Abstract
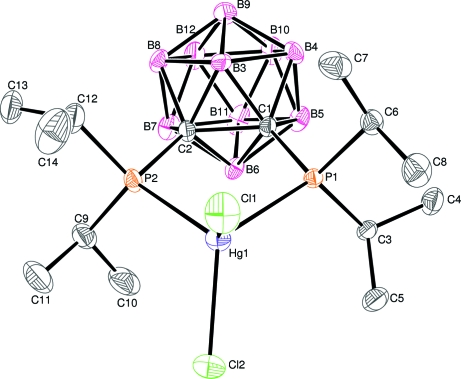
In the title complex, [HgCl2(C14H38B10P2)], the HgII atom is in a distorted HgCl2P2 tetrahedral coordination environment. The chelation of the Hg atom by two P atoms and two C atoms from the carborane skeleton results in a nearly planar five-membered ring.
Related literature
For related structures see: Mariyatra et al. (2005 ▶); Liu et al. (2004 ▶); Paavola, Kivekäs et al. (2002 ▶), Paavola, Teixidor et al. (2002a
▶,b
▶). For the synthesis and structure of the ligand, see: Kivekäs et al. (1995 ▶).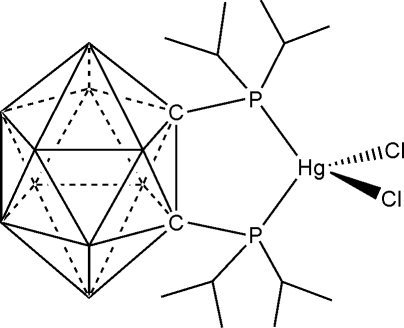
Experimental
Crystal data
[HgCl2(C14H38B10P2)]
M r = 647.97
Tetragonal,
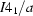
a = 21.110 (3) Å
c = 24.585 (6) Å
V = 10956 (3) Å3
Z = 16
Mo Kα radiation
μ = 5.93 mm−1
T = 298 (2) K
0.53 × 0.49 × 0.47 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2001 ▶) T min = 0.145, T max = 0.167 (expected range = 0.053–0.062)
22446 measured reflections
4815 independent reflections
3491 reflections with I > 2σ(I)
R int = 0.081
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.077
S = 1.00
4815 reflections
270 parameters
290 restraints
H-atom parameters constrained
Δρmax = 1.37 e Å−3
Δρmin = −1.23 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: SHELXTL (Bruker, 2001 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807065130/hb2648sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807065130/hb2648Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Hg1—Cl1 | 2.4482 (17) |
| Hg1—Cl2 | 2.4542 (17) |
| Hg1—P1 | 2.5200 (10) |
| Hg1—P2 | 2.5242 (16) |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project No. 20371025), the Open Research Fund Program of the Key Laboratory of Marine Drugs (Ocean University of China), the Ministry of Education [Project No. KLMD (OUC) 2004] and the Postgraduate Foundation of Taishan University (No. Yo6-2-10).
supplementary crystallographic information
Comment
The synthesis and structure of 1,2-(PiPr2)2-1,2-C2B10H10 was reported by Kivekäs et al. (1995). Since then, only a few complexes containing this ligand have been described, containing Pt(II) and Pd(II) (Paavola et al. (2002,2002a,b). We now report the structure of this ligand combined with HgII and chloride ions as the title compound, (I).
As shown in Fig. 1, The HgII atom in (I) is in a distorted HgCl2P2 tetrahedral coordination environment (Table 1). The Hg—P distances in (I) are longer than those of 2.3991Å in [Ph3PHgCl(µ-Cl)2ClHgPPh3] (Mariyatra et al., 2005). The Hg—Cl distances in (I) are also longer than the corresponding distance of 2.4015 (8)Å for in the Mariyatra et al. (2005) phase. The Cl—Hg—Cl angle in (I) of 104.81 (6) Å, is slight bigger than that of 101.19 (4)° in [(HgCl2)2((C6H11)3P)2] (Liu et al., 2004).
The chelation of the mercury(II) atom in (I) with phosphorus atoms and carbon atoms form a nearly planar five-membered ring with a maximum deviation of 0.033Å for C2. The torsion angle P1—C1—C2—P2 in (I) is 5.8 (6)°, which is smaller than that of 12.1 (2)° in the free ligand (Kivekas et al., 1995).
