Abstract
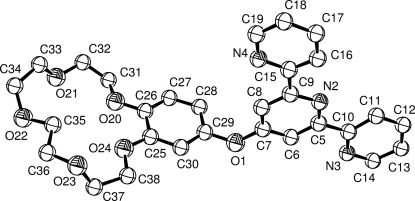
The central pyridine ring of the 2,2′:6′,2′′-terpyridine fragment of the title compound, C29H29N3O6, forms dihedral angles of 5.2 (5), 10.1 (5) and 86.0 (6)°, respectively, with the two outer pyridine rings and the benzene ring of the benzo-15-crown-5 fragment.
Related literature
For related crystal structures determined from synchrotron powder diffraction data, see Dorokhov et al. (2007 ▶). For useful applications of 2,2′:6′,2"-terpyridine derivatives, see Andres et al. (2003 ▶). For details of the synthesis of the title compound, see: Constable & Ward (1990 ▶); Chitta et al. (2004 ▶); Kobayashi (2001 ▶). For details of the indexing algorithm, see Visser (1969 ▶).
Experimental
Crystal data
C29H29N3O6
M r = 515.55
Orthorhombic,
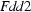
a = 58.347 (11) Å
b = 33.712 (3) Å
c = 5.3211 (8) Å
V = 10467 (3) Å3
Z = 16
Cu Kα1 radiation
λ = 1.54059 Å
μ = 0.76 mm−1
T = 295 (2) K
Specimen shape: flat sheet
15 × 1 × 1 mm
Specimen prepared at 295 (2) K and 101 kPa
Particle morphology: no specific habit, colourless
Data collection
G670 Guinier camera diffractometer
Specimen mounting: thin layer in the specimen holder of the camera
Specimen mounted in transmission mode
Scan method: continuous
Absorption correction: none
2θmin = 4.5, 2θmax = 75.0°
Increment in 2θ = 0.01°
Refinement
R p = 0.019
R wp = 0.024
R exp = 0.018
R B = 0.023
S = 1.36
Excluded region(s): none
Profile function: split-type pseudo-Voigt (Toraya, 1986 ▶)
148 parameters
173 restraints
H-atom parameters not refined
Preferred orientation correction: none
Data collection: local program (Huber, 2002 ▶); cell refinement: MRIA (Zlokazov & Chernyshev, 1992 ▶); data reduction: local program (Huber, 2002 ▶); program(s) used to solve structure: grid search (Chernyshev & Schenk, 1998 ▶); program(s) used to refine structure: MRIA; molecular graphics: PLATON (Spek, 2003 ▶); software used to prepare material for publication: MRIA, SHELXL97 (Sheldrick, 1997 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807064999/ya2062sup1.cif
Rietveld powder data: contains datablocks I. DOI: 10.1107/S1600536807064999/ya2062Isup2.rtv
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Comment
Ability of 2,2':6',2"-terpyridines to form complexes with transition metals, such as Zn(II), Fe(II), Co(II), Ni(II) and Ru(II) is widely used in supramolecular chemistry to control self organization processes. Introduction of additional fragments with various coordinating groups to a molecule of 2,2':6',2"-terpyridine opens broad prospects for purposeful design of supramolecular compounds (Andres et al., 2003). 4'-(Benzo-15-crown-5) substituted 2,2':6',2"-terpyridines represent a new interesting class of compounds involving both terpyridine fragment, which selectively binds the d-element cations, and benzo-15-crown-5 with unique ability for complexation of cations of alkaline, alkaline-earth metals as well as those of organic amines. Compounds of this class offer new opportunities for use of metal-ligand interactions for controllable assembly of the supramolecular complexes. We present here the structure of title compound, (I).
In (I) (Fig. 1), all bond lengths and angles are comparable with those reported earlier for the related compounds (Dorokhov et al., 2007). The six-membered rings N2/C5—C9 (A), N3/C10—C14 (B), N4/C15—C19 (C) and C25—C30 (D) form the following dihedral angles - A/B 5.2 (5)°, A/C 10.1 (5)° and A/D 86.0 (6)°.
