Abstract
The title compound, C41H70O12·CH4O, was prepared by microbial transformation. Within the steroid skeleton of the molecule, three six-membered rings exhibit a chair conformation, while the five -membered ring adopts an envelope conformation. The two pyranosyl rings also adopt chair conformations. The molecules are held together by an extensive O—H⋯O hydrogen-bonding network.
Related literature
For general background, see: He et al. (2005 ▶); Hu et al. (2007 ▶). For related literature, see: Li et al. (2006 ▶).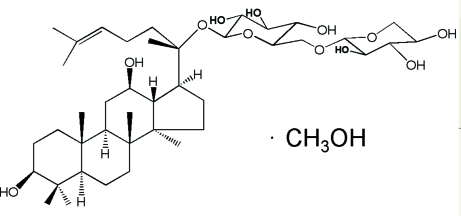
Experimental
Crystal data
C41H70O12·CH4O
M r = 787.01
Orthorhombic,
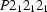
a = 8.3044 (7) Å
b = 13.2927 (11) Å
c = 38.964 (3) Å
V = 4301.1 (6) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 293 (2) K
0.43 × 0.31 × 0.21 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: none
25549 measured reflections
5269 independent reflections
3467 reflections with I > 2σ(I)
R int = 0.084
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.099
S = 0.89
5269 reflections
505 parameters
H-atom parameters constrained
Δρmax = 0.21 e Å−3
Δρmin = −0.16 e Å−3
Data collection: SMART (Bruker, 1999 ▶); cell refinement: SAINT (Bruker, 1999 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: SHELXTL (Bruker, 1997 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807063118/xu2351sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807063118/xu2351Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O11i | 0.82 | 2.38 | 2.996 (3) | 133 |
| O2—H2⋯O3 | 0.83 | 1.96 | 2.752 (3) | 160 |
| O5—H5A⋯O1i | 0.84 | 2.14 | 2.839 (3) | 140 |
| O6—H6⋯O12ii | 0.83 | 2.04 | 2.781 (3) | 147 |
| O7—H7⋯O8iii | 0.83 | 2.31 | 3.009 (3) | 142 |
| O10—H10⋯O13iv | 0.82 | 2.05 | 2.824 (4) | 156 |
| O11—H11⋯O5v | 0.81 | 1.92 | 2.703 (3) | 162 |
| O12—H12A⋯O11vi | 0.84 | 2.02 | 2.827 (3) | 162 |
| O13—H13A⋯O2vii | 0.85 | 1.98 | 2.823 (3) | 173 |
Symmetry codes: (i) 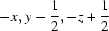 ; (ii)
; (ii) 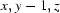 ; (iii)
; (iii) 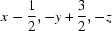 ; (iv)
; (iv) 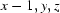 ; (v)
; (v) 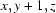 ; (vi)
; (vi) 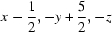 ; (vii)
; (vii) 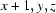 .
.
Acknowledgments
We gratefully acknowledge financial support from the SK Shanghai Foundation. We sincerely thank Dr J. Sun of Shanghai Institute of Organic Chemistry for assistance with the data collection.
supplementary crystallographic information
Comment
20-O-β-D-xylopyranosyl(1→6)-β-D-glucopyranosyl-20(S)- protopanaxadiol is a kind of rare gensenoside, found existing in notoginseng. In recent studies, the compound has been related to an anticancer agent. It is believed to have activities including: cytotoxicity to and partial reversal of multidrug resistance of human tumor cells (He et al., 2005). Besides that, the compound may be an important precurosor metabolite of Compound K, which is also a potential anticancer agent, during the process of microbial transformation of gisenoside Rb3 (Hu et al., 2007). In this article, the crystal structure is reported.
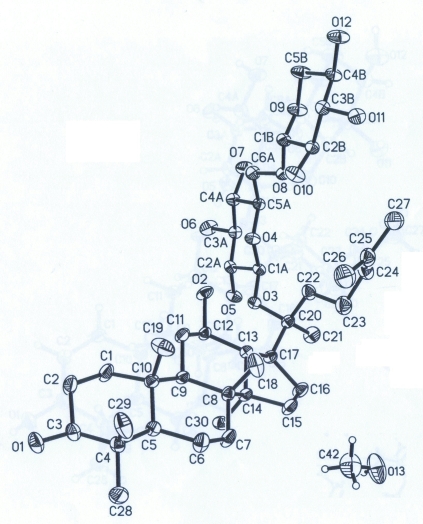
The structure mainly consists of a protopanaxadiol moiety with a disaccharide group. The bond distances and angels are normal. The C24?C25 of 1.313 (5) Å shows a typical double bound. Within the steroid skeleton of the molecule, three six membered rings all display the chair conformation, while a five membered ring displays an envelope conformation. Two pyranosyl rings are also exist in chair conformation. Extensive O—H···O hydrogen bonding occurs in the crystal structure (Table 1), which helps to stabilize the crystal structure.
Experimental
The Fermentation broth of ginsenoside Rb3 (300 mg) was centrifuged and the precipitation was extracted with EtOH for 24 h. Removal of the EtOH from the extract under reduced pressure gave crude extract. And the extract was subjected to silica gel column chromatography, eluting with HCCl3:CH3OH (10:1→7:3→5:1) to afford 12 fractions. Recrystallizing of fractions 8~10 yielded ginsenoside Rb3 100 mg. Solvent loss technique was then employed for the growth of crystals at room temperature, using methanol as the solvent.
Refinement
Hydroxyl H atoms were located in a Fourier map and refined as riding in as-found relative positions with Uiso(H) = 1.2Ueq(O). Other H atoms were placed in geometrically calculated positions with C—H = 0.93–0.98 Å and constrained to ride on their parental atoms with Uiso(H) = 1.2Ueq(C). Torsonal angles for methyl groups were refined to fit the electron density. In absence of significant anomalous scattering, Friedel pairs were meged.
Figures
Fig. 1.
The molecular structure of (I), with atom labels and 50% probability displacement ellipsoids for non-H atoms.
