | 117.90 (10) | N1—C15—H15A | 109.1 |
| C1—C6—C7 | 123.18 (11) | C16—C15—H15A | 109.1 |
| N2—C7—C6 | 116.32 (9) | N1—C15—H15B | 109.1 |
| N2—C7—H7A | 108.2 | C16—C15—H15B | 109.1 |
| C6—C7—H7A | 108.2 | H15A—C15—H15B | 107.8 |
| N2—C7—H7B | 108.2 | N2—C16—C15 | 112.95 (11) |
| C6—C7—H7B | 108.2 | N2—C16—H16A | 109.0 |
| H7A—C7—H7B | 107.4 | C15—C16—H16A | 109.0 |
| C9—C8—C13 | 119.27 (10) | N2—C16—H16B | 109.0 |
| C9—C8—N2 | 117.29 (10) | C15—C16—H16B | 109.0 |
| C13—C8—N2 | 123.43 (10) | H16A—C16—H16B | 107.8 |
| C15—N1—C1—C2 | 140.54 (11) | C16—N2—C8—C13 | −39.77 (16) |
| C14—N1—C1—C2 | −84.57 (13) | C13—C8—C9—C10 | 1.50 (18) |
| C15—N1—C1—C6 | −39.47 (15) | N2—C8—C9—C10 | −177.27 (10) |
| C14—N1—C1—C6 | 95.42 (14) | C8—C9—C10—C11 | 0.77 (18) |
| C6—C1—C2—C3 | 1.36 (18) | C9—C10—C11—C12 | −2.32 (18) |
| N1—C1—C2—C3 | −178.64 (11) | C9—C10—C11—Cl2 | 178.13 (9) |
| C1—C2—C3—C4 | −0.33 (18) | C10—C11—C12—C13 | 1.56 (18) |
| C2—C3—C4—C5 | −0.88 (18) | Cl2—C11—C12—C13 | −178.90 (9) |
| C2—C3—C4—Cl1 | 179.63 (9) | C11—C12—C13—C8 | 0.77 (18) |
| C3—C4—C5—C6 | 1.03 (17) | C11—C12—C13—C14 | 179.84 (11) |
| Cl1—C4—C5—C6 | −179.48 (8) | C9—C8—C13—C12 | −2.26 (17) |
| C4—C5—C6—C1 | 0.03 (16) | N2—C8—C13—C12 | 176.43 (10) |
| C4—C5—C6—C7 | −178.54 (10) | C9—C8—C13—C14 | 178.73 (11) |
| C2—C1—C6—C5 | −1.19 (17) | N2—C8—C13—C14 | −2.57 (18) |
| N1—C1—C6—C5 | 178.82 (10) | C1—N1—C14—C13 | −54.36 (15) |
| C2—C1—C6—C7 | 177.30 (10) | C15—N1—C14—C13 | 81.12 (14) |
| N1—C1—C6—C7 | −2.70 (17) | C12—C13—C14—N1 | 153.32 (12) |
| C8—N2—C7—C6 | −54.43 (13) | C8—C13—C14—N1 | −27.67 (18) |
| C16—N2—C7—C6 | 82.00 (12) | C1—N1—C15—C16 | 86.66 (13) |
| C5—C6—C7—N2 | 150.29 (10) | C14—N1—C15—C16 | −47.97 (14) |
| C1—C6—C7—N2 | −28.21 (15) | C8—N2—C16—C15 | 86.98 (12) |
| C7—N2—C8—C9 | −85.65 (13) | C7—N2—C16—C15 | −48.75 (14) |
| C16—N2—C8—C9 | 138.96 (11) | N1—C15—C16—N2 | −39.74 (14) |
| C7—N2—C8—C13 | 95.62 (13) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HK2402).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Coppens, P., Leiserowitz, L. & Rabinovich, D. (1965). Acta Cryst.18, 1035–1038.
- Faroughi, M., Try, A. C., Klepetko, J. & Turner, P. (2007). Tetrahedron Lett.48, 6548–6551.
- Faroughi, M., Try, A. C. & Turner, P. (2006a). Acta Cryst. E62, o3674–o3675.
- Faroughi, M., Try, A. C. & Turner, P. (2006b). Acta Cryst. E62, o3893–o3894.
- Faroughi, M., Try, A. C. & Turner, P. (2007). Acta Cryst. E63, o2695.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Hall, S. R., du Boulay, D. J. & Olthof-Hazekamp, R. (1999). Editors. The Xtal3.6 System University of Western Australia.
- Hamada, Y. & Mukai, S. (1996). Tetrahedron Asymmetry, 7, 2671–2674.
- Ishida, Y., Ito, H., Mori, D. & Saigo, K. (2005). Tetrahedron Lett.46, 109–112.
- Johnson, C. K. (1976). ORTEPII Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- Larson, S. B. & Wilcox, C. S. (1986). Acta Cryst. C42, 224–227.
- Sheldrick, G. M. (1997). SHELXL97 University of Göttingen, Germany.
- Siemens (1995). SMART, SAINT and XPREP Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Solano, C., Svensson, D., Olomi, Z., Jensen, J., Wendt, O. F. & Wärnmark, K. (2005). Eur. J. Org. Chem. pp. 3510–3517.
- Spielman, M. A. (1935). J. Am. Chem. Soc.57, 583–585.
- Tröger, J. (1887). J. Prakt. Chem.36, 225–245.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536807062642/hk2402sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807062642/hk2402Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




