Abstract
The title compound, C17H29NO, is an important hindered phenol derivative. The asymmetric unit contains two molecules. Molecules interact through O—H⋯N hydrogen bonds to form a tetramer arranged around a twofold rotation axis.
Related literature
For related literature, see: Ciba-Geigy AG (1978 ▶); Eggensperger et al. (1974 ▶, 1976 ▶); Yamazaki & Seguchi (1997 ▶). For the synthesis, see: Coffield (1965 ▶); Coffield & Mich (1965 ▶); Rieker et al. (1968 ▶).
Experimental
Crystal data
C17H29NO
M r = 263.41
Monoclinic,

a = 28.731 (9) Å
b = 8.912 (3) Å
c = 16.112 (5) Å
β = 122.965 (5)°
V = 3461.4 (19) Å3
Z = 8
Mo Kα radiation
μ = 0.06 mm−1
T = 294 (2) K
0.24 × 0.22 × 0.20 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.978, T max = 0.989
6903 measured reflections
3752 independent reflections
2317 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.112
S = 0.99
3752 reflections
359 parameters
3 restraints
H-atom parameters constrained
Δρmax = 0.12 e Å−3
Δρmin = −0.18 e Å−3
Data collection: SMART (Bruker, 1997 ▶); cell refinement: SAINT (Bruker, 1997 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: ORTEPIII (Burnett & Johnson, 1996 ▶), ORTEP-3 for Windows (Farrugia, 1997 ▶) and PLATON (Spek, 2003 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807065117/dn2296sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807065117/dn2296Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N2 | 0.85 | 2.20 | 2.836 (3) | 132 |
| O2—H2⋯N1i | 0.86 | 2.26 | 2.933 (3) | 135 |
Symmetry code: (i) 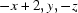 .
.
Acknowledgments
The authors gratefully acknowledge financial support from the Start Foundation for Doctors (HY07116) of Yantai University.
supplementary crystallographic information
Comment
Hindered phenol antioxidants are widely used in polymers and lubricants. It could protect polymers by increasing both their process stability and long-term stability against oxidative degradation (Yamazaki & Seguchi, 1997). Moreover, ester of 3,5-di-tert-butyl-4-hydroxyphenol acetic acid is one important kind of antioxidant derivative. An important route to prepare these compounds is to react an α-halo ester compound with the title compound in the presence of a strong base (Eggensperger et al., 1974, 1976; Eggensperger et al., 1976; Ciba-Geigy AG, 1978). The title compound is ususlly called a Mannich base. The title compound was prepared from 4-bromomethyl-2,6-di-tert-butyl-phenol and N,N-dimethylamine.It can also be easily obtained by a Mannich reaction from 2,6-di-tert-butylphenol,formaldehyde and dimethylamine (Coffield, 1965; Coffield & Mich, 1965).
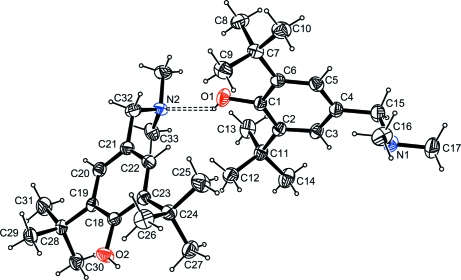
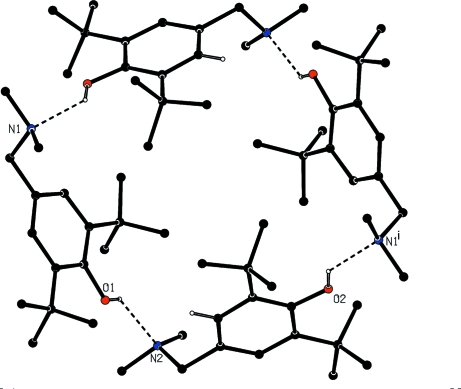
The asymmetric unit of the title compound contains two molecules which are linked by a weak O—H···N hydrogen bond (Fig. 1). Each pseudo dimer interacts with a symmetry related one to build up like a crown arranged around axis parallele to the b axis through O—H··· hydrogen bonds (Table 1, Fig. 2).
Experimental
The 4-bromomethyl-2,6-di-tert-butyl-phenol was synthesized according to the method described by Rieker(Rieker et al.,1968). Dimethylamine (2.7 g, 0.06 mol) and 4-bromomethyl-2,6-di-tert-butyl-phenol (9.0 g, 0.03 mol) were added, with stirring to THF(60 ml)at 273 K. The reaction mixture was stirred at 273 K for a further 2 h. The solvent THF was evaporated under reduced pressure and the residual was washed with water (30 ml). The product (7.39 g) was obtained in a yield of 93.6%. Suitable crystals were obtained by slow evaporation of a mixture of ethyl acetate and ethanol.