Experimental
The title compound was synthesizd by the reaction of 1 mmol HgCl2 and 1 mmol 1,2-(PiPr2)2-1,2-C2B10H10 in 10 ml dichloromethane under the protection of N2. The mixture was refluxed for 4 h, then a colourless solution formed, and colourless blocks of (I) were obtained from a dichloromethane/n-hexane solution (61.7%, m.p. 405–406 K). FTIR (KBr) \v (cm-l): 2990, 2968, 2932, 2875 (C—H); 2615, 2603, 2586, 2558 (B—H); 1072 (C—P).
Refinement
All H atoms were placed geometrically (B—H = 1.10 Å, C—H = 0.96–0.98 Å) and refined as riding with Uiso(H) = 1.2Ueq(B) or 1.5Ueq(C).
Figures
Fig. 1.
The molecular structure of (I), with 30% probability displacement ellipsoids (H atoms omitted for clarity).
Crystal data
| [HgCl2(C14H38B10P2)] | Z = 16 |
| Mr = 647.97 | F000 = 5056 |
| Tetragonal, I41/a | Dx = 1.571 Mg m−3 |
| Hall symbol: -I 4ad | Mo Kα radiation λ = 0.71073 Å |
| a = 21.110 (3) Å | Cell parameters from 5681 reflections |
| b = 21.110 (3) Å | θ = 2.3–25.3º |
| c = 24.585 (6) Å | µ = 5.93 mm−1 |
| α = 90º | T = 298 (2) K |
| β = 90º | Block, colorless |
| γ = 90º | 0.53 × 0.49 × 0.47 mm |
| V = 10956 (3) Å3 |
Data collection
| Bruker SMART CCD diffractometer | 4815 independent reflections |
| Radiation source: fine-focus sealed tube | 3491 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.081 |
| T = 298(2) K | θmax = 25.0º |
| ω scans | θmin = 1.9º |
| Absorption correction: multi-scan(SADABS; Bruker, 2001) | h = −25→23 |
| Tmin = 0.145, Tmax = 0.167 | k = −17→25 |
| 22446 measured reflections | l = −29→27 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H-atom parameters constrained |
| wR(F2) = 0.077 | w = 1/[σ2(Fo2) + (0.031P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max = 0.002 |
| 4815 reflections | Δρmax = 1.37 e Å−3 |
| 270 parameters | Δρmin = −1.23 e Å−3 |
| 290 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| P1 | 0.88138 (6) | 0.58515 (7) | 0.21383 (4) | 0.0258 (3) | |
| Hg1 | 0.906748 (11) | 0.554286 (12) | 0.117243 (8) | 0.03997 (11) | |
| P2 | 1.02256 (7) | 0.53987 (8) | 0.13960 (5) | 0.0344 (4) | |
| Cl1 | 0.87446 (8) | 0.63978 (9) | 0.05593 (7) | 0.0653 (5) | |
| Cl2 | 0.85996 (8) | 0.45816 (8) | 0.07802 (7) | 0.0598 (5) | |
| B3 | 1.0185 (3) | 0.6349 (3) | 0.2328 (2) | 0.0303 (16) | |
| H3 | 1.0115 | 0.6728 | 0.2028 | 0.036* | |
| B4 | 0.9818 (3) | 0.6364 (4) | 0.2987 (2) | 0.0391 (19) | |
| H4 | 0.9519 | 0.6761 | 0.3122 | 0.047* | |