Experimental
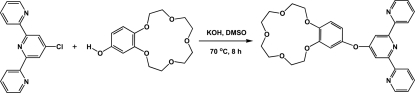
The synthesis of 2,2':6',2"-terpyridine was carried out according to Constable & Ward (1990). 4'-Hydroxy-benzo-15-crown-5 was obtained on the basis of commercially accessible benzo-15-crown-5 by the formylation reaction (Chitta et al., 2004) and the subsequent oxidizing decarbonylation (Kobayashi, 2001). 4'-(4'''-Benzo-15-crown-5)-oxy-2,2':6',2''-terpyridine has been synthesized using reaction of nucleophilic replacement of 4'-chloro-2,2':6',2''-terpyridine with 4'-hydroxy-benzo-15-crown-5 in the dry DMSO in the presence of a base (KOH).
The synthesis of 4'-(4'''-benzo-15-crown-5)-oxy-2,2':6',2''-terpyridine (Scheme 2): to a stirred suspension of powdered KOH (890 mg, 15,90 mmol) in dry DMSO (12 ml) in the argon atmosphere at 70 °C, the 4'-hydroxy-benzo-15-crown-5 (928 mg, 3,269 mmol) was added. After 30 min, 4'-chloro-2,2':6',2''-terpyridine (873 mg, 3,269 mmol) was added and the mixture was stirred for 8 h at 70 °C and then poured into 150 ml of ice water. The water phase was extracted with chloroform (150 x 100 x 50 x 50 ml), dried over MgSO4 and evaporated in vacuum. The residue (20 ml) was purified by column chromatography on the neutral Al2O3 (CHCl3). After evaporation, the product, obtained as a yellowish oil, was crystallized from diethyl ether, then filtered and washed on the filter by the cooled diethyl ether, recrystallized from methyl alcohol (25 ml) and dried in vacuum at 60 °C. The yield of the target product as white solid was 623 mg (37,2%). (M. p. = 151 °C)
Refinement
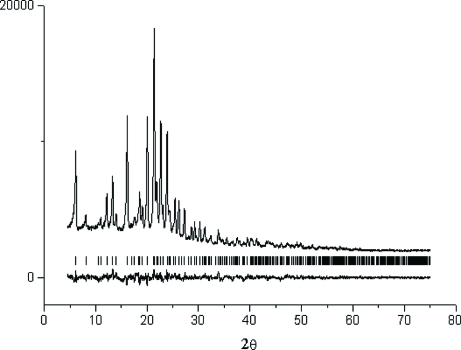
During the exposure, the specimen was spun in its plane to improve particle statistics. The orthorhombic unit-cell dimensions were determined with the indexing program ITO (Visser, 1969), M20=44, using the first 35 peak positions. The space group Fdd2 was chosen on the basis of systematic extinction rules and confirmed later by the crystal structure solution. The structure of (I) was solved by the systematic grid search procedure (Chernyshev & Schenk, 1998) and refined following the methodology described in detail elsewhere (Dorokhov et al., 2007) by the subsequent bond-restrained Rietveld refinement with the program MRIA (Zlokazov & Chernyshev, 1992). All O atoms were refined isotropically with the overall Uiso parameter. The Uiso for the rest of non-H atoms were fixed at 0.051 Å2. All H atoms were placed in geometrically calculated positions and not refined. The diffraction profiles and the differences between the measured and calculated profiles are shown in Fig. 2.
Figures
Fig. 1.
The molecular structure of (I) with the atomic numbering and 50% displacement spheres. H atoms omitted for clarity.
Fig. 2.
The Rietveld plot, showing the observed and difference profiles for (I). The reflection positions are shown above the difference profile.
Fig. 3.
The formation of the title compound.