Refinement
All H atoms attached to C atoms were fixed geometrically and treated as riding with C—H = 0.93 Å (aromatic) and 0.96 Å (methyle) with Uiso(H) = 1.2(aromatic) or 1.5(methyle)Ueq(C). H atoms of hydroxyle group were located in difference Fourier maps and included in the subsequent refinement using restraints (O—H= 0.85 (1) Å) with Uiso(H) = 1.5Ueq(O). In the final stage of refinement, they were treated as riding on their parent O atoms.
In the absence of significant anomalous scattering, the absolute configuration could not be reliably determined and then the Friedel pairs were merged and any references to the Flack parameter were removed.
Figures
Fig. 1.
View of the two crystallygraphically independent molecules with the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level. Hydrogen bond is shown as dashed line. H atoms are represented as small spheres of arbitrary radii.
Fig. 2.
View of the crown formed by the assembly of four molecules through O—H···N hydrogen bonds. Dashed lines indicate the hydrogen bonds.H atoms not involved in hydrogen bonding have been omitted for clarity.
Crystal data
| C17H29NO | F000 = 1168 |
| Mr = 263.41 | Dx = 1.011 Mg m−3 |
| Monoclinic, C2 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: C 2y | Cell parameters from 2365 reflections |
| a = 28.731 (9) Å | θ = 2.4–21.0º |
| b = 8.912 (3) Å | µ = 0.06 mm−1 |
| c = 16.112 (5) Å | T = 294 (2) K |
| β = 122.965 (5)º | Block, colourless |
| V = 3461.4 (19) Å3 | 0.24 × 0.22 × 0.20 mm |
| Z = 8 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3752 independent reflections |
| Radiation source: fine-focus sealed tube | 2317 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.040 |
| T = 294(2) K | θmax = 26.4º |
| φ and ω scans | θmin = 1.5º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 1996) | h = −35→29 |
| Tmin = 0.978, Tmax = 0.989 | k = −11→11 |
| 6903 measured reflections | l = 0→20 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.044 | H-atom parameters constrained |
| wR(F2) = 0.112 | w = 1/[σ2(Fo2) + (0.0567P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.99 | (Δ/σ)max = 0.001 |
| 3752 reflections | Δρmax = 0.12 e Å−3 |
| 359 parameters | Δρmin = −0.18 e Å−3 |
| 3 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.96396 (9) | −0.0075 (3) | 0.26691 (14) | 0.0632 (7) | |
| H1 | 0.9775 | −0.0898 | 0.2629 | 0.076* | |
| O2 | 1.20963 (9) | 0.0785 (3) | 0.24701 (16) | 0.0604 (6) | |
| H2 | 1.1990 | 0.1526 | 0.2069 | 0.073* | |
| N1 | 0.76397 (10) | 0.2845 (3) | −0.13626 (17) | 0.0531 (7) | |
| N2 | 1.05111 (10) | −0.2181 (3) | 0.37179 (17) | 0.0466 (6) | |
