Abstract
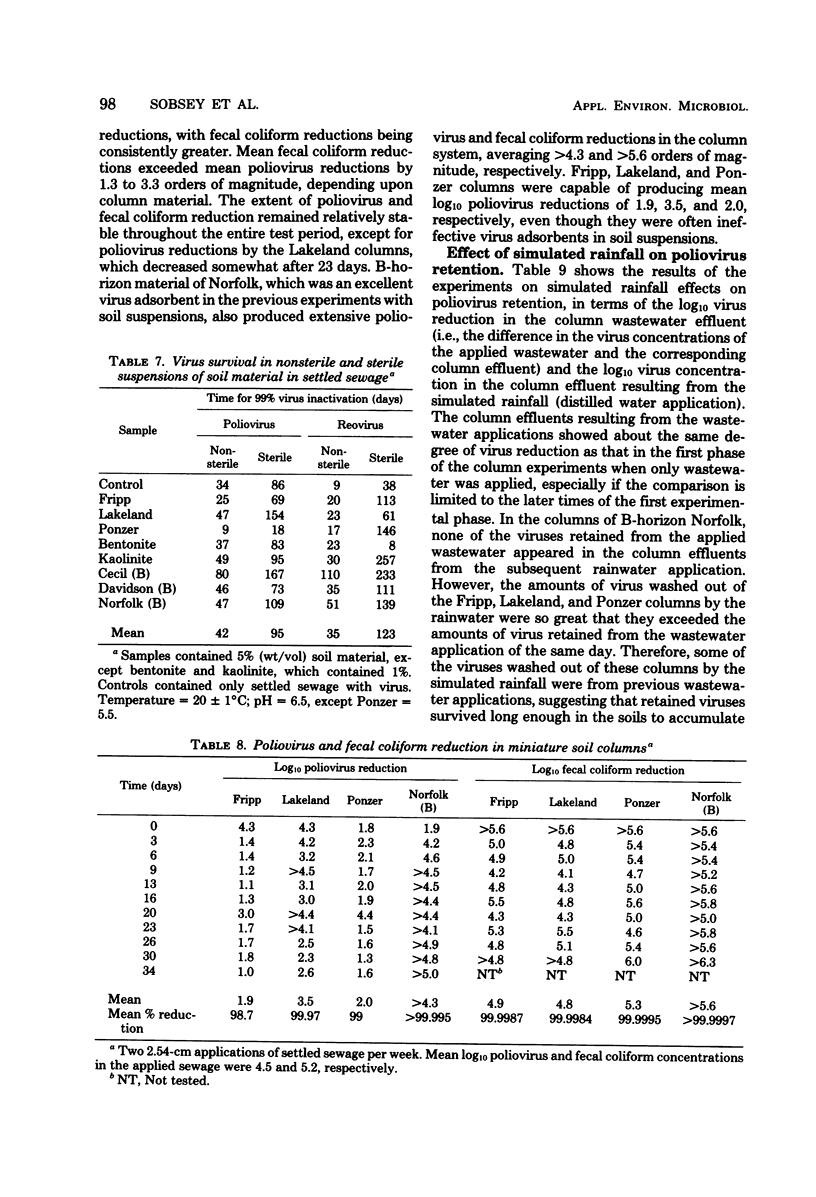
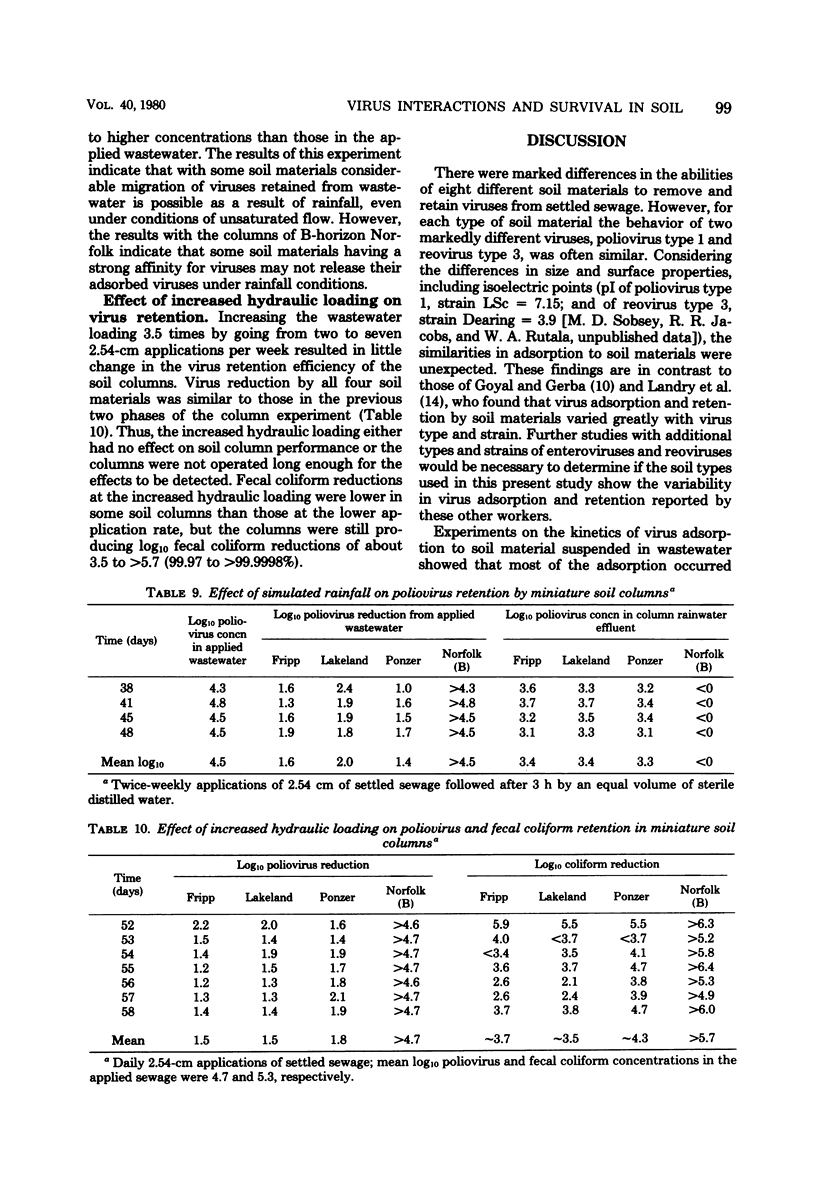
There were marked differences in the abilities of eight different soil materials to remove and retain viruses from settled sewage, but for each soil material the behavior of two different viruses, poliovirus type 1 and reovirus type 3, was often similar. Virus adsorption to soil materials was rapid, the majority occurring within 15 min. Clayey materials efficiently adsorbed both viruses from wastewater over a range of pH and total dissolved solids levels. Sands and organic soil materials were comparatively poor adsorbents, but in some cases their ability to adsorb viruses increased at low pH and with the addition of total dissolved solids or divalent cations. Viruses in suspensions of soil material in settled sewage survived for considerable time periods, despite microbial activity. In some cases virus survival was prolonged in suspensions of soil materials compared to soil-free controls. Although sandy and organic soil materials were poor virus adsorbents when suspended in wastewater, they gave ≥95% virus removal from intermittently applied wastewater as unsaturated, 10-cm-deep columns. However, considerable quantities of the retained viruses were washed from the columns by simulated rainfall. Under the same conditions, clayey soil material removed ≥99.9995% of the viruses from applied wastewater, and none were washed from the columns by simulated rainfall.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duboise S. M., Moore B. E., Sagik B. P. Poliovirus survival and movement in a sandy forest soil. Appl Environ Microbiol. 1976 Apr;31(4):536–543. doi: 10.1128/aem.31.4.536-543.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Schaiberger G. E. Effect of particulates on virus survival in seawater. J Water Pollut Control Fed. 1975 Jan;47(1):93–103. [PubMed] [Google Scholar]