Crystal data
| C29H29N3O6 | Dx = 1.309 Mg m−3 |
| Mr = 515.55 | Cu Kα1 radiation λ = 1.54059 Å |
| Orthorhombic, Fdd2 | µ = 0.76 mm−1 |
| a = 58.347 (11) Å | T = 295 (2) K |
| b = 33.712 (3) Å | Specimen shape: flat_sheet |
| c = 5.3211 (8) Å | 15 × 1 × 1 mm |
| V = 10467 (3) Å3 | Specimen prepared at 101 kPa |
| Z = 16 | Specimen prepared at 295(2) K |
| F000 = 4352 | Particle morphology: no specific habit, colourless |
Data collection
| Guinier camera G670 diffractometer | Scan method: continuous |
| Radiation source: line-focus sealed tube | T = 295(2) K |
| Monochromator: Curved Germanium (111) | 2θmin = 4.50, 2θmax = 75.00º |
| Specimen mounting: thin layer in the specimen holder of the camera | Increment in 2θ = 0.01º |
| Specimen mounted in transmission mode | Increment in 2θ = 0.01º |
Refinement
| Refinement on Inet | Profile function: split-type pseudo-Voigt (Toraya, 1986) |
| Least-squares matrix: full with fixed elements per cycle | 148 parameters |
| Rp = 0.019 | 173 restraints |
| Rwp = 0.024 | 7 constraints |
| Rexp = 0.018 | H-atom parameters not refined |
| RB = 0.023 | Weighting scheme based on measured s.u.'s ? |
| S = 1.36 | (Δ/σ)max = 0.007 |
| Wavelength of incident radiation: 1.54059 Å | Extinction correction: none |
| Excluded region(s): none | Preferred orientation correction: none |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.0046 (3) | 0.6614 (4) | 0.1346 | 0.067 (3)* | |
| N2 | 0.0097 (3) | 0.5679 (6) | 0.655 (3) | 0.051* | |
| N3 | 0.0633 (3) | 0.5704 (6) | 0.288 (4) | 0.051* | |
| N4 | −0.0478 (3) | 0.6009 (6) | 0.858 (4) | 0.051* | |
| C5 | 0.0253 (4) | 0.5800 (7) | 0.478 (4) | 0.051* | |
| C6 | 0.0209 (4) | 0.6110 (7) | 0.301 (4) | 0.051* | |
| H6 | 0.0319 | 0.6180 | 0.1824 | 0.061* | |
| C7 | 0.0000 (4) | 0.6304 (7) | 0.310 (4) | 0.051* | |
| C8 | −0.0160 (4) | 0.6196 (7) | 0.486 (4) | 0.051* | |
| H8 | −0.0301 | 0.6325 | 0.4940 | 0.061* | |
| C9 | −0.0108 (4) | 0.5887 (7) | 0.654 (4) | 0.051* | |
| C10 | 0.0482 (4) | 0.5595 (7) | 0.471 (4) | 0.051* | |
| C11 | 0.0544 (4) | 0.5322 (6) | 0.660 (4) | 0.051* | |
| H11 | 0.0445 | 0.5270 | 0.7934 | 0.061* | |
| C12 | 0.0756 (3) | 0.5132 (7) | 0.645 (4) | 0.051* | |
| H12 | 0.0798 | 0.4945 | 0.7643 | 0.061* | |
| C13 | 0.0905 (4) | 0.5225 (7) | 0.448 (4) | 0.051* | |
| H13 | 0.1045 | 0.5096 | 0.4299 | 0.061* | |
| C14 | 0.0837 (4) | 0.5518 (7) | 0.278 (4) | 0.051* | |
| H14 | 0.0938 | 0.5589 | 0.1510 | 0.061* | |
| C15 | −0.0286 (3) | 0.5771 (6) | 0.840 (3) | 0.051* | |
| C16 | −0.0263 (4) | 0.5425 (7) | 0.984 (4) | 0.051* | |
| H16 | −0.0137 | 0.5259 | 0.9615 | 0.061* | |
| C17 | −0.0431 (4) | 0.5332 (7) | 1.161 (4) | 0.051* | |