| C1 | 0.91498 (12) | 0.0341 (3) | 0.1819 (2) | 0.0442 (7) | |
| C2 | 0.89225 (12) | −0.0421 (3) | 0.0907 (2) | 0.0442 (7) | |
| C3 | 0.84136 (12) | 0.0109 (4) | 0.0118 (2) | 0.0478 (8) | |
| H3 | 0.8253 | −0.0377 | −0.0488 | 0.057* | |
| C4 | 0.81353 (12) | 0.1321 (4) | 0.0193 (2) | 0.0486 (8) | |
| C5 | 0.83809 (12) | 0.2039 (4) | 0.1103 (2) | 0.0488 (8) | |
| H5 | 0.8198 | 0.2850 | 0.1163 | 0.059* | |
| C6 | 0.88894 (12) | 0.1598 (3) | 0.1930 (2) | 0.0445 (8) | |
| C7 | 0.91528 (13) | 0.2442 (4) | 0.2931 (2) | 0.0503 (8) | |
| C8 | 0.91655 (15) | 0.1407 (4) | 0.3709 (2) | 0.0657 (10) | |
| H8A | 0.9389 | 0.0542 | 0.3808 | 0.099* | |
| H8B | 0.8795 | 0.1093 | 0.3481 | 0.099* | |
| H8C | 0.9320 | 0.1940 | 0.4322 | 0.099* | |
| C9 | 0.97393 (13) | 0.3002 (4) | 0.3292 (3) | 0.0663 (10) | |
| H9A | 0.9881 | 0.3577 | 0.3887 | 0.099* | |
| H9B | 0.9726 | 0.3621 | 0.2792 | 0.099* | |
| H9C | 0.9978 | 0.2158 | 0.3424 | 0.099* | |
| C10 | 0.88150 (15) | 0.3852 (4) | 0.2835 (3) | 0.0685 (10) | |
| H10A | 0.8984 | 0.4338 | 0.3467 | 0.103* | |
| H10B | 0.8443 | 0.3565 | 0.2614 | 0.103* | |
| H10C | 0.8808 | 0.4530 | 0.2365 | 0.103* | |
| C11 | 0.92034 (13) | −0.1804 (4) | 0.0773 (2) | 0.0517 (8) | |
| C12 | 0.97975 (13) | −0.1438 (4) | 0.1057 (2) | 0.0610 (9) | |
| H12A | 0.9948 | −0.2285 | 0.0913 | 0.091* | |
| H12B | 1.0025 | −0.1214 | 0.1751 | 0.091* | |
| H12C | 0.9789 | −0.0585 | 0.0684 | 0.091* | |
| C13 | 0.91972 (15) | −0.3122 (4) | 0.1393 (3) | 0.0674 (10) | |
| H13A | 0.8821 | −0.3347 | 0.1180 | 0.101* | |
| H13B | 0.9403 | −0.2846 | 0.2080 | 0.101* | |
| H13C | 0.9364 | −0.3990 | 0.1305 | 0.101* | |
| C14 | 0.88828 (15) | −0.2344 (5) | −0.0307 (2) | 0.0774 (11) | |
| H14A | 0.8867 | −0.1550 | −0.0724 | 0.116* | |
| H14B | 0.8513 | −0.2623 | −0.0507 | 0.116* | |
| H14C | 0.9068 | −0.3195 | −0.0364 | 0.116* | |
| C15 | 0.75856 (13) | 0.1836 (4) | −0.0695 (2) | 0.0594 (9) | |
| H15A | 0.7373 | 0.0961 | −0.1066 | 0.071* | |
| H15B | 0.7379 | 0.2349 | −0.0463 | 0.071* | |
| C16 | 0.78926 (17) | 0.4270 (4) | −0.0877 (3) | 0.0712 (11) | |
| H16A | 0.7927 | 0.4902 | −0.1323 | 0.107* | |
| H16B | 0.8253 | 0.4087 | −0.0296 | 0.107* | |
| H16C | 0.7663 | 0.4757 | −0.0694 | 0.107* | |
| C17 | 0.70858 (14) | 0.3107 (5) | −0.2253 (3) | 0.0816 (12) | |
| H17A | 0.6856 | 0.3598 | −0.2071 | 0.122* | |
| H17B | 0.6922 | 0.2164 | −0.2563 | 0.122* | |
| H17C | 0.7118 | 0.3730 | −0.2706 | 0.122* | |
| C18 | 1.17964 (12) | 0.0384 (3) | 0.2871 (2) | 0.0412 (7) | |
| C19 | 1.20150 (11) | −0.0867 (3) | 0.3518 (2) | 0.0404 (7) | |
| C20 | 1.17375 (11) | −0.1308 (4) | 0.3964 (2) | 0.0458 (7) | |
| H20 | 1.1880 | −0.2108 | 0.4406 | 0.055* | |