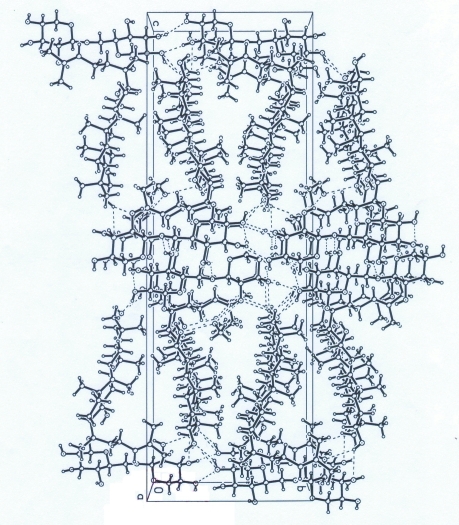
Fig. 2.
The packing of (I), viewed down the c axis. H atoms not involved in hydrogen bonding have been omitted.
Crystal data
| C41H70O12·C1H4O1 | F000 = 1720 |
| Mr = 787.01 | Dx = 1.215 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 3975 reflections |
| a = 8.3044 (7) Å | θ = 2.2–20.3º |
| b = 13.2927 (11) Å | µ = 0.09 mm−1 |
| c = 38.964 (3) Å | T = 293 (2) K |
| V = 4301.1 (6) Å3 | Prismatic, colorless |
| Z = 4 | 0.43 × 0.31 × 0.21 mm |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 3467 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.084 |
| Monochromator: graphite | θmax = 27.0º |
| T = 293(2) K | θmin = 1.6º |
| φ and ω scans | h = −8→10 |
| Absorption correction: none | k = −16→16 |
| 25549 measured reflections | l = −49→36 |
| 5269 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.099 | w = 1/[σ2(Fo2) + (0.0447P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.89 | (Δ/σ)max = 0.001 |
| 5269 reflections | Δρmax = 0.21 e Å−3 |
| 505 parameters | Δρmin = −0.16 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.1448 (3) | 0.78606 (18) | 0.37658 (5) | 0.0555 (6) | |
| H1 | −0.1513 | 0.7395 | 0.3903 | 0.067* | |
| O2 | 0.0242 (2) | 0.70266 (16) | 0.15197 (5) | 0.0478 (6) | |
| H2 | 0.0585 | 0.6714 | 0.1351 | 0.057* | |
| O3 | 0.2082 (2) | 0.59757 (13) | 0.10544 (5) | 0.0332 (5) | |
| O4 | 0.0549 (2) | 0.69683 (12) | 0.07077 (5) | 0.0355 (5) | |
| O5 | 0.1065 (3) | 0.42488 (12) | 0.06866 (5) | 0.0410 (5) | |
| H5A | 0.1328 | 0.4117 | 0.0891 | 0.049* | |
| O6 | −0.1631 (3) | 0.45653 (15) | 0.02402 (6) | 0.0564 (7) | |
| H6 | −0.1048 | 0.4060 | 0.0217 | 0.068* | |
| O7 | −0.1595 (3) | 0.65240 (15) | −0.00867 (6) | 0.0554 (6) | |
| H7 | −0.2445 | 0.6200 | −0.0101 | 0.066* | |
| O8 | 0.0245 (2) | 0.89395 (12) | 0.04299 (5) | 0.0383 (5) | |
| O9 | −0.0675 (3) | 0.99980 (13) | 0.00267 (5) | 0.0494 (6) | |
| O10 | 0.0865 (3) | 1.06672 (15) | 0.08776 (5) | 0.0536 (6) | |
| H10 | 0.0749 | 1.0105 | 0.0963 | 0.064* | |
| O11 | 0.1701 (2) | 1.23411 (14) | 0.04880 (5) | 0.0436 (5) | |
| H11 | 0.1346 | 1.2905 | 0.0517 | 0.052* | |
| O12 | −0.0620 (3) | 1.27492 (13) | −0.00414 (6) | 0.0588 (7) | |
| H12A | −0.1258 | 1.2720 | −0.0208 | 0.071* | |
| O13 | 1.0284 (5) | 0.9088 (2) | 0.13462 (9) | 0.1152 (12) | |
| H13A | 1.0334 | 0.8464 | 0.1387 | 0.138* | |
| C1 | −0.0982 (4) | 0.7430 (3) | 0.28168 (8) | 0.0577 (10) | |
| H1A | −0.1708 | 0.7610 | 0.2632 | 0.069* | |
| H1B | −0.0866 | 0.6704 | 0.2816 | 0.069* | |
| C2 | −0.1738 (4) | 0.7753 (3) | 0.31584 (8) | 0.0598 (10) | |
| H2A | −0.1978 | 0.8467 | 0.3150 | 0.072* | |
| H2B | −0.2744 | 0.7395 | 0.3191 | 0.072* | |
| C3 | −0.0647 (4) | 0.7546 (2) | 0.34554 (8) | 0.0437 (8) | |
| H3 | −0.0490 | 0.6816 | 0.3469 | 0.052* | |
| C4 | 0.1004 (4) | 0.8032 (2) | 0.34246 (7) | 0.0388 (7) | |
| C5 | 0.1721 (3) | 0.7717 (2) | 0.30679 (7) | 0.0353 (7) | |
| H5 | 0.1791 | 0.6982 | 0.3081 | 0.042* | |
| C6 | 0.3460 (4) | 0.8051 (3) | 0.30089 (8) | 0.0550 (9) | |
| H6A | 0.3482 | 0.8764 | 0.2956 | 0.066* | |
| H6B | 0.4080 | 0.7945 | 0.3217 | 0.066* | |
| C7 | 0.4216 (4) | 0.7463 (3) | 0.27159 (8) | 0.0556 (10) | |
| H7A | 0.4276 | 0.6760 | 0.2781 | 0.067* | |
| H7B | 0.5309 | 0.7702 | 0.2682 | 0.067* | |
| C8 | 0.3295 (3) | 0.7549 (2) | 0.23745 (7) | 0.0361 (7) | |
| C9 | 0.1476 (3) | 0.7339 (2) | 0.24388 (7) | 0.0326 (7) | |