| H17 | −0.0415 | 0.5110 | 1.2628 | 0.061* | |
| C18 | −0.0624 (4) | 0.5579 (7) | 1.183 (4) | 0.051* | |
| H18 | −0.0744 | 0.5516 | 1.2909 | 0.061* | |
| C19 | −0.0630 (4) | 0.5925 (7) | 1.035 (4) | 0.051* | |
| H19 | −0.0748 | 0.6106 | 1.0631 | 0.061* | |
| O20 | −0.0608 (2) | 0.7962 (4) | 0.187 (3) | 0.067 (3)* | |
| O21 | −0.0911 (2) | 0.8612 (4) | 0.246 (3) | 0.067 (3)* | |
| O22 | −0.1263 (3) | 0.8329 (4) | −0.111 (3) | 0.067 (3)* | |
| O23 | −0.1184 (2) | 0.7296 (4) | −0.314 (3) | 0.067 (3)* | |
| O24 | −0.0706 (2) | 0.7416 (4) | −0.138 (3) | 0.067 (3)* | |
| C25 | −0.0511 (4) | 0.7344 (7) | −0.001 (4) | 0.051* | |
| C26 | −0.0454 (4) | 0.7653 (7) | 0.176 (4) | 0.051* | |
| C27 | −0.0263 (3) | 0.7608 (7) | 0.327 (4) | 0.051* | |
| H27 | −0.0215 | 0.7816 | 0.4291 | 0.061* | |
| C28 | −0.0139 (4) | 0.7242 (7) | 0.325 (4) | 0.051* | |
| H28 | −0.0025 | 0.7196 | 0.4433 | 0.061* | |
| C29 | −0.0190 (4) | 0.6956 (8) | 0.146 (4) | 0.051* | |
| C30 | −0.0376 (3) | 0.6998 (6) | −0.014 (4) | 0.051* | |
| H30 | −0.0411 | 0.6800 | −0.1292 | 0.061* | |
| C31 | −0.0540 (4) | 0.8312 (7) | 0.328 (4) | 0.051* | |
| H31A | −0.0444 | 0.8235 | 0.4689 | 0.061* | |
| H31B | −0.0453 | 0.8490 | 0.2215 | 0.061* | |
| C32 | −0.0743 (4) | 0.8510 (7) | 0.419 (4) | 0.051* | |
| H32A | −0.0813 | 0.8341 | 0.5447 | 0.061* | |
| H32B | −0.0695 | 0.8751 | 0.5038 | 0.061* | |
| C33 | −0.1144 (3) | 0.8536 (7) | 0.324 (4) | 0.051* | |
| H33A | −0.1188 | 0.8739 | 0.4437 | 0.061* | |
| H33B | −0.1147 | 0.8284 | 0.4119 | 0.061* | |
| C34 | −0.1324 (4) | 0.8524 (7) | 0.116 (4) | 0.051* | |
| H34A | −0.1460 | 0.8396 | 0.1823 | 0.061* | |
| H34B | −0.1366 | 0.8795 | 0.0750 | 0.061* | |
| C35 | −0.1172 (4) | 0.7936 (7) | −0.091 (4) | 0.051* | |
| H35A | −0.1202 | 0.7829 | 0.0754 | 0.061* | |
| H35B | −0.1008 | 0.7940 | −0.1177 | 0.061* | |
| C36 | −0.1289 (4) | 0.7680 (7) | −0.291 (4) | 0.051* | |
| H36A | −0.1449 | 0.7647 | −0.2477 | 0.061* | |
| H36B | −0.1281 | 0.7815 | −0.4518 | 0.061* | |
| C37 | −0.0982 (4) | 0.7268 (6) | −0.462 (4) | 0.051* | |
| H37A | −0.0945 | 0.7529 | −0.5273 | 0.061* | |
| H37B | −0.1012 | 0.7095 | −0.6039 | 0.061* | |
| C38 | −0.0776 (4) | 0.7110 (6) | −0.319 (4) | 0.051* | |
| H38A | −0.0816 | 0.6867 | −0.2314 | 0.061* | |
| H38B | −0.0651 | 0.7053 | −0.4341 | 0.061* |
Geometric parameters (Å, °)
| O1—C7 | 1.43 (3) | C28—C29 | 1.39 (3) |
| O1—C29 | 1.43 (3) | C29—C30 | 1.39 (3) |
| O20—C26 | 1.38 (3) | C31—C32 | 1.44 (3) |
| O20—C31 | 1.45 (3) | C33—C34 | 1.53 (3) |
| O21—C32 | 1.39 (3) | C35—C36 | 1.53 (3) |
| O21—C33 | 1.45 (2) | C37—C38 | 1.52 (3) |
| O22—C34 | 1.42 (3) | C6—H6 | 0.93 |
| O22—C35 | 1.43 (3) | C8—H8 | 0.93 |