| B5 | 0.9646 (3) | 0.5571 (3) | 0.3155 (2) | 0.0401 (19) | |
| H5 | 0.9232 | 0.5447 | 0.3406 | 0.048* | |
| B6 | 0.9896 (3) | 0.5093 (3) | 0.2589 (2) | 0.0319 (16) | |
| H6 | 0.9638 | 0.4667 | 0.2457 | 0.038* | |
| B7 | 1.0743 (3) | 0.5145 (4) | 0.2565 (3) | 0.0413 (19) | |
| H7 | 1.1044 | 0.4750 | 0.2430 | 0.050* | |
| B8 | 1.0922 (3) | 0.5945 (3) | 0.2405 (3) | 0.0379 (18) | |
| H8 | 1.1341 | 0.6074 | 0.2164 | 0.046* | |
| B9 | 1.0649 (3) | 0.6428 (4) | 0.2937 (3) | 0.046 (2) | |
| H9 | 1.0894 | 0.6872 | 0.3041 | 0.056* | |
| B10 | 1.0309 (3) | 0.5941 (4) | 0.3432 (3) | 0.050 (2) | |
| H10 | 1.0334 | 0.6065 | 0.3866 | 0.060* | |
| B11 | 1.0350 (3) | 0.5142 (4) | 0.3206 (3) | 0.047 (2) | |
| H11 | 1.0393 | 0.4738 | 0.3486 | 0.057* | |
| B12 | 1.0978 (3) | 0.5680 (4) | 0.3086 (3) | 0.050 (2) | |
| H12 | 1.1436 | 0.5629 | 0.3294 | 0.060* | |
| C1 | 0.9610 (2) | 0.5824 (3) | 0.24933 (18) | 0.0280 (12) | |
| C2 | 1.0262 (2) | 0.5577 (3) | 0.21524 (19) | 0.0301 (12) | |
| C3 | 0.8303 (3) | 0.5299 (3) | 0.2536 (2) | 0.0326 (13) | |
| H3A | 0.8588 | 0.4975 | 0.2683 | 0.039* | |
| C4 | 0.7960 (3) | 0.5584 (3) | 0.3027 (2) | 0.0508 (17) | |
| H4A | 0.7646 | 0.5880 | 0.2903 | 0.076* | |
| H4B | 0.8260 | 0.5799 | 0.3255 | 0.076* | |
| H4C | 0.7758 | 0.5252 | 0.3230 | 0.076* | |
| C5 | 0.7850 (3) | 0.4955 (3) | 0.2166 (2) | 0.0515 (18) | |
| H5A | 0.7646 | 0.4620 | 0.2364 | 0.077* | |
| H5B | 0.8079 | 0.4778 | 0.1864 | 0.077* | |
| H5C | 0.7537 | 0.5246 | 0.2034 | 0.077* | |
| C6 | 0.8465 (3) | 0.6652 (3) | 0.2224 (2) | 0.0380 (14) | |
| H6A | 0.8417 | 0.6741 | 0.2613 | 0.046* | |
| C7 | 0.8844 (3) | 0.7185 (3) | 0.1962 (3) | 0.0577 (18) | |
| H7A | 0.8959 | 0.7066 | 0.1598 | 0.087* | |
| H7B | 0.9220 | 0.7263 | 0.2171 | 0.087* | |
| H7C | 0.8590 | 0.7563 | 0.1951 | 0.087* | |
| C8 | 0.7808 (3) | 0.6633 (3) | 0.1959 (3) | 0.0584 (18) | |
| H8A | 0.7606 | 0.7037 | 0.2001 | 0.088* | |
| H8B | 0.7556 | 0.6312 | 0.2131 | 0.088* | |
| H8C | 0.7851 | 0.6537 | 0.1579 | 0.088* | |
| C9 | 1.0537 (3) | 0.4602 (3) | 0.1261 (3) | 0.0523 (16) | |
| H9A | 1.0981 | 0.4580 | 0.1375 | 0.063* | |
| C10 | 1.0167 (4) | 0.4082 (3) | 0.1536 (3) | 0.078 (2) | |
| H10A | 0.9722 | 0.4173 | 0.1511 | 0.116* | |
| H10B | 1.0288 | 0.4057 | 0.1912 | 0.116* | |
| H10C | 1.0255 | 0.3686 | 0.1360 | 0.116* | |
| C11 | 1.0495 (4) | 0.4506 (4) | 0.0638 (3) | 0.085 (2) | |
| H11A | 1.0616 | 0.4080 | 0.0549 | 0.128* | |
| H11B | 1.0774 | 0.4798 | 0.0459 | 0.128* | |