| H9 | 0.1429 | 0.6627 | 0.2503 | 0.039* | |
| C10 | 0.0674 (4) | 0.7909 (2) | 0.27473 (7) | 0.0368 (7) | |
| C11 | 0.0535 (4) | 0.7403 (2) | 0.21037 (7) | 0.0433 (8) | |
| H11A | −0.0575 | 0.7222 | 0.2150 | 0.052* | |
| H11B | 0.0542 | 0.8098 | 0.2027 | 0.052* | |
| C12 | 0.1155 (3) | 0.6744 (2) | 0.18123 (7) | 0.0343 (7) | |
| H12 | 0.0924 | 0.6039 | 0.1867 | 0.041* | |
| C13 | 0.2970 (3) | 0.6870 (2) | 0.17658 (7) | 0.0299 (6) | |
| H13 | 0.3148 | 0.7565 | 0.1691 | 0.036* | |
| C14 | 0.3875 (3) | 0.6743 (2) | 0.21106 (7) | 0.0348 (7) | |
| C15 | 0.5622 (4) | 0.6825 (3) | 0.19860 (8) | 0.0508 (9) | |
| H15A | 0.6353 | 0.6525 | 0.2151 | 0.061* | |
| H15B | 0.5921 | 0.7523 | 0.1952 | 0.061* | |
| C16 | 0.5670 (4) | 0.6248 (3) | 0.16445 (8) | 0.0510 (9) | |
| H16A | 0.6102 | 0.5577 | 0.1679 | 0.061* | |
| H16B | 0.6344 | 0.6598 | 0.1480 | 0.061* | |
| C17 | 0.3895 (3) | 0.6188 (2) | 0.15113 (7) | 0.0349 (7) | |
| H17 | 0.3529 | 0.5499 | 0.1555 | 0.042* | |
| C18 | 0.3600 (5) | 0.8615 (2) | 0.22343 (9) | 0.0596 (10) | |
| H18A | 0.2832 | 0.8762 | 0.2057 | 0.089* | |
| H18B | 0.4669 | 0.8652 | 0.2141 | 0.089* | |
| H18C | 0.3490 | 0.9095 | 0.2417 | 0.089* | |
| C19 | 0.0399 (5) | 0.9034 (2) | 0.26662 (9) | 0.0659 (11) | |
| H19A | 0.0007 | 0.9103 | 0.2436 | 0.099* | |
| H19B | 0.1397 | 0.9392 | 0.2689 | 0.099* | |
| H19C | −0.0378 | 0.9306 | 0.2823 | 0.099* | |
| C20 | 0.3724 (3) | 0.6361 (2) | 0.11227 (7) | 0.0355 (7) | |
| C21 | 0.4888 (4) | 0.5676 (2) | 0.09287 (8) | 0.0553 (9) | |
| H21A | 0.4776 | 0.4998 | 0.1011 | 0.083* | |
| H21B | 0.5973 | 0.5901 | 0.0966 | 0.083* | |
| H21C | 0.4647 | 0.5698 | 0.0688 | 0.083* | |
| C22 | 0.3795 (4) | 0.7460 (2) | 0.10016 (8) | 0.0459 (8) | |
| H22A | 0.3657 | 0.7460 | 0.0754 | 0.055* | |
| H22B | 0.2871 | 0.7805 | 0.1098 | 0.055* | |
| C23 | 0.5271 (5) | 0.8082 (3) | 0.10821 (10) | 0.0629 (10) | |
| H23A | 0.5320 | 0.8208 | 0.1327 | 0.075* | |
| H23B | 0.6229 | 0.7711 | 0.1017 | 0.075* | |
| C24 | 0.5236 (4) | 0.9067 (3) | 0.08935 (10) | 0.0587 (10) | |
| H24 | 0.5410 | 0.9030 | 0.0658 | 0.070* | |
| C25 | 0.4993 (4) | 0.9973 (3) | 0.10175 (9) | 0.0549 (9) | |
| C26 | 0.4625 (6) | 1.0201 (3) | 0.13858 (11) | 0.0854 (13) | |
| H26A | 0.3543 | 1.0450 | 0.1405 | 0.128* | |
| H26B | 0.5362 | 1.0702 | 0.1469 | 0.128* | |
| H26C | 0.4734 | 0.9600 | 0.1520 | 0.128* | |
| C27 | 0.5084 (5) | 1.0876 (3) | 0.07860 (10) | 0.0718 (12) | |
| H27A | 0.6062 | 1.1238 | 0.0831 | 0.108* | |
| H27B | 0.4178 | 1.1307 | 0.0828 | 0.108* | |
| H27C | 0.5071 | 1.0660 | 0.0551 | 0.108* | |
| C28 | 0.2058 (4) | 0.7593 (3) | 0.37114 (8) | 0.0678 (11) | |
| H28A | 0.3092 | 0.7916 | 0.3709 | 0.102* | |
| H28B | 0.2194 | 0.6884 | 0.3675 | 0.102* | |
| H28C | 0.1549 | 0.7705 | 0.3929 | 0.102* | |
| C29 | 0.0899 (5) | 0.9178 (2) | 0.34767 (9) | 0.0641 (11) | |
| H29A | −0.0138 | 0.9413 | 0.3403 | 0.096* | |
| H29B | 0.1725 | 0.9502 | 0.3344 | 0.096* | |
| H29C | 0.1045 | 0.9333 | 0.3715 | 0.096* | |
| C30 | 0.3678 (4) | 0.5653 (2) | 0.22474 (8) | 0.0505 (9) | |
| H30A | 0.3915 | 0.5184 | 0.2067 | 0.076* | |
| H30B | 0.2591 | 0.5555 | 0.2324 | 0.076* | |
| H30C | 0.4406 | 0.5547 | 0.2435 | 0.076* | |
| C42 | 0.8920 (6) | 0.9444 (3) | 0.15107 (13) | 0.1016 (17) | |
| H42A | 0.8403 | 0.8901 | 0.1630 | 0.152* | |
| H42B | 0.8192 | 0.9721 | 0.1344 | 0.152* | |
| H42C | 0.9220 | 0.9957 | 0.1672 | 0.152* | |