| C21 | 1.12606 (12) | −0.0621 (4) | 0.3786 (2) | 0.0446 (7) | |
| C22 | 1.10584 (12) | 0.0591 (4) | 0.3143 (2) | 0.0441 (7) | |
| H22 | 1.0739 | 0.1067 | 0.3023 | 0.053* | |
| C23 | 1.13113 (12) | 0.1136 (3) | 0.2665 (2) | 0.0412 (7) | |
| C24 | 1.10825 (12) | 0.2547 (3) | 0.2001 (2) | 0.0483 (8) | |
| C25 | 1.05452 (14) | 0.3120 (4) | 0.1893 (3) | 0.0717 (10) | |
| H25A | 1.0268 | 0.2348 | 0.1605 | 0.108* | |
| H25B | 1.0414 | 0.3991 | 0.1475 | 0.108* | |
| H25C | 1.0620 | 0.3379 | 0.2533 | 0.108* | |
| C26 | 1.15096 (15) | 0.3832 (4) | 0.2467 (3) | 0.0664 (10) | |
| H26A | 1.1587 | 0.4053 | 0.3114 | 0.100* | |
| H26B | 1.1362 | 0.4709 | 0.2058 | 0.100* | |
| H26C | 1.1846 | 0.3534 | 0.2520 | 0.100* | |
| C27 | 1.09303 (14) | 0.2209 (4) | 0.0937 (2) | 0.0634 (10) | |
| H27A | 1.1259 | 0.1938 | 0.0956 | 0.095* | |
| H27B | 1.0768 | 0.3085 | 0.0532 | 0.095* | |
| H27C | 1.0670 | 0.1395 | 0.0664 | 0.095* | |
| C28 | 1.25469 (11) | −0.1685 (3) | 0.3744 (2) | 0.0468 (8) | |
| C29 | 1.30491 (13) | −0.0619 (5) | 0.4262 (2) | 0.0657 (10) | |
| H29A | 1.3382 | −0.1173 | 0.4467 | 0.099* | |
| H29B | 1.3076 | −0.0179 | 0.4830 | 0.099* | |
| H29C | 1.3003 | 0.0159 | 0.3811 | 0.099* | |
| C30 | 1.24665 (13) | −0.2338 (4) | 0.2788 (2) | 0.0596 (9) | |
| H30A | 1.2413 | −0.1533 | 0.2348 | 0.089* | |
| H30B | 1.2147 | −0.2981 | 0.2473 | 0.089* | |
| H30C | 1.2789 | −0.2904 | 0.2947 | 0.089* | |
| C31 | 1.26844 (15) | −0.3037 (5) | 0.4444 (3) | 0.0738 (11) | |
| H31A | 1.3006 | −0.3546 | 0.4547 | 0.111* | |
| H31B | 1.2376 | −0.3717 | 0.4152 | 0.111* | |
| H31C | 1.2757 | −0.2688 | 0.5067 | 0.111* | |
| C32 | 1.09783 (13) | −0.1136 (4) | 0.4303 (2) | 0.0552 (9) | |
| H32A | 1.1252 | −0.1622 | 0.4919 | 0.066* | |
| H32B | 1.0843 | −0.0261 | 0.4466 | 0.066* | |
| C33 | 1.06835 (14) | −0.3538 (4) | 0.3442 (3) | 0.0620 (9) | |
| H33A | 1.0960 | −0.4056 | 0.4029 | 0.093* | |
| H33B | 1.0835 | −0.3266 | 0.3059 | 0.093* | |
| H33C | 1.0368 | −0.4181 | 0.3057 | 0.093* | |
| C34 | 1.02793 (14) | −0.2575 (5) | 0.4305 (3) | 0.0706 (11) | |
| H34A | 0.9968 | −0.3233 | 0.3929 | 0.106* | |
| H34B | 1.0161 | −0.1680 | 0.4470 | 0.106* | |
| H34C | 1.0557 | −0.3072 | 0.4902 | 0.106* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0632 (14) | 0.0591 (14) | 0.0435 (12) | 0.0226 (12) | 0.0135 (11) | −0.0036 (11) |
| O2 | 0.0607 (14) | 0.0630 (14) | 0.0759 (15) | 0.0133 (12) | 0.0490 (12) | 0.0255 (13) |
| N1 | 0.0567 (16) | 0.0494 (16) | 0.0451 (14) | 0.0094 (14) | 0.0225 (13) | 0.0080 (13) |
| N2 | 0.0505 (15) | 0.0470 (15) | 0.0540 (14) | 0.0018 (13) | 0.0359 (13) | 0.0041 (13) |
| C1 | 0.0402 (17) | 0.0464 (19) | 0.0407 (16) | 0.0025 (15) | 0.0185 (14) | 0.0058 (15) |