| O23—C36 | 1.44 (3) | C11—H11 | 0.93 |
| O23—C37 | 1.42 (3) | C12—H12 | 0.93 |
| O24—C25 | 1.37 (3) | C13—H13 | 0.93 |
| O24—C38 | 1.47 (3) | C14—H14 | 0.93 |
| N2—C5 | 1.38 (3) | C16—H16 | 0.93 |
| N2—C9 | 1.39 (3) | C17—H17 | 0.93 |
| N3—C10 | 1.36 (3) | C18—H18 | 0.93 |
| N3—C14 | 1.35 (3) | C19—H19 | 0.93 |
| N4—C15 | 1.38 (3) | C27—H27 | 0.93 |
| N4—C19 | 1.32 (3) | C28—H28 | 0.93 |
| C5—C6 | 1.43 (3) | C30—H30 | 0.93 |
| C5—C10 | 1.50 (3) | C31—H31A | 0.97 |
| C6—C7 | 1.38 (3) | C31—H31B | 0.97 |
| C7—C8 | 1.37 (3) | C32—H32A | 0.97 |
| C8—C9 | 1.41 (3) | C32—H32B | 0.97 |
| C9—C15 | 1.49 (3) | C33—H33A | 0.97 |
| C10—C11 | 1.41 (3) | C33—H33B | 0.97 |
| C11—C12 | 1.40 (3) | C34—H34A | 0.97 |
| C12—C13 | 1.40 (3) | C34—H34B | 0.97 |
| C13—C14 | 1.39 (3) | C35—H35A | 0.97 |
| C15—C16 | 1.40 (3) | C35—H35B | 0.97 |
| C16—C17 | 1.40 (3) | C36—H36A | 0.97 |
| C17—C18 | 1.41 (3) | C36—H36B | 0.97 |
| C18—C19 | 1.41 (3) | C37—H37A | 0.97 |
| C25—C26 | 1.44 (3) | C37—H37B | 0.97 |
| C25—C30 | 1.41 (3) | C38—H38A | 0.97 |
| C26—C27 | 1.38 (3) | C38—H38B | 0.97 |
| C27—C28 | 1.43 (3) | ||
| C7—O1—C29 | 132.4 (14) | C11—C12—H12 | 120 |
| C26—O20—C31 | 117.3 (16) | C13—C12—H12 | 120 |
| C32—O21—C33 | 115.2 (16) | C12—C13—H13 | 121 |
| C34—O22—C35 | 117.3 (16) | C14—C13—H13 | 121 |
| C36—O23—C37 | 117.4 (15) | N3—C14—H14 | 118 |
| C25—O24—C38 | 117.0 (16) | C13—C14—H14 | 118 |
| C5—N2—C9 | 114.7 (19) | C15—C16—H16 | 120 |
| C10—N3—C14 | 118.3 (19) | C17—C16—H16 | 120 |
| C15—N4—C19 | 117.9 (19) | C16—C17—H17 | 120 |
| N2—C5—C6 | 123 (2) | C18—C17—H17 | 121 |
| N2—C5—C10 | 117.8 (19) | C17—C18—H18 | 121 |
| C6—C5—C10 | 118.8 (19) | C19—C18—H18 | 121 |
| C5—C6—C7 | 119 (2) | N4—C19—H19 | 118 |
| O1—C7—C6 | 119.3 (19) | C18—C19—H19 | 118 |
| O1—C7—C8 | 121 (2) | C26—C27—H27 | 120 |
| C6—C7—C8 | 120 (2) | C28—C27—H27 | 120 |
| C7—C8—C9 | 119 (2) | C27—C28—H28 | 120 |
| N2—C9—C8 | 124 (2) | C29—C28—H28 | 120 |
| N2—C9—C15 | 117.8 (19) | C25—C30—H30 | 120 |
| C8—C9—C15 | 118 (2) | C29—C30—H30 | 120 |
| N3—C10—C5 | 117.7 (16) | O20—C31—H31A | 110 |
| N3—C10—C11 | 121 (2) | O20—C31—H31B | 110 |
| C5—C10—C11 | 120.8 (19) | C32—C31—H31A | 110 |
| C10—C11—C12 | 119 (2) | C32—C31—H31B | 110 |
| C11—C12—C13 | 119 (2) | H31A—C31—H31B | 108 |
| C12—C13—C14 | 118 (2) | O21—C32—H32A | 108 |
| N3—C14—C13 | 124 (2) | O21—C32—H32B | 108 |
| N4—C15—C9 | 117.4 (18) | C31—C32—H32A | 108 |
| N4—C15—C16 | 121.5 (18) | C31—C32—H32B | 108 |
| C9—C15—C16 | 121.0 (18) | H32A—C32—H32B | 107 |
| C15—C16—C17 | 119 (2) | O21—C33—H33A | 108 |
| C16—C17—C18 | 119 (2) | O21—C33—H33B | 108 |
| C17—C18—C19 | 118 (2) | C34—C33—H33A | 108 |
| N4—C19—C18 | 124 (2) | C34—C33—H33B | 108 |