| C1A | 0.1450 (3) | 0.60535 (18) | 0.07231 (7) | 0.0301 (7) | |
| H1A1 | 0.2311 | 0.6051 | 0.0551 | 0.036* | |
| C2A | 0.0281 (4) | 0.52007 (18) | 0.06611 (7) | 0.0316 (7) | |
| H2A1 | −0.0585 | 0.5235 | 0.0832 | 0.038* | |
| C3A | −0.0432 (4) | 0.53004 (18) | 0.03063 (7) | 0.0331 (7) | |
| H3A | 0.0430 | 0.5229 | 0.0136 | 0.040* | |
| C4A | −0.1214 (4) | 0.6325 (2) | 0.02629 (7) | 0.0366 (7) | |
| H4A | −0.2199 | 0.6355 | 0.0401 | 0.044* | |
| C5A | −0.0067 (3) | 0.71584 (18) | 0.03752 (7) | 0.0317 (7) | |
| H5A1 | 0.0830 | 0.7198 | 0.0212 | 0.038* | |
| C6A | −0.0913 (4) | 0.8157 (2) | 0.03872 (9) | 0.0416 (8) | |
| H6A1 | −0.1508 | 0.8262 | 0.0176 | 0.050* | |
| H6A2 | −0.1670 | 0.8167 | 0.0577 | 0.050* | |
| C1B | −0.0405 (4) | 0.98966 (18) | 0.03842 (8) | 0.0361 (7) | |
| H1B1 | −0.1422 | 0.9959 | 0.0510 | 0.043* | |
| C2B | 0.0804 (4) | 1.06560 (19) | 0.05159 (7) | 0.0337 (7) | |
| H2B1 | 0.1873 | 1.0487 | 0.0426 | 0.040* | |
| C3B | 0.0367 (4) | 1.17108 (18) | 0.04037 (7) | 0.0347 (7) | |
| H3B | −0.0572 | 1.1934 | 0.0536 | 0.042* | |
| C4B | −0.0042 (4) | 1.17607 (19) | 0.00276 (8) | 0.0400 (8) | |
| H4B | 0.0935 | 1.1640 | −0.0107 | 0.048* | |
| C5B | −0.1275 (5) | 1.0974 (2) | −0.00591 (9) | 0.0551 (10) | |
| H5B1 | −0.2259 | 1.1104 | 0.0067 | 0.066* | |
| H5B2 | −0.1521 | 1.1002 | −0.0302 | 0.066* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0617 (15) | 0.0692 (16) | 0.0356 (13) | −0.0109 (13) | 0.0146 (11) | −0.0030 (11) |
| O2 | 0.0348 (12) | 0.0773 (15) | 0.0312 (11) | 0.0163 (11) | −0.0092 (10) | −0.0133 (11) |
| O3 | 0.0385 (11) | 0.0318 (10) | 0.0293 (11) | 0.0001 (9) | −0.0021 (9) | −0.0018 (9) |
| O4 | 0.0495 (12) | 0.0212 (9) | 0.0356 (11) | 0.0047 (9) | −0.0054 (10) | −0.0023 (9) |
| O5 | 0.0643 (15) | 0.0236 (10) | 0.0350 (11) | 0.0072 (10) | −0.0016 (11) | −0.0019 (9) |
| O6 | 0.0563 (15) | 0.0287 (11) | 0.0840 (18) | −0.0062 (11) | −0.0208 (14) | −0.0112 (12) |
| O7 | 0.0634 (16) | 0.0416 (12) | 0.0613 (16) | −0.0077 (12) | −0.0271 (13) | 0.0009 (11) |
| O8 | 0.0368 (11) | 0.0198 (9) | 0.0585 (14) | −0.0011 (9) | −0.0072 (10) | 0.0026 (9) |
| O9 | 0.0730 (16) | 0.0220 (10) | 0.0533 (14) | −0.0001 (11) | −0.0240 (13) | −0.0018 (10) |
| O10 | 0.0816 (17) | 0.0382 (12) | 0.0410 (13) | −0.0050 (12) | −0.0082 (13) | 0.0031 (10) |
| O11 | 0.0510 (13) | 0.0217 (10) | 0.0580 (14) | −0.0023 (10) | −0.0099 (11) | −0.0066 (10) |
| O12 | 0.0804 (17) | 0.0240 (11) | 0.0721 (16) | 0.0042 (11) | −0.0285 (14) | 0.0032 (11) |
| O13 | 0.132 (3) | 0.078 (2) | 0.136 (3) | 0.024 (2) | 0.057 (2) | 0.036 (2) |
| C1 | 0.0291 (17) | 0.105 (3) | 0.0393 (19) | −0.0002 (19) | −0.0025 (16) | −0.012 (2) |
| C2 | 0.0330 (18) | 0.106 (3) | 0.041 (2) | −0.003 (2) | 0.0072 (16) | −0.007 (2) |
| C3 | 0.0475 (19) | 0.0480 (18) | 0.0355 (18) | −0.0041 (16) | 0.0082 (16) | −0.0025 (15) |
| C4 | 0.0415 (18) | 0.0449 (18) | 0.0299 (16) | −0.0049 (15) | 0.0018 (14) | −0.0048 (14) |
| C5 | 0.0366 (17) | 0.0378 (16) | 0.0315 (16) | −0.0015 (14) | −0.0014 (13) | −0.0074 (14) |
| C6 | 0.041 (2) | 0.087 (3) | 0.0374 (19) | −0.0151 (19) | −0.0019 (16) | −0.0193 (19) |
| C7 | 0.0280 (17) | 0.099 (3) | 0.0398 (19) | −0.0020 (19) | −0.0025 (15) | −0.019 (2) |
| C8 | 0.0297 (16) | 0.0453 (18) | 0.0332 (16) | −0.0040 (14) | −0.0014 (13) | −0.0067 (14) |
| C9 | 0.0279 (15) | 0.0382 (16) | 0.0316 (16) | 0.0010 (13) | −0.0045 (13) | −0.0024 (13) |