| C2 | 0.0463 (18) | 0.0427 (18) | 0.0411 (17) | −0.0050 (15) | 0.0222 (15) | 0.0025 (15) |
| C3 | 0.0455 (18) | 0.0478 (19) | 0.0395 (16) | −0.0097 (15) | 0.0162 (15) | 0.0009 (14) |
| C4 | 0.0409 (17) | 0.054 (2) | 0.0487 (18) | −0.0009 (16) | 0.0227 (15) | 0.0098 (16) |
| C5 | 0.0469 (18) | 0.0479 (18) | 0.058 (2) | 0.0057 (15) | 0.0327 (17) | 0.0112 (16) |
| C6 | 0.0457 (18) | 0.0461 (19) | 0.0457 (17) | −0.0019 (15) | 0.0276 (16) | 0.0043 (15) |
| C7 | 0.0529 (19) | 0.050 (2) | 0.0565 (19) | −0.0006 (16) | 0.0356 (16) | −0.0011 (16) |
| C8 | 0.080 (3) | 0.071 (2) | 0.055 (2) | 0.004 (2) | 0.0430 (19) | 0.0030 (19) |
| C9 | 0.063 (2) | 0.065 (2) | 0.076 (2) | −0.0129 (19) | 0.0405 (19) | −0.015 (2) |
| C10 | 0.080 (2) | 0.063 (2) | 0.072 (2) | 0.010 (2) | 0.048 (2) | −0.003 (2) |
| C11 | 0.0546 (19) | 0.0449 (18) | 0.0439 (17) | 0.0024 (16) | 0.0193 (15) | −0.0038 (15) |
| C12 | 0.065 (2) | 0.062 (2) | 0.0564 (19) | 0.0093 (19) | 0.0335 (17) | −0.0006 (18) |
| C13 | 0.071 (2) | 0.044 (2) | 0.072 (2) | −0.0013 (18) | 0.029 (2) | 0.0050 (18) |
| C14 | 0.084 (3) | 0.065 (2) | 0.056 (2) | 0.006 (2) | 0.0209 (19) | −0.014 (2) |
| C15 | 0.0464 (19) | 0.065 (2) | 0.058 (2) | 0.0038 (17) | 0.0227 (16) | 0.0119 (18) |
| C16 | 0.101 (3) | 0.049 (2) | 0.070 (2) | 0.006 (2) | 0.050 (2) | 0.0000 (18) |
| C17 | 0.070 (2) | 0.093 (3) | 0.060 (2) | 0.028 (2) | 0.021 (2) | 0.019 (2) |
| C18 | 0.0420 (17) | 0.0431 (17) | 0.0387 (15) | −0.0036 (14) | 0.0220 (14) | −0.0010 (14) |
| C19 | 0.0360 (16) | 0.0428 (18) | 0.0347 (15) | −0.0041 (13) | 0.0144 (13) | −0.0039 (13) |
| C20 | 0.0453 (18) | 0.0444 (17) | 0.0399 (16) | −0.0064 (15) | 0.0182 (14) | 0.0009 (14) |
| C21 | 0.0435 (18) | 0.0500 (19) | 0.0414 (16) | −0.0099 (16) | 0.0238 (14) | −0.0088 (15) |
| C22 | 0.0419 (17) | 0.0453 (18) | 0.0465 (16) | −0.0054 (15) | 0.0248 (15) | −0.0100 (15) |
| C23 | 0.0392 (16) | 0.0386 (17) | 0.0423 (16) | −0.0044 (14) | 0.0199 (14) | −0.0079 (13) |
| C24 | 0.0528 (18) | 0.0432 (18) | 0.0509 (17) | 0.0055 (16) | 0.0296 (15) | −0.0011 (15) |
| C25 | 0.075 (2) | 0.059 (2) | 0.089 (3) | 0.023 (2) | 0.050 (2) | 0.012 (2) |
| C26 | 0.080 (2) | 0.0441 (19) | 0.076 (2) | −0.0067 (19) | 0.043 (2) | −0.0042 (19) |
| C27 | 0.067 (2) | 0.067 (2) | 0.0489 (19) | 0.016 (2) | 0.0262 (17) | 0.0066 (18) |
| C28 | 0.0370 (16) | 0.0526 (19) | 0.0404 (17) | 0.0061 (15) | 0.0144 (14) | 0.0058 (15) |
| C29 | 0.0404 (18) | 0.081 (3) | 0.062 (2) | −0.0071 (18) | 0.0188 (16) | −0.013 (2) |
| C30 | 0.0496 (18) | 0.061 (2) | 0.059 (2) | 0.0088 (18) | 0.0236 (17) | −0.0061 (18) |
| C31 | 0.063 (2) | 0.074 (3) | 0.076 (2) | 0.021 (2) | 0.032 (2) | 0.028 (2) |