| H11C | 1.0068 | 0.4580 | 0.0519 | 0.128* | |
| C12 | 1.0783 (3) | 0.5968 (3) | 0.1081 (2) | 0.0502 (16) | |
| H12A | 1.0793 | 0.6333 | 0.1326 | 0.060* | |
| C13 | 1.1470 (3) | 0.5732 (4) | 0.1045 (3) | 0.069 (2) | |
| H13A | 1.1509 | 0.5437 | 0.0750 | 0.104* | |
| H13B | 1.1584 | 0.5527 | 0.1380 | 0.104* | |
| H13C | 1.1748 | 0.6086 | 0.0983 | 0.104* | |
| C14 | 1.0553 (4) | 0.6221 (4) | 0.0543 (3) | 0.093 (3) | |
| H14A | 1.0866 | 0.6501 | 0.0393 | 0.140* | |
| H14B | 1.0164 | 0.6448 | 0.0596 | 0.140* | |
| H14C | 1.0483 | 0.5875 | 0.0297 | 0.140* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P1 | 0.0229 (8) | 0.0301 (9) | 0.0245 (7) | −0.0001 (7) | −0.0025 (6) | −0.0018 (6) |
| Hg1 | 0.03668 (17) | 0.05346 (19) | 0.02979 (13) | 0.00074 (13) | −0.00920 (11) | −0.00648 (11) |
| P2 | 0.0269 (9) | 0.0513 (10) | 0.0249 (7) | 0.0046 (8) | −0.0005 (7) | −0.0113 (7) |
| Cl1 | 0.0625 (12) | 0.0856 (14) | 0.0479 (9) | 0.0170 (11) | −0.0108 (9) | 0.0162 (10) |
| Cl2 | 0.0628 (12) | 0.0618 (12) | 0.0547 (10) | −0.0097 (10) | −0.0096 (9) | −0.0179 (9) |
| B3 | 0.030 (4) | 0.032 (4) | 0.030 (3) | −0.008 (3) | −0.001 (3) | −0.006 (3) |
| B4 | 0.037 (4) | 0.052 (5) | 0.029 (3) | 0.002 (4) | 0.000 (3) | −0.018 (3) |
| B5 | 0.035 (4) | 0.062 (5) | 0.023 (3) | −0.005 (4) | 0.002 (3) | 0.009 (3) |
| B6 | 0.026 (4) | 0.033 (4) | 0.036 (3) | 0.001 (3) | 0.002 (3) | 0.011 (3) |
| B7 | 0.029 (4) | 0.056 (5) | 0.038 (4) | 0.006 (4) | −0.007 (3) | 0.009 (4) |
| B8 | 0.022 (4) | 0.060 (5) | 0.032 (3) | −0.008 (4) | −0.001 (3) | −0.005 (4) |
| B9 | 0.033 (4) | 0.061 (6) | 0.045 (4) | −0.005 (4) | −0.008 (4) | −0.020 (4) |
| B10 | 0.036 (5) | 0.093 (7) | 0.020 (3) | 0.003 (5) | −0.011 (3) | −0.012 (4) |
| B11 | 0.033 (4) | 0.075 (6) | 0.034 (4) | 0.006 (4) | −0.004 (3) | 0.028 (4) |
| B12 | 0.031 (4) | 0.089 (7) | 0.031 (4) | 0.003 (5) | −0.008 (3) | −0.008 (4) |
| C1 | 0.023 (3) | 0.038 (3) | 0.023 (2) | −0.001 (2) | 0.001 (2) | −0.002 (2) |
| C2 | 0.026 (3) | 0.037 (3) | 0.027 (2) | 0.004 (2) | 0.002 (2) | −0.003 (2) |
| C3 | 0.027 (3) | 0.040 (3) | 0.030 (3) | −0.008 (3) | 0.001 (2) | −0.002 (2) |
| C4 | 0.038 (4) | 0.068 (4) | 0.046 (3) | −0.004 (3) | 0.009 (3) | −0.001 (3) |
| C5 | 0.041 (4) | 0.066 (4) | 0.047 (3) | −0.018 (4) | 0.003 (3) | −0.005 (3) |
| C6 | 0.033 (3) | 0.045 (3) | 0.037 (3) | 0.005 (3) | −0.003 (3) | −0.004 (3) |
| C7 | 0.063 (4) | 0.039 (4) | 0.071 (4) | 0.007 (4) | −0.009 (4) | −0.002 (3) |