- Gilbert R. G., Gerba C. P., Rice R. C., Bouwer H., Wallis C., Melnick J. L. Virus and bacteria removal from wastewater by land treatment. Appl Environ Microbiol. 1976 Sep;32(3):333–338. doi: 10.1128/aem.32.3.333-338.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979 Aug;38(2):241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance J. C., Gerba C. P., Melnick J. L. Virus movement in soil columns flooded with secondary sewage effluent. Appl Environ Microbiol. 1976 Oct;32(4):520–526. doi: 10.1128/aem.32.4.520-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E. F., Vaughn J. M., Thomas M. Z., Beckwith C. A. Adsorption of enteroviruses to soil cores and their subsequent elution by artificial rainwater. Appl Environ Microbiol. 1979 Oct;38(4):680–687. doi: 10.1128/aem.38.4.680-687.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S. A., Sorber C. A. Virus and bacteria removal from wastewater by rapid infiltration through soil. Appl Environ Microbiol. 1977 Mar;33(3):609–619. doi: 10.1128/aem.33.3.609-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. H., Wallis C., Ward C. H. Inactivation of clay-associated bacteriophage MS-2 by chlorine. Appl Environ Microbiol. 1977 Feb;33(2):385–391. doi: 10.1128/aem.33.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W., Pierce L. V. Demonstration of virus in groundwater after effluent discharge onto soil. Appl Microbiol. 1975 Jun;29(6):751–757. doi: 10.1128/am.29.6.751-757.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