| C32 | 0.062 (2) | 0.061 (2) | 0.0483 (18) | −0.0064 (18) | 0.0341 (17) | −0.0044 (17) |
| C33 | 0.052 (2) | 0.049 (2) | 0.081 (2) | 0.0040 (17) | 0.0329 (18) | 0.0002 (19) |
| C34 | 0.073 (2) | 0.086 (3) | 0.072 (2) | 0.003 (2) | 0.052 (2) | 0.020 (2) |
Geometric parameters (Å, °)
| O1—C1 | 1.377 (3) | C16—H16B | 0.9600 |
| O1—H1 | 0.8495 | C16—H16C | 0.9600 |
| O2—C18 | 1.376 (3) | C17—H17A | 0.9600 |
| O2—H2 | 0.8557 | C17—H17B | 0.9600 |
| N1—C16 | 1.461 (4) | C17—H17C | 0.9600 |
| N1—C17 | 1.470 (4) | C18—C23 | 1.413 (4) |
| N1—C15 | 1.474 (4) | C18—C19 | 1.419 (4) |
| N2—C33 | 1.465 (4) | C19—C20 | 1.389 (4) |
| N2—C34 | 1.466 (4) | C19—C28 | 1.547 (4) |
| N2—C32 | 1.477 (4) | C20—C21 | 1.381 (4) |
| C1—C6 | 1.411 (4) | C20—H20 | 0.9300 |
| C1—C2 | 1.415 (4) | C21—C22 | 1.387 (4) |
| C2—C3 | 1.398 (4) | C21—C32 | 1.515 (4) |
| C2—C11 | 1.552 (5) | C22—C23 | 1.403 (4) |
| C3—C4 | 1.387 (4) | C22—H22 | 0.9300 |
| C3—H3 | 0.9300 | C23—C24 | 1.547 (4) |
| C4—C5 | 1.390 (4) | C24—C26 | 1.542 (4) |
| C4—C15 | 1.514 (4) | C24—C25 | 1.543 (4) |
| C5—C6 | 1.395 (4) | C24—C27 | 1.551 (4) |
| C5—H5 | 0.9300 | C25—H25A | 0.9600 |
| C6—C7 | 1.552 (4) | C25—H25B | 0.9600 |
| C7—C9 | 1.536 (4) | C25—H25C | 0.9600 |
| C7—C8 | 1.541 (4) | C26—H26A | 0.9600 |
| C7—C10 | 1.543 (5) | C26—H26B | 0.9600 |
| C8—H8A | 0.9600 | C26—H26C | 0.9600 |
| C8—H8B | 0.9600 | C27—H27A | 0.9600 |
| C8—H8C | 0.9600 | C27—H27B | 0.9600 |
| C9—H9A | 0.9600 | C27—H27C | 0.9600 |
| C9—H9B | 0.9600 | C28—C29 | 1.540 (4) |
| C9—H9C | 0.9600 | C28—C30 | 1.543 (4) |
| C10—H10A | 0.9600 | C28—C31 | 1.547 (5) |
| C10—H10B | 0.9600 | C29—H29A | 0.9600 |
| C10—H10C | 0.9600 | C29—H29B | 0.9600 |
| C11—C14 | 1.537 (4) | C29—H29C | 0.9600 |
| C11—C12 | 1.542 (4) | C30—H30A | 0.9600 |
| C11—C13 | 1.549 (5) | C30—H30B | 0.9600 |
| C12—H12A | 0.9600 | C30—H30C | 0.9600 |
| C12—H12B | 0.9600 | C31—H31A | 0.9600 |
| C12—H12C | 0.9600 | C31—H31B | 0.9600 |
| C13—H13A | 0.9600 | C31—H31C | 0.9600 |
| C13—H13B | 0.9600 | C32—H32A | 0.9700 |
| C13—H13C | 0.9600 | C32—H32B | 0.9700 |
| C14—H14A | 0.9600 | C33—H33A | 0.9600 |
| C14—H14B | 0.9600 | C33—H33B | 0.9600 |
| C14—H14C | 0.9600 | C33—H33C | 0.9600 |
| C15—H15A | 0.9700 | C34—H34A | 0.9600 |
| C15—H15B | 0.9700 | C34—H34B | 0.9600 |
| C16—H16A | 0.9600 | C34—H34C | 0.9600 |
| C1—O1—H1 | 114.8 | H17A—C17—H17B | 109.5 |
| C18—O2—H2 | 119.8 | N1—C17—H17C | 109.5 |
| C16—N1—C17 | 110.1 (3) | H17A—C17—H17C | 109.5 |
| C16—N1—C15 | 111.0 (2) | H17B—C17—H17C | 109.5 |
| C17—N1—C15 | 108.7 (3) | O2—C18—C23 | 123.8 (2) |
| C33—N2—C34 | 110.2 (3) | O2—C18—C19 | 114.1 (2) |