| C10 | 0.0335 (17) | 0.0454 (18) | 0.0315 (16) | 0.0072 (14) | −0.0002 (13) | −0.0004 (14) |
| C11 | 0.0276 (16) | 0.066 (2) | 0.0360 (18) | 0.0071 (16) | −0.0014 (14) | −0.0041 (16) |
| C12 | 0.0250 (15) | 0.0467 (18) | 0.0311 (16) | −0.0025 (13) | −0.0038 (13) | −0.0037 (14) |
| C13 | 0.0284 (15) | 0.0306 (15) | 0.0306 (16) | 0.0018 (13) | −0.0016 (13) | −0.0036 (13) |
| C14 | 0.0273 (16) | 0.0434 (17) | 0.0339 (17) | 0.0031 (14) | −0.0044 (13) | −0.0042 (14) |
| C15 | 0.0286 (18) | 0.080 (2) | 0.043 (2) | 0.0047 (18) | −0.0049 (15) | −0.0126 (19) |
| C16 | 0.0325 (18) | 0.070 (2) | 0.051 (2) | 0.0158 (18) | 0.0010 (16) | −0.0043 (18) |
| C17 | 0.0328 (16) | 0.0348 (16) | 0.0372 (17) | 0.0065 (13) | −0.0019 (14) | −0.0043 (14) |
| C18 | 0.075 (3) | 0.049 (2) | 0.054 (2) | −0.023 (2) | 0.022 (2) | −0.0168 (18) |
| C19 | 0.101 (3) | 0.055 (2) | 0.041 (2) | 0.033 (2) | 0.015 (2) | 0.0047 (17) |
| C20 | 0.0318 (16) | 0.0403 (16) | 0.0345 (17) | 0.0049 (14) | −0.0002 (14) | −0.0065 (14) |
| C21 | 0.054 (2) | 0.071 (2) | 0.042 (2) | 0.0104 (19) | 0.0031 (18) | −0.0154 (18) |
| C22 | 0.048 (2) | 0.054 (2) | 0.0360 (18) | −0.0120 (17) | −0.0008 (15) | 0.0009 (16) |
| C23 | 0.060 (2) | 0.066 (2) | 0.063 (2) | −0.015 (2) | 0.001 (2) | 0.002 (2) |
| C24 | 0.064 (2) | 0.056 (2) | 0.056 (2) | −0.018 (2) | 0.001 (2) | 0.0047 (19) |
| C25 | 0.048 (2) | 0.061 (2) | 0.056 (2) | −0.0144 (18) | −0.0009 (18) | 0.001 (2) |
| C26 | 0.091 (3) | 0.078 (3) | 0.087 (3) | −0.011 (3) | 0.004 (3) | −0.005 (3) |
| C27 | 0.066 (3) | 0.061 (2) | 0.089 (3) | −0.011 (2) | −0.016 (2) | 0.012 (2) |
| C28 | 0.055 (2) | 0.114 (3) | 0.0339 (19) | 0.005 (2) | −0.0049 (17) | −0.003 (2) |
| C29 | 0.089 (3) | 0.054 (2) | 0.049 (2) | −0.020 (2) | 0.025 (2) | −0.0180 (18) |
| C30 | 0.058 (2) | 0.053 (2) | 0.0408 (19) | 0.0216 (18) | −0.0010 (17) | 0.0010 (16) |
| C42 | 0.098 (4) | 0.079 (3) | 0.128 (4) | 0.024 (3) | 0.011 (4) | 0.027 (3) |
| C1A | 0.0401 (17) | 0.0214 (14) | 0.0289 (16) | 0.0023 (13) | 0.0021 (14) | −0.0015 (12) |
| C2A | 0.0420 (17) | 0.0181 (13) | 0.0348 (16) | 0.0017 (13) | 0.0071 (14) | −0.0037 (12) |
| C3A | 0.0397 (17) | 0.0236 (14) | 0.0359 (17) | −0.0028 (13) | −0.0034 (14) | −0.0079 (13) |
| C4A | 0.0425 (18) | 0.0263 (14) | 0.0410 (18) | −0.0012 (14) | −0.0051 (15) | −0.0031 (13) |
| C5A | 0.0357 (16) | 0.0266 (14) | 0.0329 (16) | −0.0017 (13) | −0.0021 (13) | −0.0001 (12) |
| C6A | 0.0417 (18) | 0.0252 (15) | 0.058 (2) | −0.0049 (14) | −0.0047 (16) | 0.0017 (15) |
| C1B | 0.0426 (18) | 0.0196 (14) | 0.0460 (19) | −0.0010 (13) | −0.0028 (15) | 0.0020 (13) |
| C2B | 0.0388 (17) | 0.0283 (15) | 0.0340 (17) | 0.0006 (13) | −0.0004 (14) | −0.0013 (13) |
| C3B | 0.0406 (18) | 0.0214 (14) | 0.0420 (18) | −0.0009 (13) | −0.0003 (15) | −0.0062 (13) |
| C4B | 0.055 (2) | 0.0183 (14) | 0.0469 (19) | 0.0024 (14) | −0.0043 (16) | 0.0011 (13) |
| C5B | 0.079 (3) | 0.0264 (16) | 0.059 (2) | 0.0001 (17) | −0.028 (2) | 0.0042 (16) |
Geometric parameters (Å, °)
| O1—C3 | 1.442 (3) | C16—C17 | 1.565 (4) |
| O1—H1 | 0.8206 | C16—H16A | 0.9700 |
| O2—C12 | 1.419 (3) | C16—H16B | 0.9700 |
| O2—H2 | 0.8267 | C17—C20 | 1.538 (4) |
| O3—C1A | 1.398 (3) | C17—H17 | 0.9800 |
| O3—C20 | 1.481 (3) | C18—H18A | 0.9600 |
| O4—C5A | 1.416 (3) | C18—H18B | 0.9600 |
| O4—C1A | 1.429 (3) | C18—H18C | 0.9600 |
| O5—C2A | 1.426 (3) | C19—H19A | 0.9600 |
