Abstract
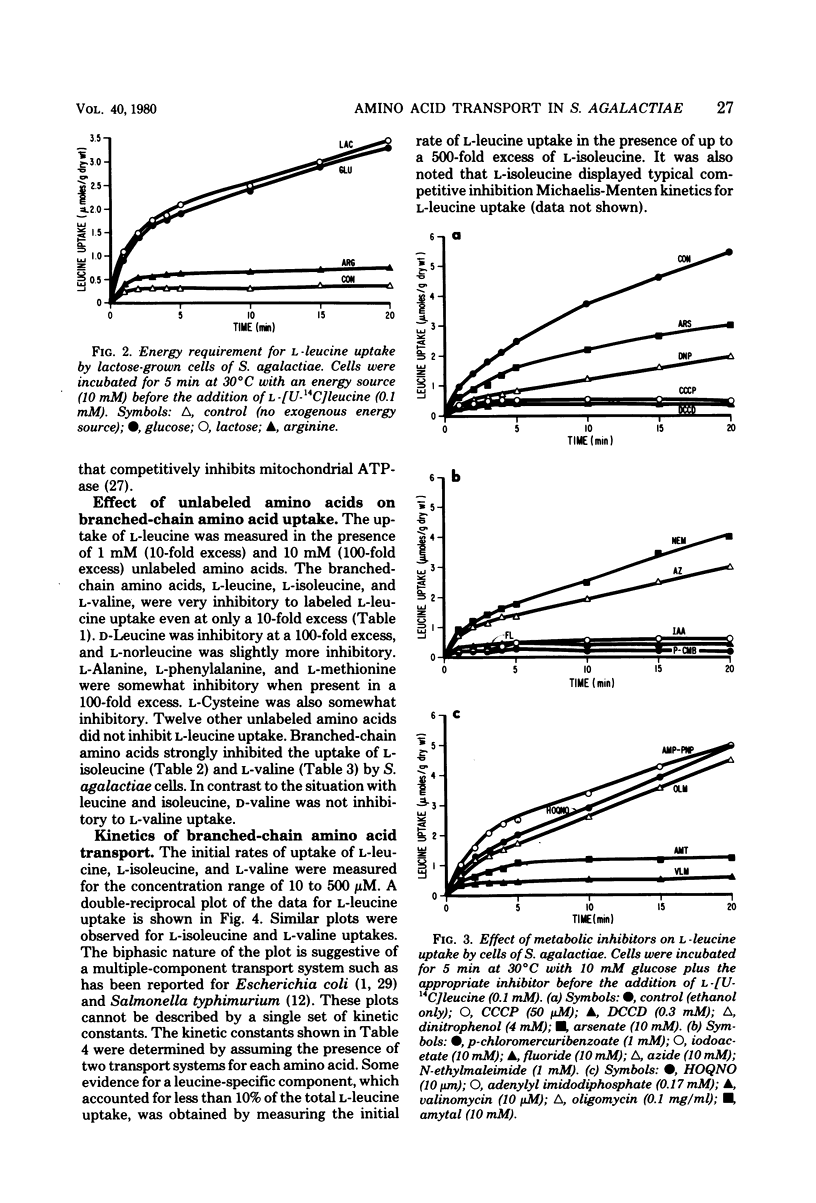
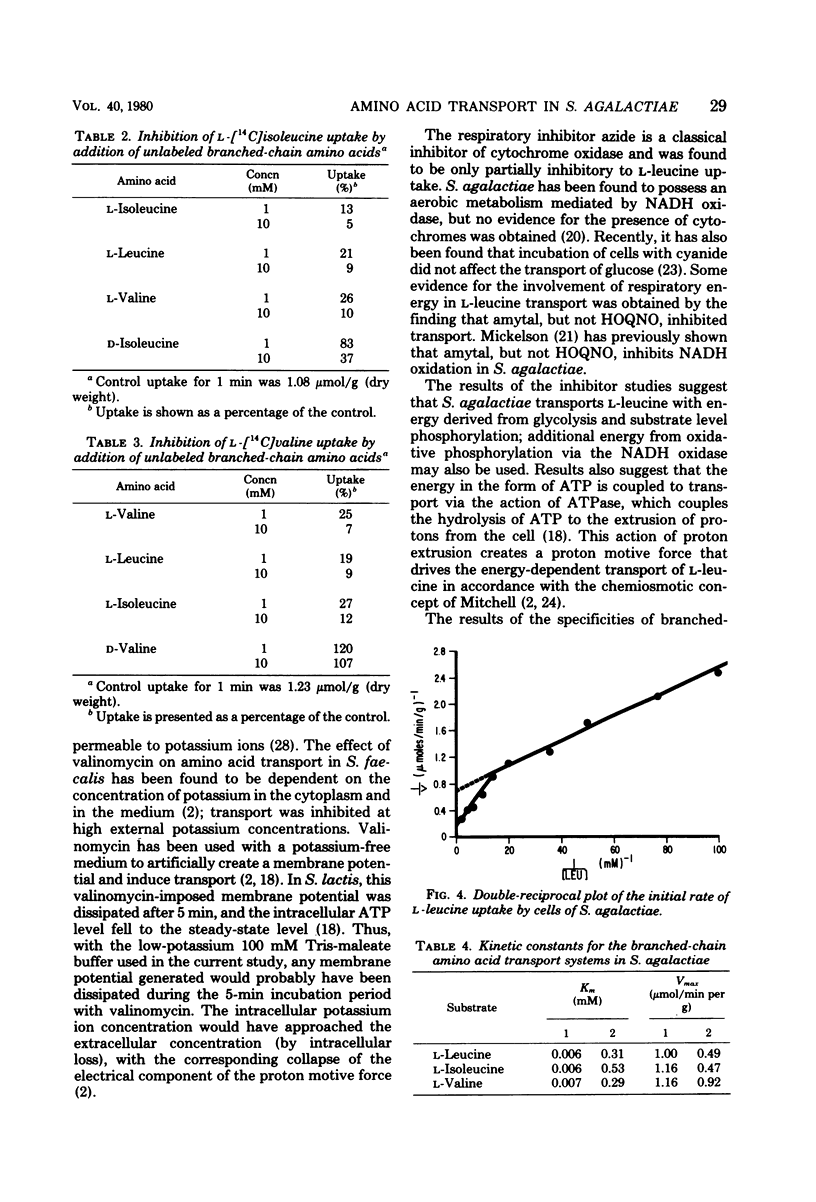
The transport of the branched-chain amino acids in Streptococcus agalactiae was characterized. Glucose-grown cells were able to utilize only glucose as an energy source for transport of L-leucine, whereas lactose-grown cells could utilize both glucose and lactose. It was determined from metabolic inhibitor studies that energy from glycolysis and substrate level phosphorylation was required for active transport. Energy was found to be coupled to transport by the action of adenosine triphosphatase and the generation of a proton motive force. The branched-chain amino acids were found to share a common transport system that may consist of multiple components.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- BROCK T. D., MOO-PENN G. An amino acid transport system in Streptococcus faecium. Arch Biochem Biophys. 1962 Aug;98:183–190. doi: 10.1016/0003-9861(62)90171-6. [DOI] [PubMed] [Google Scholar]
- Brown R. W. Compounds affecting Streptococcus agalactiae growth in milk. J Dairy Sci. 1974 Jul;57(7):797–802. doi: 10.3168/jds.S0022-0302(74)84967-2. [DOI] [PubMed] [Google Scholar]
- Brown R. W., Mickelson M. N. Lactoperoxidase, thiocyanate, and free cystine in bovine mammary secretions in early dry period and at the start of lactation and their effect on Streptococcus agalactiae growth. Am J Vet Res. 1979 Feb;40(2):250–255. [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Interaction of arsenate with phosphate-transport systems in wild- type and mutant Streptococcus faecalis. J Bacteriol. 1966 Jun;91(6):2257–2262. doi: 10.1128/jb.91.6.2257-2262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelínkoá J. Group B streptococci in the human population. Curr Top Microbiol Immunol. 1977;76:127–165. [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Kiritani K., Ohnishi K. Repression and inhibition of transport systems for branched-chain amino acids in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):589–598. doi: 10.1128/jb.129.2.589-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger M. J., Rothenburg B. A. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979 Jan 26;203(4378):374–376. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Kochan I., Wasynczuk J., McCabe M. A. Effects of injected iron and siderophores on infections in normal and immune mice. Infect Immun. 1978 Nov;22(2):560–567. doi: 10.1128/iai.22.2.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. ATP synthesis driven by a protonmotive force in Streptococcus lactis. J Membr Biol. 1975;25(3-4):285–310. doi: 10.1007/BF01868580. [DOI] [PubMed] [Google Scholar]
- McDonald J. S., McDonald T. J., Stark D. R. Antibiograms of streptococci isolated from bovine intramammary infections. Am J Vet Res. 1976 Oct;37(10):1185–1188. [PubMed] [Google Scholar]
- Mickelson M. N. Aerobic metabolism of Streptococcus agalactiae. J Bacteriol. 1967 Jul;94(1):184–191. doi: 10.1128/jb.94.1.184-191.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Effect of uncoupling agents and respiratory inhibitors on the growth of Streptococcus agalactiae. J Bacteriol. 1974 Nov;120(2):733–740. doi: 10.1128/jb.120.2.733-740.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Effects of nutritional characteristics of Streptococcus agalactiae on inhibition of growth by lactoperoxidase-thiocyanate-hydrogen peroxide in chemically defined culture medium. Appl Environ Microbiol. 1976 Aug;32(2):238–244. doi: 10.1128/aem.32.2.238-244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J Bacteriol. 1977 Nov;132(2):541–548. doi: 10.1128/jb.132.2.541-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Differential effects of adenylyl imidodiphosphate on adenosine triphosphate synthesis and the partial reactions of oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3579–3585. [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett N. P., Morse G. E. Long-chain fatty acid inhibition of growth of Streptococcus agalactiae in a chemically defined medium. J Bacteriol. 1966 Jun;91(6):2245–2250. doi: 10.1128/jb.91.6.2245-2250.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