| C33—N2—C32 | 112.0 (2) | C23—C18—C19 | 122.1 (3) |
| C34—N2—C32 | 108.3 (2) | C20—C19—C18 | 117.0 (3) |
| O1—C1—C6 | 114.4 (2) | C20—C19—C28 | 121.1 (3) |
| O1—C1—C2 | 123.2 (3) | C18—C19—C28 | 121.9 (3) |
| C6—C1—C2 | 122.4 (3) | C21—C20—C19 | 123.4 (3) |
| C3—C2—C1 | 116.4 (3) | C21—C20—H20 | 118.3 |
| C3—C2—C11 | 120.5 (3) | C19—C20—H20 | 118.3 |
| C1—C2—C11 | 123.1 (3) | C20—C21—C22 | 117.8 (3) |
| C4—C3—C2 | 123.5 (3) | C20—C21—C32 | 121.2 (3) |
| C4—C3—H3 | 118.3 | C22—C21—C32 | 121.0 (3) |
| C2—C3—H3 | 118.3 | C21—C22—C23 | 123.3 (3) |
| C3—C4—C5 | 117.6 (3) | C21—C22—H22 | 118.4 |
| C3—C4—C15 | 120.6 (3) | C23—C22—H22 | 118.4 |
| C5—C4—C15 | 121.8 (3) | C22—C23—C18 | 116.5 (3) |
| C4—C5—C6 | 123.0 (3) | C22—C23—C24 | 120.6 (3) |
| C4—C5—H5 | 118.5 | C18—C23—C24 | 122.8 (3) |
| C6—C5—H5 | 118.5 | C26—C24—C25 | 106.9 (3) |
| C5—C6—C1 | 117.0 (3) | C26—C24—C23 | 109.9 (2) |
| C5—C6—C7 | 121.4 (3) | C25—C24—C23 | 111.7 (3) |
| C1—C6—C7 | 121.6 (3) | C26—C24—C27 | 110.7 (3) |
| C9—C7—C8 | 110.5 (3) | C25—C24—C27 | 106.1 (3) |
| C9—C7—C10 | 105.9 (3) | C23—C24—C27 | 111.3 (3) |
| C8—C7—C10 | 107.7 (3) | C24—C25—H25A | 109.5 |
| C9—C7—C6 | 111.4 (2) | C24—C25—H25B | 109.5 |
| C8—C7—C6 | 109.7 (2) | H25A—C25—H25B | 109.5 |
| C10—C7—C6 | 111.5 (3) | C24—C25—H25C | 109.5 |
| C7—C8—H8A | 109.5 | H25A—C25—H25C | 109.5 |
| C7—C8—H8B | 109.5 | H25B—C25—H25C | 109.5 |
| H8A—C8—H8B | 109.5 | C24—C26—H26A | 109.5 |
| C7—C8—H8C | 109.5 | C24—C26—H26B | 109.5 |
| H8A—C8—H8C | 109.5 | H26A—C26—H26B | 109.5 |
| H8B—C8—H8C | 109.5 | C24—C26—H26C | 109.5 |
| C7—C9—H9A | 109.5 | H26A—C26—H26C | 109.5 |
| C7—C9—H9B | 109.5 | H26B—C26—H26C | 109.5 |
| H9A—C9—H9B | 109.5 | C24—C27—H27A | 109.5 |
| C7—C9—H9C | 109.5 | C24—C27—H27B | 109.5 |
| H9A—C9—H9C | 109.5 | H27A—C27—H27B | 109.5 |
| H9B—C9—H9C | 109.5 | C24—C27—H27C | 109.5 |
| C7—C10—H10A | 109.5 | H27A—C27—H27C | 109.5 |
| C7—C10—H10B | 109.5 | H27B—C27—H27C | 109.5 |
| H10A—C10—H10B | 109.5 | C29—C28—C30 | 111.1 (3) |
| C7—C10—H10C | 109.5 | C29—C28—C19 | 110.6 (3) |
| H10A—C10—H10C | 109.5 | C30—C28—C19 | 110.5 (2) |
| H10B—C10—H10C | 109.5 | C29—C28—C31 | 107.1 (2) |
| C14—C11—C12 | 106.7 (3) | C30—C28—C31 | 105.9 (3) |
| C14—C11—C13 | 106.5 (3) | C19—C28—C31 | 111.5 (3) |
| C12—C11—C13 | 111.6 (3) | C28—C29—H29A | 109.5 |
| C14—C11—C2 | 111.7 (3) | C28—C29—H29B | 109.5 |
| C12—C11—C2 | 111.5 (3) | H29A—C29—H29B | 109.5 |
| C13—C11—C2 | 108.8 (2) | C28—C29—H29C | 109.5 |
| C11—C12—H12A | 109.5 | H29A—C29—H29C | 109.5 |
| C11—C12—H12B | 109.5 | H29B—C29—H29C | 109.5 |
| H12A—C12—H12B | 109.5 | C28—C30—H30A | 109.5 |
| C11—C12—H12C | 109.5 | C28—C30—H30B | 109.5 |