| O5—H5A | 0.8444 | C19—H19B | 0.9600 |
| O6—C3A | 1.418 (3) | C19—H19C | 0.9600 |
| O6—H6 | 0.8326 | C20—C21 | 1.528 (4) |
| O7—C4A | 1.423 (3) | C20—C22 | 1.536 (4) |
| O7—H7 | 0.8293 | C21—H21A | 0.9600 |
| O8—C1B | 1.393 (3) | C21—H21B | 0.9600 |
| O8—C6A | 1.426 (3) | C21—H21C | 0.9600 |
| O9—C1B | 1.418 (3) | C22—C23 | 1.512 (4) |
| O9—C5B | 1.429 (3) | C22—H22A | 0.9700 |
| O10—C2B | 1.410 (3) | C22—H22B | 0.9700 |
| O10—H10 | 0.8225 | C23—C24 | 1.501 (5) |
| O11—C3B | 1.427 (3) | C23—H23A | 0.9700 |
| O11—H11 | 0.8128 | C23—H23B | 0.9700 |
| O12—C4B | 1.424 (3) | C24—C25 | 1.313 (5) |
| O12—H12A | 0.8392 | C24—H24 | 0.9300 |
| O13—C42 | 1.385 (5) | C25—C26 | 1.498 (5) |
| O13—H13A | 0.8463 | C25—C27 | 1.503 (5) |
| C1—C2 | 1.533 (4) | C26—H26A | 0.9600 |
| C1—C10 | 1.539 (4) | C26—H26B | 0.9600 |
| C1—H1A | 0.9700 | C26—H26C | 0.9600 |
| C1—H1B | 0.9700 | C27—H27A | 0.9600 |
| C2—C3 | 1.495 (4) | C27—H27B | 0.9600 |
| C2—H2A | 0.9700 | C27—H27C | 0.9600 |
| C2—H2B | 0.9700 | C28—H28A | 0.9600 |
| C3—C4 | 1.520 (4) | C28—H28B | 0.9600 |
| C3—H3 | 0.9800 | C28—H28C | 0.9600 |
| C4—C28 | 1.535 (4) | C29—H29A | 0.9600 |
| C4—C29 | 1.539 (4) | C29—H29B | 0.9600 |
| C4—C5 | 1.569 (4) | C29—H29C | 0.9600 |
| C5—C6 | 1.529 (4) | C30—H30A | 0.9600 |
| C5—C10 | 1.543 (4) | C30—H30B | 0.9600 |
| C5—H5 | 0.9800 | C30—H30C | 0.9600 |
| C6—C7 | 1.519 (4) | C42—H42A | 0.9600 |
| C6—H6A | 0.9700 | C42—H42B | 0.9600 |
| C6—H6B | 0.9700 | C42—H42C | 0.9600 |
| C7—C8 | 1.539 (4) | C1A—C2A | 1.511 (4) |
| C7—H7A | 0.9700 | C1A—H1A1 | 0.9800 |
| C7—H7B | 0.9700 | C2A—C3A | 1.510 (4) |
| C8—C18 | 1.539 (4) | C2A—H2A1 | 0.9800 |
| C8—C9 | 1.557 (4) | C3A—C4A | 1.518 (4) |
| C8—C14 | 1.562 (4) | C3A—H3A | 0.9800 |
| C9—C11 | 1.524 (4) | C4A—C5A | 1.526 (4) |
| C9—C10 | 1.569 (4) | C4A—H4A | 0.9800 |
| C9—H9 | 0.9800 | C5A—C6A | 1.503 (4) |
| C10—C19 | 1.545 (4) | C5A—H5A1 | 0.9800 |
| C11—C12 | 1.524 (4) | C6A—H6A1 | 0.9700 |
| C11—H11A | 0.9700 | C6A—H6A2 | 0.9700 |
| C11—H11B | 0.9700 | C1B—C2B | 1.513 (4) |
| C12—C13 | 1.528 (4) | C1B—H1B1 | 0.9800 |
| C12—H12 | 0.9800 | C2B—C3B | 1.513 (4) |
| C13—C17 | 1.547 (4) | C2B—H2B1 | 0.9800 |
| C13—C14 | 1.549 (4) | C3B—C4B | 1.506 (4) |
| C13—H13 | 0.9800 | C3B—H3B | 0.9800 |
| C14—C15 | 1.534 (4) | C4B—C5B | 1.502 (4) |
| C14—C30 | 1.552 (4) | C4B—H4B | 0.9800 |
| C15—C16 | 1.536 (4) | C5B—H5B1 | 0.9700 |
| C15—H15A | 0.9700 | C5B—H5B2 | 0.9700 |
| C15—H15B | 0.9700 | ||
| C3—O1—H1 | 111.1 | O3—C20—C17 | 102.1 (2) |
| C12—O2—H2 | 108.6 | C21—C20—C17 | 109.8 (2) |
| C1A—O3—C20 | 119.1 (2) | C22—C20—C17 | 116.2 (2) |
| C5A—O4—C1A | 112.29 (19) | C20—C21—H21A | 109.5 |
| C2A—O5—H5A | 111.6 | C20—C21—H21B | 109.5 |
| C3A—O6—H6 | 99.6 | H21A—C21—H21B | 109.5 |
| C4A—O7—H7 | 99.1 | C20—C21—H21C | 109.5 |
| C1B—O8—C6A | 113.0 (2) | H21A—C21—H21C | 109.5 |
| C1B—O9—C5B | 111.8 (2) | H21B—C21—H21C | 109.5 |
| C2B—O10—H10 | 112.8 | C23—C22—C20 | 119.2 (3) |
| C3B—O11—H11 | 106.9 | C23—C22—H22A | 107.5 |
| C4B—O12—H12A | 108.5 | C20—C22—H22A | 107.5 |
| C42—O13—H13A | 106.7 | C23—C22—H22B | 107.5 |
| C2—C1—C10 | 113.8 (3) | C20—C22—H22B | 107.5 |
| C2—C1—H1A | 108.8 | H22A—C22—H22B | 107.0 |
| C10—C1—H1A | 108.8 | C24—C23—C22 | 111.1 (3) |
| C2—C1—H1B | 108.8 | C24—C23—H23A | 109.4 |
| C10—C1—H1B | 108.8 | C22—C23—H23A | 109.4 |
| H1A—C1—H1B | 107.7 | C24—C23—H23B | 109.4 |
| C3—C2—C1 | 111.8 (3) | C22—C23—H23B | 109.4 |
