Abstract
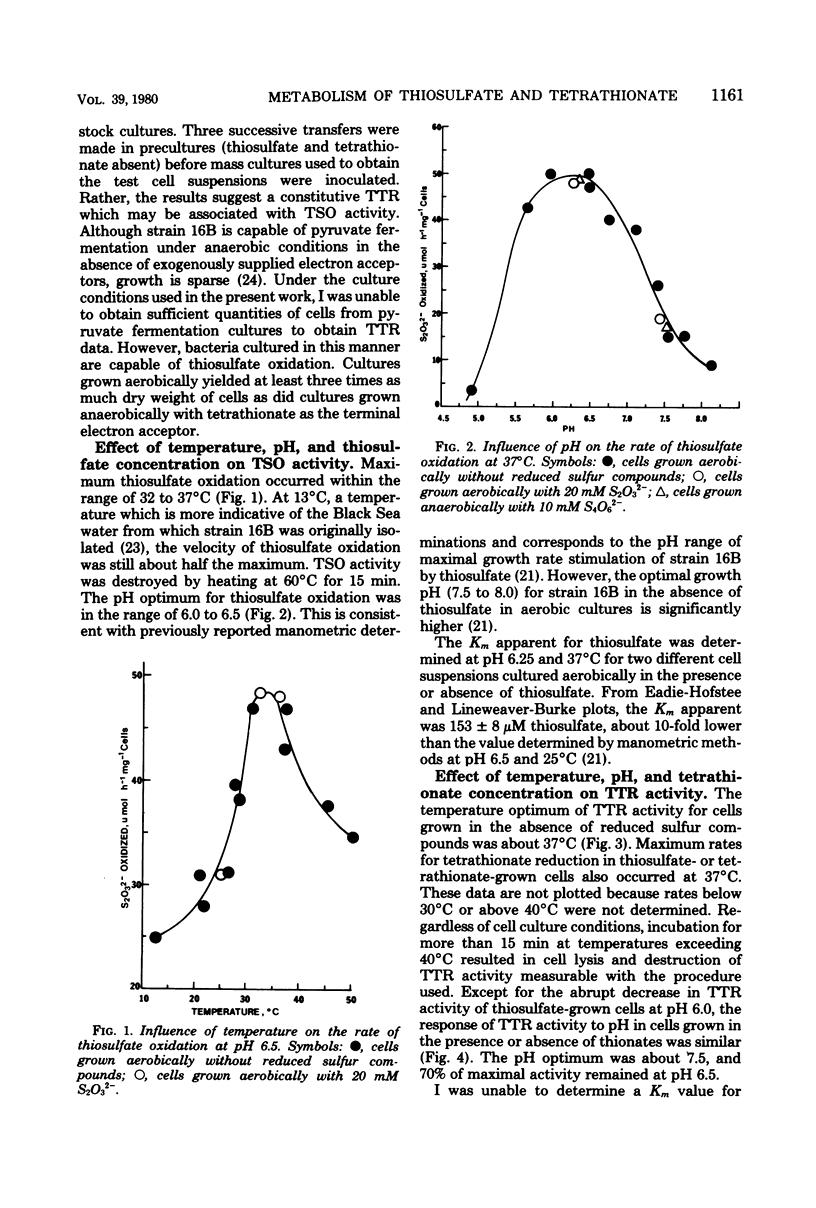
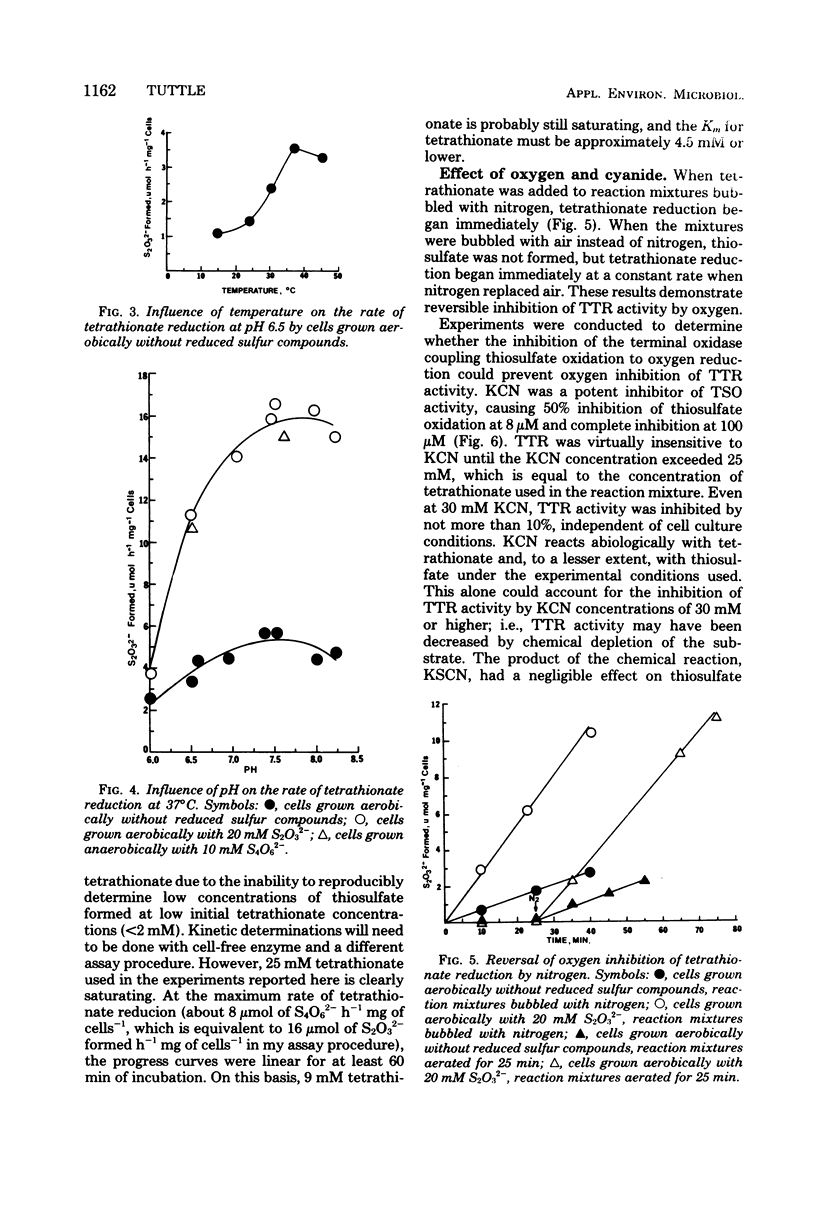
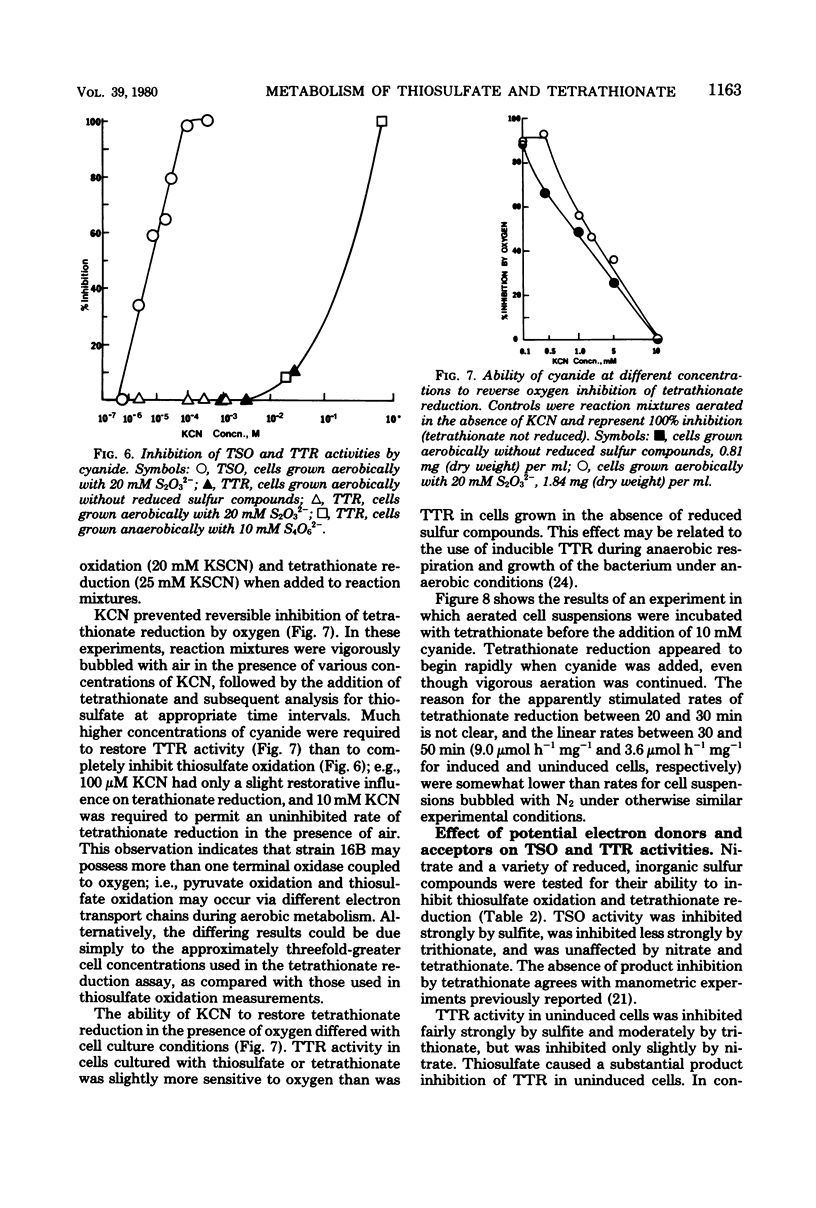
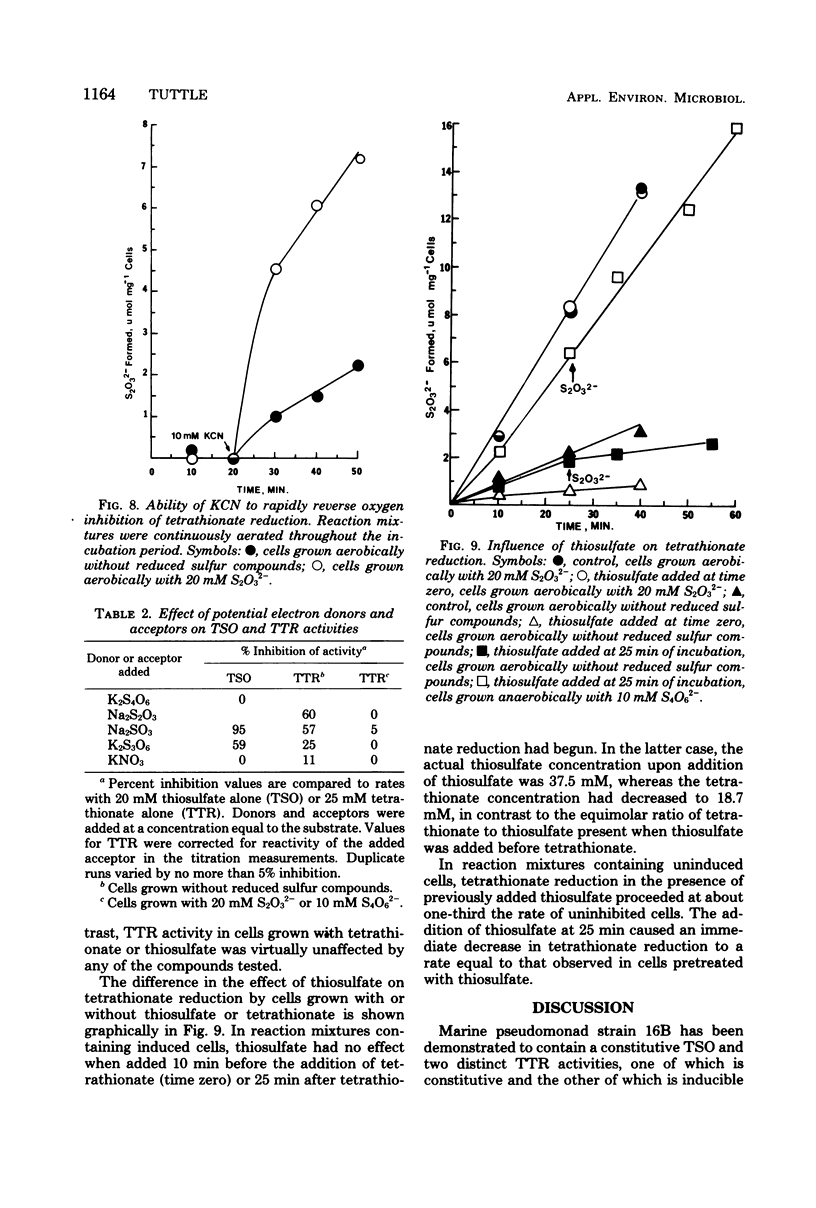
Levels of thiosulfate-oxidizing enzyme (TSO) and tetrathionate reductase (TTR) were measured in washed cell suspensions of a heterotrophic marine thiosulfate-oxidizing bacterium, strain 16B. TSO activity remained virtually constant in aerobically and anaerobically grown cells and was unaffected by the presence or absence of thiosulfate and tetrathionate in the growth medium. TTR was also present in cells grown aerobically and anaerobically, but its activity was threefold greater in cells cultured in media containing tetrathionate or thiosulfate. Tetrathionate appears to be the inducer of increased TTR activity in both aerobically and anaerobically grown cells. TTR (constitutive or induced) and TSO have different pH and temperature optima. Both TTR activities were unaffected by 10 mM KCN, which reversed oxygen inhibition of tetrathionate reduction. TSO