| C8 | 0.047 (4) | 0.064 (4) | 0.064 (4) | 0.017 (4) | −0.011 (3) | 0.002 (4) |
| C9 | 0.042 (3) | 0.060 (4) | 0.055 (3) | 0.010 (3) | −0.004 (3) | −0.021 (3) |
| C10 | 0.083 (5) | 0.054 (5) | 0.096 (5) | 0.018 (4) | −0.004 (5) | −0.028 (4) |
| C11 | 0.073 (5) | 0.109 (5) | 0.075 (4) | 0.014 (4) | 0.001 (4) | −0.052 (4) |
| C12 | 0.038 (3) | 0.073 (4) | 0.039 (3) | −0.003 (3) | 0.006 (3) | 0.000 (3) |
| C13 | 0.041 (4) | 0.105 (5) | 0.062 (4) | −0.007 (4) | 0.016 (3) | −0.015 (4) |
| C14 | 0.081 (5) | 0.142 (7) | 0.057 (4) | −0.012 (5) | 0.005 (4) | 0.029 (5) |
Geometric parameters (Å, °)
| P1—C6 | 1.856 (6) | B10—B12 | 1.739 (10) |
| P1—C3 | 1.865 (5) | B10—B11 | 1.778 (11) |
| P1—C1 | 1.896 (5) | B10—H10 | 1.1000 |
| Hg1—Cl1 | 2.4482 (17) | B11—B12 | 1.769 (10) |
| Hg1—Cl2 | 2.4542 (17) | B11—H11 | 1.1000 |
| Hg1—P1 | 2.5200 (10) | B12—H12 | 1.1000 |
| Hg1—P2 | 2.5242 (16) | C1—C2 | 1.694 (6) |
| P2—C9 | 1.836 (7) | C3—C5 | 1.507 (7) |
| P2—C12 | 1.851 (6) | C3—C4 | 1.531 (7) |
| P2—C2 | 1.899 (5) | C3—H3A | 0.9800 |
| B3—C1 | 1.691 (8) | C4—H4A | 0.9600 |
| B3—C2 | 1.693 (8) | C4—H4B | 0.9600 |
| B3—B8 | 1.783 (9) | C4—H4C | 0.9600 |
| B3—B4 | 1.795 (8) | C5—H5A | 0.9600 |
| B3—B9 | 1.798 (9) | C5—H5B | 0.9600 |
| B3—H3 | 1.1000 | C5—H5C | 0.9600 |
| B4—C1 | 1.722 (8) | C6—C7 | 1.523 (8) |
| B4—B10 | 1.752 (10) | C6—C8 | 1.532 (7) |
| B4—B5 | 1.763 (10) | C6—H6A | 0.9800 |
| B4—B9 | 1.764 (10) | C7—H7A | 0.9600 |
| B4—H4 | 1.1000 | C7—H7B | 0.9600 |
| B5—C1 | 1.713 (7) | C7—H7C | 0.9600 |
| B5—B10 | 1.743 (10) | C8—H8A | 0.9600 |
| B5—B11 | 1.745 (10) | C8—H8B | 0.9600 |
| B5—B6 | 1.797 (9) | C8—H8C | 0.9600 |
| B5—H5 | 1.1000 | C9—C10 | 1.507 (9) |
| B6—C2 | 1.673 (8) | C9—C11 | 1.548 (9) |
| B6—C1 | 1.674 (8) | C9—H9A | 0.9800 |
| B6—B7 | 1.793 (9) | C10—H10A | 0.9600 |
| B6—B11 | 1.797 (9) | C10—H10B | 0.9600 |
| B6—H6 | 1.1000 | C10—H10C | 0.9600 |
| B7—C2 | 1.701 (8) | C11—H11A | 0.9600 |
| B7—B8 | 1.775 (10) | C11—H11B | 0.9600 |
| B7—B12 | 1.778 (10) | C11—H11C | 0.9600 |
| B7—B11 | 1.781 (9) | C12—C14 | 1.507 (8) |
| B7—H7 | 1.1000 | C12—C13 | 1.537 (8) |
| B8—C2 | 1.711 (8) | C12—H12A | 0.9800 |
| B8—B9 | 1.756 (9) | C13—H13A | 0.9600 |
| B8—B12 | 1.770 (9) | C13—H13B | 0.9600 |
| B8—H8 | 1.1000 | C13—H13C | 0.9600 |
| B9—B10 | 1.748 (11) | C14—H14A | 0.9600 |
| B9—B12 | 1.765 (11) | C14—H14B | 0.9600 |
| B9—H9 | 1.1000 | C14—H14C | 0.9600 |
| C6—P1—C3 | 106.3 (3) | B9—B10—H10 | 121.1 |