| C3—C2—H2A | 109.2 | H23A—C23—H23B | 108.0 |
| C1—C2—H2A | 109.2 | C25—C24—C23 | 128.5 (4) |
| C3—C2—H2B | 109.2 | C25—C24—H24 | 115.7 |
| C1—C2—H2B | 109.2 | C23—C24—H24 | 115.7 |
| H2A—C2—H2B | 107.9 | C24—C25—C26 | 124.7 (4) |
| O1—C3—C2 | 108.4 (3) | C24—C25—C27 | 120.3 (3) |
| O1—C3—C4 | 111.0 (2) | C26—C25—C27 | 115.0 (3) |
| C2—C3—C4 | 114.0 (3) | C25—C26—H26A | 109.5 |
| O1—C3—H3 | 107.7 | C25—C26—H26B | 109.5 |
| C2—C3—H3 | 107.7 | H26A—C26—H26B | 109.5 |
| C4—C3—H3 | 107.7 | C25—C26—H26C | 109.5 |
| C3—C4—C28 | 107.2 (3) | H26A—C26—H26C | 109.5 |
| C3—C4—C29 | 111.0 (3) | H26B—C26—H26C | 109.5 |
| C28—C4—C29 | 108.2 (3) | C25—C27—H27A | 109.5 |
| C3—C4—C5 | 107.4 (2) | C25—C27—H27B | 109.5 |
| C28—C4—C5 | 109.1 (2) | H27A—C27—H27B | 109.5 |
| C29—C4—C5 | 113.7 (3) | C25—C27—H27C | 109.5 |
| C6—C5—C10 | 111.3 (3) | H27A—C27—H27C | 109.5 |
| C6—C5—C4 | 114.5 (2) | H27B—C27—H27C | 109.5 |
| C10—C5—C4 | 117.3 (2) | C4—C28—H28A | 109.5 |
| C6—C5—H5 | 104.0 | C4—C28—H28B | 109.5 |
| C10—C5—H5 | 104.0 | H28A—C28—H28B | 109.5 |
| C4—C5—H5 | 104.0 | C4—C28—H28C | 109.5 |
| C7—C6—C5 | 110.7 (3) | H28A—C28—H28C | 109.5 |
| C7—C6—H6A | 109.5 | H28B—C28—H28C | 109.5 |
| C5—C6—H6A | 109.5 | C4—C29—H29A | 109.5 |
| C7—C6—H6B | 109.5 | C4—C29—H29B | 109.5 |
| C5—C6—H6B | 109.5 | H29A—C29—H29B | 109.5 |
| H6A—C6—H6B | 108.1 | C4—C29—H29C | 109.5 |
| C6—C7—C8 | 114.0 (3) | H29A—C29—H29C | 109.5 |
| C6—C7—H7A | 108.8 | H29B—C29—H29C | 109.5 |
| C8—C7—H7A | 108.8 | C14—C30—H30A | 109.5 |
| C6—C7—H7B | 108.8 | C14—C30—H30B | 109.5 |
| C8—C7—H7B | 108.8 | H30A—C30—H30B | 109.5 |
| H7A—C7—H7B | 107.7 | C14—C30—H30C | 109.5 |
| C7—C8—C18 | 107.1 (3) | H30A—C30—H30C | 109.5 |
| C7—C8—C9 | 109.3 (2) | H30B—C30—H30C | 109.5 |
| C18—C8—C9 | 112.5 (3) | O13—C42—H42A | 109.5 |
| C7—C8—C14 | 111.4 (3) | O13—C42—H42B | 109.5 |
| C18—C8—C14 | 110.3 (2) | H42A—C42—H42B | 109.5 |
| C9—C8—C14 | 106.4 (2) | O13—C42—H42C | 109.5 |
| C11—C9—C8 | 110.4 (2) | H42A—C42—H42C | 109.5 |
| C11—C9—C10 | 114.3 (2) | H42B—C42—H42C | 109.5 |
| C8—C9—C10 | 116.6 (2) | O3—C1A—O4 | 107.4 (2) |
| C11—C9—H9 | 104.7 | O3—C1A—C2A | 109.5 (2) |
| C8—C9—H9 | 104.7 | O4—C1A—C2A | 107.2 (2) |
| C10—C9—H9 | 104.7 | O3—C1A—H1A1 | 110.9 |
| C1—C10—C5 | 107.0 (2) | O4—C1A—H1A1 | 110.9 |
| C1—C10—C19 | 107.7 (3) | C2A—C1A—H1A1 | 110.9 |
| C5—C10—C19 | 114.1 (3) | O5—C2A—C3A | 108.7 (2) |
| C1—C10—C9 | 108.3 (2) | O5—C2A—C1A | 111.2 (2) |
| C5—C10—C9 | 107.5 (2) | C3A—C2A—C1A | 109.4 (2) |
| C19—C10—C9 | 111.9 (2) | O5—C2A—H2A1 | 109.2 |
| C12—C11—C9 | 115.7 (2) | C3A—C2A—H2A1 | 109.2 |
| C12—C11—H11A | 108.4 | C1A—C2A—H2A1 | 109.2 |
| C9—C11—H11A | 108.4 | O6—C3A—C2A | 112.4 (2) |
| C12—C11—H11B | 108.4 | O6—C3A—C4A | 107.3 (2) |
| C9—C11—H11B | 108.4 | C2A—C3A—C4A | 110.4 (2) |
| H11A—C11—H11B | 107.4 | O6—C3A—H3A | 108.9 |
| O2—C12—C11 | 105.4 (2) | C2A—C3A—H3A | 108.9 |
| O2—C12—C13 | 113.7 (2) | C4A—C3A—H3A | 108.9 |
| C11—C12—C13 | 111.0 (2) | O7—C4A—C3A | 111.6 (2) |
| O2—C12—H12 | 108.8 | O7—C4A—C5A | 106.1 (2) |
| C11—C12—H12 | 108.8 | C3A—C4A—C5A | 110.6 (2) |
| C13—C12—H12 | 108.8 | O7—C4A—H4A | 109.5 |
| C12—C13—C17 | 120.1 (2) | C3A—C4A—H4A | 109.5 |
| C12—C13—C14 | 111.3 (2) | C5A—C4A—H4A | 109.5 |
| C17—C13—C14 | 104.6 (2) | O4—C5A—C6A | 107.4 (2) |
| C12—C13—H13 | 106.7 | O4—C5A—C4A | 111.0 (2) |
| C17—C13—H13 | 106.7 | C6A—C5A—C4A | 111.0 (2) |