was partially inhibited by 5 μM KCN and completely inhibited by 90 μM KCN. These findings and results of experiments to determine the influence of several inorganic electron donors and acceptors on TSO and TTR activities suggest that constitutive TSO and TTR represent reverse activities of the same enzyme, whereas inducible TTR is a separate enzyme used by strain 16B only for anaerobic respiration of tetrathionate. The bacterium appears well adapted to growth in environments characterized by low oxygen tension, dilute organic carbon concentrations, and the presence of a variety of reduced, inorganic sulfur compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kaprálek F., Pichinoty F. The effect of oxygen on tetrathionate reductase activity and biosynthesis. J Gen Microbiol. 1970 Jul;62(1):95–105. doi: 10.1099/00221287-62-1-95. [DOI] [PubMed] [Google Scholar]
- Kaprálek F. The physiological role of tetrathionate respiration in growing citrobacter. J Gen Microbiol. 1972 Jun;71(1):133–139. doi: 10.1099/00221287-71-1-133. [DOI] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. 3. Properties of thiosulfate-oxidizing enzyme and proposed pathway of thiosulfate oxidation. Can J Biochem. 1970 Mar;48(3):355–363. doi: 10.1139/o70-058. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., BIGLIARDI-ROUVIER J. [Research on tetrathionate reductase of a facultative anaerobic bacterium]. Biochim Biophys Acta. 1963 Mar 12;67:366–378. doi: 10.1016/0006-3002(63)91843-2. [DOI] [PubMed] [Google Scholar]
- Papavassiliou J., Samaraki-Lyberopoulou V., Piperakis G. Production of tetrathionate reductase by Salmonella. Can J Microbiol. 1969 Feb;15(2):238–240. doi: 10.1139/m69-041. [DOI] [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. The thiosulfate-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 Oct;46(10):1215–1220. doi: 10.1139/o68-181. [DOI] [PubMed] [Google Scholar]
- Starkey R. L. The Production of Polythionates from Thiosulfate by Microörganisms. J Bacteriol. 1934 Oct;28(4):387–400. doi: 10.1128/jb.28.4.387-400.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Influence of hydrogen acceptors on growth and energy production of Proteus mirabilis. Antonie Van Leeuwenhoek. 1972;38(1):81–90. doi: 10.1007/BF02328079. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 2. Thiosulphate-oxidizing enzyme. Biochem J. 1961 Apr;78:680–686. doi: 10.1042/bj0780680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A. Metabolism of thiosulfate and tetrathionate by heterotrophic bacteria from soil. J Bacteriol. 1967 Feb;93(2):550–559. doi: 10.1128/jb.93.2.550-559.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H., Holmes P. E., Jannasch H. W. Growth rate stimulation of marine pseudomonads by thiosulfate. Arch Microbiol. 1974;99(1):1–14. doi: 10.1007/BF00696218. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H., Jannasch H. W. Dissimilatory reduction of inorganic sulfur by facultatively anaerobic marine bacteria. J Bacteriol. 1973 Sep;115(3):732–737. doi: 10.1128/jb.115.3.732-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