| C6—P1—C1 | 109.1 (2) | B4—B10—H10 | 120.9 |
| C3—P1—C1 | 104.6 (2) | B11—B10—H10 | 121.8 |
| C6—P1—Hg1 | 115.26 (18) | B5—B11—B12 | 107.1 (5) |
| C3—P1—Hg1 | 117.07 (17) | B5—B11—B10 | 59.3 (4) |
| C1—P1—Hg1 | 103.73 (13) | B12—B11—B10 | 58.7 (4) |
| Cl1—Hg1—Cl2 | 104.81 (6) | B5—B11—B7 | 109.3 (5) |
| Cl1—Hg1—P1 | 109.29 (6) | B12—B11—B7 | 60.1 (4) |
| Cl2—Hg1—P1 | 119.90 (5) | B10—B11—B7 | 107.3 (5) |
| Cl1—Hg1—P2 | 119.51 (6) | B5—B11—B6 | 60.9 (4) |
| Cl2—Hg1—P2 | 112.06 (6) | B12—B11—B6 | 107.3 (4) |
| P1—Hg1—P2 | 91.82 (4) | B10—B11—B6 | 107.0 (5) |
| C9—P2—C12 | 107.0 (3) | B7—B11—B6 | 60.1 (4) |
| C9—P2—C2 | 110.2 (3) | B5—B11—H11 | 121.2 |
| C12—P2—C2 | 104.8 (3) | B12—B11—H11 | 122.6 |
| C9—P2—Hg1 | 114.7 (2) | B10—B11—H11 | 123.0 |
| C12—P2—Hg1 | 116.5 (2) | B7—B11—H11 | 121.1 |
| C2—P2—Hg1 | 103.19 (15) | B6—B11—H11 | 121.8 |
| C1—B3—C2 | 60.0 (3) | B10—B12—B9 | 59.8 (4) |
| C1—B3—B8 | 106.7 (5) | B10—B12—B11 | 60.9 (4) |
| C2—B3—B8 | 58.9 (4) | B9—B12—B11 | 108.3 (5) |
| C1—B3—B4 | 59.1 (3) | B10—B12—B8 | 107.9 (5) |
| C2—B3—B4 | 106.8 (5) | B9—B12—B8 | 59.6 (4) |
| B8—B3—B4 | 106.8 (5) | B11—B12—B8 | 108.1 (5) |
| C1—B3—B9 | 104.6 (5) | B10—B12—B7 | 109.1 (5) |
| C2—B3—B9 | 104.5 (5) | B9—B12—B7 | 108.0 (5) |
| B8—B3—B9 | 58.7 (4) | B11—B12—B7 | 60.3 (4) |
| B4—B3—B9 | 58.8 (4) | B8—B12—B7 | 60.0 (4) |
| C1—B3—H3 | 122.8 | B10—B12—H12 | 121.2 |
| C2—B3—H3 | 122.9 | B9—B12—H12 | 122.0 |
| B8—B3—H3 | 122.5 | B11—B12—H12 | 121.3 |
| B4—B3—H3 | 122.3 | B8—B12—H12 | 122.0 |
| B9—B3—H3 | 124.4 | B7—B12—H12 | 121.3 |
| C1—B4—B10 | 104.7 (5) | B6—C1—B3 | 112.3 (4) |
| C1—B4—B5 | 58.9 (3) | B6—C1—C2 | 59.6 (3) |
| B10—B4—B5 | 59.4 (4) | B3—C1—C2 | 60.0 (3) |
| C1—B4—B9 | 104.8 (4) | B6—C1—B5 | 64.1 (4) |
| B10—B4—B9 | 59.6 (4) | B3—C1—B5 | 113.7 (4) |
| B5—B4—B9 | 107.1 (5) | C2—C1—B5 | 109.8 (4) |
| C1—B4—B3 | 57.5 (3) | B6—C1—B4 | 114.8 (4) |
| B10—B4—B3 | 107.4 (5) | B3—C1—B4 | 63.5 (4) |
| B5—B4—B3 | 106.4 (5) | C2—C1—B4 | 110.2 (4) |
| B9—B4—B3 | 60.7 (4) | B5—C1—B4 | 61.8 (4) |
| C1—B4—H4 | 124.8 | B6—C1—P1 | 114.3 (4) |
| B10—B4—H4 | 122.6 | B3—C1—P1 | 120.3 (4) |
| B5—B4—H4 | 122.3 | C2—C1—P1 | 120.1 (3) |
| B9—B4—H4 | 122.2 | B5—C1—P1 | 119.0 (3) |
| B3—B4—H4 | 122.2 | B4—C1—P1 | 122.0 (3) |
| C1—B5—B10 | 105.4 (5) | B6—C2—C1 | 59.6 (3) |
| C1—B5—B11 | 105.5 (4) | B6—C2—B3 | 112.3 (4) |
| B10—B5—B11 | 61.3 (4) | C1—C2—B3 | 60.0 (3) |
| C1—B5—B4 | 59.4 (3) | B6—C2—B7 | 64.2 (4) |