| H12A—C12—H12C | 109.5 | H30A—C30—H30B | 109.5 |
| H12B—C12—H12C | 109.5 | C28—C30—H30C | 109.5 |
| C11—C13—H13A | 109.5 | H30A—C30—H30C | 109.5 |
| C11—C13—H13B | 109.5 | H30B—C30—H30C | 109.5 |
| H13A—C13—H13B | 109.5 | C28—C31—H31A | 109.5 |
| C11—C13—H13C | 109.5 | C28—C31—H31B | 109.5 |
| H13A—C13—H13C | 109.5 | H31A—C31—H31B | 109.5 |
| H13B—C13—H13C | 109.5 | C28—C31—H31C | 109.5 |
| C11—C14—H14A | 109.5 | H31A—C31—H31C | 109.5 |
| C11—C14—H14B | 109.5 | H31B—C31—H31C | 109.5 |
| H14A—C14—H14B | 109.5 | N2—C32—C21 | 114.4 (2) |
| C11—C14—H14C | 109.5 | N2—C32—H32A | 108.7 |
| H14A—C14—H14C | 109.5 | C21—C32—H32A | 108.7 |
| H14B—C14—H14C | 109.5 | N2—C32—H32B | 108.7 |
| N1—C15—C4 | 113.8 (3) | C21—C32—H32B | 108.7 |
| N1—C15—H15A | 108.8 | H32A—C32—H32B | 107.6 |
| C4—C15—H15A | 108.8 | N2—C33—H33A | 109.5 |
| N1—C15—H15B | 108.8 | N2—C33—H33B | 109.5 |
| C4—C15—H15B | 108.8 | H33A—C33—H33B | 109.5 |
| H15A—C15—H15B | 107.7 | N2—C33—H33C | 109.5 |
| N1—C16—H16A | 109.5 | H33A—C33—H33C | 109.5 |
| N1—C16—H16B | 109.5 | H33B—C33—H33C | 109.5 |
| H16A—C16—H16B | 109.5 | N2—C34—H34A | 109.5 |
| N1—C16—H16C | 109.5 | N2—C34—H34B | 109.5 |
| H16A—C16—H16C | 109.5 | H34A—C34—H34B | 109.5 |
| H16B—C16—H16C | 109.5 | N2—C34—H34C | 109.5 |
| N1—C17—H17A | 109.5 | H34A—C34—H34C | 109.5 |
| N1—C17—H17B | 109.5 | H34B—C34—H34C | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N2 | 0.85 | 2.20 | 2.836 (3) | 132 |
| O2—H2···N1i | 0.86 | 2.26 | 2.933 (3) | 135 |
Symmetry codes: (i) −x+2, y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: DN2296).
References
- Bruker (1997). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Burnett, M. N. & Johnson, C. K. (1996). ORTEPIII. Report ORNL-6895. Oak Ridge National Laboratory, Tennessee, USA.
- Ciba-Geigy AG (1978). Swiss Patent CH597 297.
- Coffield, T. H. (1965). US Patent 3 208 859.
- Coffield, T. H. & Mich, F. (1965). US Patent 3 225 099.
- Eggensperger, H., Franzen, V. & Kloss, W. (1974). US Patent 3 950 382.
- Eggensperger, H., Franzen, V. & Kloss, W. (1976). US Patent 3 856 846.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Rieker, A., Kaufmann, H., Brück, D., Workman, R. & Müller, E. (1968). Tetrahedron, 24, 103–115.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (1997). SHELXS97 and SHELXL97 University of Göttingen, Germany.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Yamazaki, T. & Seguchi, T. (1997). J. Polym. Sci. Part A Polym. Chem.35, 2431–2439.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807065117/dn2296sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807065117/dn2296Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report