| C14—C13—H13 | 106.7 | O4—C5A—H5A1 | 109.1 |
| C15—C14—C13 | 100.2 (2) | C6A—C5A—H5A1 | 109.1 |
| C15—C14—C30 | 105.9 (3) | C4A—C5A—H5A1 | 109.1 |
| C13—C14—C30 | 110.4 (2) | O8—C6A—C5A | 109.5 (2) |
| C15—C14—C8 | 116.8 (2) | O8—C6A—H6A1 | 109.8 |
| C13—C14—C8 | 110.3 (2) | C5A—C6A—H6A1 | 109.8 |
| C30—C14—C8 | 112.5 (2) | O8—C6A—H6A2 | 109.8 |
| C14—C15—C16 | 105.3 (2) | C5A—C6A—H6A2 | 109.8 |
| C14—C15—H15A | 110.7 | H6A1—C6A—H6A2 | 108.2 |
| C16—C15—H15A | 110.7 | O8—C1B—O9 | 105.9 (2) |
| C14—C15—H15B | 110.7 | O8—C1B—C2B | 108.0 (2) |
| C16—C15—H15B | 110.7 | O9—C1B—C2B | 112.0 (2) |
| H15A—C15—H15B | 108.8 | O8—C1B—H1B1 | 110.3 |
| C15—C16—C17 | 106.7 (2) | O9—C1B—H1B1 | 110.3 |
| C15—C16—H16A | 110.4 | C2B—C1B—H1B1 | 110.3 |
| C17—C16—H16A | 110.4 | O10—C2B—C3B | 106.7 (2) |
| C15—C16—H16B | 110.4 | O10—C2B—C1B | 111.7 (2) |
| C17—C16—H16B | 110.4 | C3B—C2B—C1B | 111.2 (2) |
| H16A—C16—H16B | 108.6 | O10—C2B—H2B1 | 109.1 |
| C20—C17—C13 | 119.8 (2) | C3B—C2B—H2B1 | 109.1 |
| C20—C17—C16 | 114.0 (2) | C1B—C2B—H2B1 | 109.1 |
| C13—C17—C16 | 103.0 (2) | O11—C3B—C4B | 111.9 (2) |
| C20—C17—H17 | 106.4 | O11—C3B—C2B | 106.9 (2) |
| C13—C17—H17 | 106.4 | C4B—C3B—C2B | 112.1 (2) |
| C16—C17—H17 | 106.4 | O11—C3B—H3B | 108.6 |
| C8—C18—H18A | 109.5 | C4B—C3B—H3B | 108.6 |
| C8—C18—H18B | 109.5 | C2B—C3B—H3B | 108.6 |
| H18A—C18—H18B | 109.5 | O12—C4B—C5B | 111.7 (3) |
| C8—C18—H18C | 109.5 | O12—C4B—C3B | 107.5 (2) |
| H18A—C18—H18C | 109.5 | C5B—C4B—C3B | 110.0 (3) |
| H18B—C18—H18C | 109.5 | O12—C4B—H4B | 109.2 |
| C10—C19—H19A | 109.5 | C5B—C4B—H4B | 109.2 |
| C10—C19—H19B | 109.5 | C3B—C4B—H4B | 109.2 |
| H19A—C19—H19B | 109.5 | O9—C5B—C4B | 110.0 (3) |
| C10—C19—H19C | 109.5 | O9—C5B—H5B1 | 109.7 |
| H19A—C19—H19C | 109.5 | C4B—C5B—H5B1 | 109.7 |
| H19B—C19—H19C | 109.5 | O9—C5B—H5B2 | 109.7 |
| O3—C20—C21 | 106.7 (2) | C4B—C5B—H5B2 | 109.7 |
| O3—C20—C22 | 108.0 (2) | H5B1—C5B—H5B2 | 108.2 |
| C21—C20—C22 | 113.0 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O11i | 0.82 | 2.38 | 2.996 (3) | 133 |
| O2—H2···O3 | 0.83 | 1.96 | 2.752 (3) | 160 |
| O5—H5A···O1i | 0.84 | 2.14 | 2.839 (3) | 140 |
| O6—H6···O12ii | 0.83 | 2.04 | 2.781 (3) | 147 |
| O7—H7···O8iii | 0.83 | 2.31 | 3.009 (3) | 142 |
| O10—H10···O13iv | 0.82 | 2.05 | 2.824 (4) | 156 |
| O11—H11···O5v | 0.81 | 1.92 | 2.703 (3) | 162 |
| O12—H12A···O11vi | 0.84 | 2.02 | 2.827 (3) | 162 |
| O13—H13A···O2vii | 0.85 | 1.98 | 2.823 (3) | 173 |
Symmetry codes: (i) −x, y−1/2, −z+1/2; (ii) x, y−1, z; (iii) x−1/2, −y+3/2, −z; (iv) x−1, y, z; (v) x, y+1, z; (vi) x−1/2, −y+5/2, −z; (vii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU2351).
References
- Bruker (1997). SHELXTL Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (1999). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- He, K. J., Liu, Y., Yang, Y., Li, P. & Yang, L. (2005). Chem. Pharm. Bull.53, 177–179. [DOI] [PubMed]
- Hu, Y., Luan, H. W., Hao, D. C., Xiao, H. B., Yang, S. L. & Yang, L. (2007). Enzym. Microb. Technol.40, 1358–1366.
- Li, H. Z., Zhang, Y. J. & Yang, C. R. (2006). Tianran Chanwu Yanjiu Yu Kaifa, 18, 549–554.
- Sheldrick, G. M. (1997). SHELXS97 and SHELXL97, University of Göttingen, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807063118/xu2351sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807063118/xu2351Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report