| B10—B5—B4 | 60.0 (4) | C1—C2—B7 | 110.8 (4) |
| B11—B5—B4 | 109.5 (5) | B3—C2—B7 | 114.9 (5) |
| C1—B5—B6 | 56.9 (3) | B6—C2—B8 | 114.9 (4) |
| B10—B5—B6 | 108.6 (5) | C1—C2—B8 | 110.0 (4) |
| B11—B5—B6 | 61.0 (4) | B3—C2—B8 | 63.2 (4) |
| B4—B5—B6 | 107.0 (4) | B7—C2—B8 | 62.7 (4) |
| C1—B5—H5 | 124.9 | B6—C2—P2 | 119.3 (4) |
| B10—B5—H5 | 121.6 | C1—C2—P2 | 120.9 (3) |
| B11—B5—H5 | 120.9 | B3—C2—P2 | 115.9 (4) |
| B4—B5—H5 | 121.4 | B7—C2—P2 | 120.1 (3) |
| B6—B5—H5 | 122.3 | B8—C2—P2 | 118.6 (3) |
| C2—B6—C1 | 60.8 (3) | C5—C3—C4 | 111.4 (5) |
| C2—B6—B7 | 58.7 (3) | C5—C3—P1 | 110.6 (4) |
| C1—B6—B7 | 107.3 (5) | C4—C3—P1 | 116.1 (4) |
| C2—B6—B5 | 106.8 (5) | C5—C3—H3A | 106.0 |
| C1—B6—B5 | 59.0 (3) | C4—C3—H3A | 106.0 |
| B7—B6—B5 | 106.5 (5) | P1—C3—H3A | 106.0 |
| C2—B6—B11 | 105.0 (5) | C3—C4—H4A | 109.5 |
| C1—B6—B11 | 104.9 (5) | C3—C4—H4B | 109.5 |
| B7—B6—B11 | 59.5 (4) | H4A—C4—H4B | 109.5 |
| B5—B6—B11 | 58.1 (4) | C3—C4—H4C | 109.5 |
| C2—B6—H6 | 122.6 | H4A—C4—H4C | 109.5 |
| C1—B6—H6 | 122.3 | H4B—C4—H4C | 109.5 |
| B7—B6—H6 | 122.3 | C3—C5—H5A | 109.5 |
| B5—B6—H6 | 122.8 | C3—C5—H5B | 109.5 |
| B11—B6—H6 | 124.1 | H5A—C5—H5B | 109.5 |
| C2—B7—B8 | 58.9 (4) | C3—C5—H5C | 109.5 |
| C2—B7—B12 | 104.8 (5) | H5A—C5—H5C | 109.5 |
| B8—B7—B12 | 59.8 (4) | H5B—C5—H5C | 109.5 |
| C2—B7—B11 | 104.6 (5) | C7—C6—C8 | 108.3 (5) |
| B8—B7—B11 | 107.3 (5) | C7—C6—P1 | 114.6 (4) |
| B12—B7—B11 | 59.6 (4) | C8—C6—P1 | 106.7 (4) |
| C2—B7—B6 | 57.1 (3) | C7—C6—H6A | 109.0 |
| B8—B7—B6 | 106.1 (5) | C8—C6—H6A | 109.0 |
| B12—B7—B6 | 107.1 (5) | P1—C6—H6A | 109.0 |
| B11—B7—B6 | 60.4 (4) | C6—C7—H7A | 109.5 |
| C2—B7—H7 | 124.8 | C6—C7—H7B | 109.5 |
| B8—B7—H7 | 122.1 | H7A—C7—H7B | 109.5 |
| B12—B7—H7 | 122.5 | C6—C7—H7C | 109.5 |
| B11—B7—H7 | 122.3 | H7A—C7—H7C | 109.5 |
| B6—B7—H7 | 122.7 | H7B—C7—H7C | 109.5 |
| C2—B8—B9 | 105.5 (5) | C6—C8—H8A | 109.5 |
| C2—B8—B12 | 104.7 (5) | C6—C8—H8B | 109.5 |
| B9—B8—B12 | 60.1 (4) | H8A—C8—H8B | 109.5 |
| C2—B8—B7 | 58.4 (3) | C6—C8—H8C | 109.5 |
| B9—B8—B7 | 108.5 (5) | H8A—C8—H8C | 109.5 |
| B12—B8—B7 | 60.2 (4) | H8B—C8—H8C | 109.5 |
| C2—B8—B3 | 57.9 (3) | C10—C9—C11 | 108.6 (6) |
| B9—B8—B3 | 61.1 (4) | C10—C9—P2 | 113.6 (5) |
| B12—B8—B3 | 108.1 (5) | C11—C9—P2 | 106.2 (5) |
| B7—B8—B3 | 107.0 (5) | C10—C9—H9A | 109.4 |
| C2—B8—H8 | 124.9 | C11—C9—H9A | 109.4 |
| B9—B8—H8 | 121.4 | P2—C9—H9A | 109.4 |
| B12—B8—H8 | 122.3 | C9—C10—H10A | 109.5 |