| O24—C25—C26 | 114.2 (19) | H33A—C33—H33B | 107 |
| O24—C25—C30 | 125.7 (19) | O22—C34—H34A | 108 |
| C26—C25—C30 | 120 (2) | O22—C34—H34B | 108 |
| O20—C26—C25 | 115.1 (19) | C33—C34—H34A | 108 |
| O20—C26—C27 | 126 (2) | C33—C34—H34B | 108 |
| C25—C26—C27 | 119 (2) | H34A—C34—H34B | 107 |
| C26—C27—C28 | 120 (2) | O22—C35—H35A | 110 |
| C27—C28—C29 | 120 (2) | O22—C35—H35B | 110 |
| O1—C29—C28 | 117.6 (19) | C36—C35—H35A | 110 |
| O1—C29—C30 | 121 (2) | C36—C35—H35B | 110 |
| C28—C29—C30 | 121 (2) | H35A—C35—H35B | 108 |
| C25—C30—C29 | 119 (2) | O23—C36—H36A | 109 |
| O20—C31—C32 | 108.9 (18) | O23—C36—H36B | 109 |
| O21—C32—C31 | 118.0 (18) | C35—C36—H36A | 109 |
| O21—C33—C34 | 116.2 (17) | C35—C36—H36B | 109 |
| O22—C34—C33 | 117.2 (19) | H36A—C36—H36B | 108 |
| O22—C35—C36 | 107.7 (18) | O23—C37—H37A | 109 |
| O23—C36—C35 | 112.1 (18) | O23—C37—H37B | 109 |
| O23—C37—C38 | 113.7 (17) | C38—C37—H37A | 109 |
| O24—C38—C37 | 107.6 (16) | C38—C37—H37B | 109 |
| C5—C6—H6 | 120 | H37A—C37—H37B | 108 |
| C7—C6—H6 | 120 | O24—C38—H38A | 110 |
| C7—C8—H8 | 120 | O24—C38—H38B | 110 |
| C9—C8—H8 | 120 | C37—C38—H38A | 110 |
| C10—C11—H11 | 120 | C37—C38—H38B | 110 |
| C12—C11—H11 | 120 | H38A—C38—H38B | 108 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: YA2062).
References
- Andres, P. R., Hofmeier, H., Lohmeijer Bas, G. G. & Schubert, U. S. (2003). Synthesis, 18, 2865–2871.
- Chernyshev, V. V. & Schenk, H. (1998). Z. Kristallogr.213, 1–3.
- Chitta, R., Rogers, L. M., Wanklin, A., Karr, P. A., Kahol, P. K., Zandler, M. E. & D’Souza, F. (2004). Inorg. Chem.43, 6969–6978. [DOI] [PubMed]
- Constable, E. C. & Ward, M. D. (1990). J. Chem. Soc. Dalton Trans. pp. 1405–1409.
- Dorokhov, A. V., Chernyshov, D. Y., Burlov, A. S., Garnovskii, A. D., Ivanova, I. S., Pyatova, E. N., Tsivadze, A. Y., Aslanov, L. A. & Chernyshev, V. V. (2007). Acta Cryst. B63, 402–410. [DOI] [PubMed]
- Huber (2002). Software for G670 Imaging Plate Guinier Camera Version 4.3.16. Huber Diffraktionstechnik GmbH, Rimsting, Germany.
- Kobayashi, N. (2001). Coord. Chem. Rev.219–221, 99–123.
- Sheldrick, G. M. (1997). SHELXL97 University of Göttingen, Germany.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Toraya, H. (1986). J. Appl. Cryst.19, 440–447.
- Visser, J. W. (1969). J. Appl. Cryst.2, 89–95.
- Zlokazov, V. B. & Chernyshev, V. V. (1992). J. Appl. Cryst.25, 447–451.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536807064999/ya2062sup1.cif
Rietveld powder data: contains datablocks I. DOI: 10.1107/S1600536807064999/ya2062Isup2.rtv
Additional supplementary materials: crystallographic information; 3D view; checkCIF report