| B7—B8—H8 | 121.7 | C9—C10—H10B | 109.5 |
| B3—B8—H8 | 121.8 | H10A—C10—H10B | 109.5 |
| B10—B9—B8 | 108.2 (6) | C9—C10—H10C | 109.5 |
| B10—B9—B4 | 59.9 (4) | H10A—C10—H10C | 109.5 |
| B8—B9—B4 | 109.4 (5) | H10B—C10—H10C | 109.5 |
| B10—B9—B12 | 59.4 (4) | C9—C11—H11A | 109.5 |
| B8—B9—B12 | 60.4 (4) | C9—C11—H11B | 109.5 |
| B4—B9—B12 | 108.0 (6) | H11A—C11—H11B | 109.5 |
| B10—B9—B3 | 107.5 (5) | C9—C11—H11C | 109.5 |
| B8—B9—B3 | 60.2 (4) | H11A—C11—H11C | 109.5 |
| B4—B9—B3 | 60.5 (3) | H11B—C11—H11C | 109.5 |
| B12—B9—B3 | 107.7 (5) | C14—C12—C13 | 111.6 (5) |
| B10—B9—H9 | 122.2 | C14—C12—P2 | 113.2 (5) |
| B8—B9—H9 | 120.9 | C13—C12—P2 | 114.5 (5) |
| B4—B9—H9 | 121.1 | C14—C12—H12A | 105.6 |
| B12—B9—H9 | 122.1 | C13—C12—H12A | 105.6 |
| B3—B9—H9 | 121.9 | P2—C12—H12A | 105.6 |
| B12—B10—B5 | 108.6 (5) | C12—C13—H13A | 109.5 |
| B12—B10—B9 | 60.8 (4) | C12—C13—H13B | 109.5 |
| B5—B10—B9 | 108.7 (5) | H13A—C13—H13B | 109.5 |
| B12—B10—B4 | 109.6 (5) | C12—C13—H13C | 109.5 |
| B5—B10—B4 | 60.6 (4) | H13A—C13—H13C | 109.5 |
| B9—B10—B4 | 60.5 (4) | H13B—C13—H13C | 109.5 |
| B12—B10—B11 | 60.4 (4) | C12—C14—H14A | 109.5 |
| B5—B10—B11 | 59.4 (4) | C12—C14—H14B | 109.5 |
| B9—B10—B11 | 108.7 (5) | H14A—C14—H14B | 109.5 |
| B4—B10—B11 | 108.5 (5) | C12—C14—H14C | 109.5 |
| B12—B10—H10 | 120.8 | H14A—C14—H14C | 109.5 |
| B5—B10—H10 | 121.6 | H14B—C14—H14C | 109.5 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB2648).
References
- Bruker (2001). SMART, SAINT, SADABS and SHELXTL Bruker AXS Inc., Madison, Wisconsin, USA.
- Kivekäs, R., Sillanpää, R., Teixidor, F., Viñas, C., Nuñez, R. & Abad, M. (1995). Acta Cryst. C51, 1864–1868.
- Liu, L., Zhang, Q.-F. & Leung, W.-H. (2004). Acta Cryst. E60, m394–m395.
- Mariyatra, M. B., Panchanatheswaran, K., Low, J. N. & Glidewell, C. (2005). Acta Cryst. C61, m211–m214. [DOI] [PubMed]
- Paavola, S., Kivekäs, R., Teixidor, F. & Viñas, C. (2002). J. Organomet. Chem.606, 183–187.
- Paavola, S., Teixidor, F., Viñas, C. & Kivekäs, R. (2002a). Acta Cryst. C58, m237–m239. [DOI] [PubMed]
- Paavola, S., Teixidor, F., Viñas, C. & Kivekäs, R. (2002b). J. Organomet. Chem.645, 39–46.
- Sheldrick, G. M. (1997). SHELXL97 and SHELXS97 University of Göttingen, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807065130/hb2648sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807065130/hb2648Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report