Abstract
The title molecule (alternative name: pyridine-3-carbohydrazide; C6H7N3O) was obtained from the reaction of ethyl nicotinate with hydrazine hydrate in methanol. In the amide group, the C—N bond is relatively short, suggesting some degree of electronic delocalization in the molecule. The stabilized conformation may be compared with those of isomeric compounds picolinohydrazide (pyridine-2-carbohydrazide) and isonicotinohydrazide (pyridine-4-carbohydrazide). In the title isomer, the pyridine ring forms an angle of 33.79 (9)° with the plane of the non-H atoms of the hydrazide group. This lack of coplanarity between the hydrazide functionality and the pyridine ring is considerably greater than that observed in isonicotinohydrazide (dihedral angle = 17.14°), while picolinohydrazide is almost fully planar. The title isomer forms intermolecular N—H⋯O and N—H⋯N hydrogen bonds, which stabilize the crystal structure.
Related literature
The structure of the same compound has been determined independently and is reported in the following paper (Portalone & Colapietro, 2008 ▶). The structures of picolinohydrazide (Zareef et al., 2006 ▶) and isonicotinohydrazide (Jensen, 1954 ▶; Bhat et al., 1974) have been published. For related literature on the biological activity of these molecules, see: Ouelleta et al. (2004 ▶); Zhao et al. (2007 ▶). For related literature, see: Bhat et al. (1974 ▶); Zareef et al. (2006 ▶).
Experimental
Crystal data
C6H7N3O
M r = 137.15
Orthorhombic,
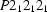
a = 3.8855 (7) Å
b = 10.5191 (5) Å
c = 15.9058 (9) Å
V = 650.10 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 293 (2) K
0.46 × 0.30 × 0.20 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: none
1534 measured reflections
1051 independent reflections
866 reflections with I > 2σ(I)
R int = 0.015
3 standard reflections every 200 reflections intensity decay: <1%
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.087
S = 1.09
1051 reflections
92 parameters
H-atom parameters constrained
Δρmax = 0.18 e Å−3
Δρmin = −0.15 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1994 ▶); cell refinement: CAD-4 EXPRESS; data reduction: HELENA (Spek, 1996 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: PLATON (Spek, 2003 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680706655X/bh2145sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680706655X/bh2145Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2N⋯N1i | 0.88 | 2.11 | 2.975 (2) | 166 |
| N3—H3NA⋯O1ii | 0.87 | 2.22 | 3.045 (2) | 157 |
| N3—H3NB⋯O1iii | 0.85 | 2.55 | 3.155 (2) | 130 |
Symmetry codes: (i) 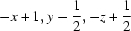 ; (ii)
; (ii) 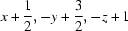 ; (iii)
; (iii) 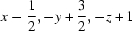 .
.
Table 2. Selected bond lengths (Å) of nicotinohydrazide (I), picolinic acid hydrazide (II) and isonicotinohydrazide (III).
| (I) | (II) | (III) | |
|---|---|---|---|
| N2—N3 | 1.418 (2) | 1.422 | 1.429 |
| C6—N2 | 1.335 (2) | 1.334 | 1.346 |
| C6—O1 | 1.231 (2) | 1.235 | 1.235 |
| C6—C2 | 1.503 (2) | 1.507 | 1.513 |
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC), Financiadora de Estudos e Projetos (FINEP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
supplementary crystallographic information
Comment
The importance of aromatic hydrazides is closely related to their biological activity and to the fact that they can be used for the syntheses of several other biologically active compounds. Nicotinohydrazide, (I), for example, is an efficient peroxidase-activated inhibitor of the POX activity of PGHS-2 (Ouelleta et al., 2004). On the other hand, the isomer isonicotinohydrazide, (III, scheme 2), is not a potent inhibitor, with an IC50 of 129 mM against 15 mM for (I).
Structure also plays a major role in the activity of the anti-tuberculosis drug isonicotinohydrazide, which requires Mycobacterium tuberculosis catalase-peroxidase (KatG) activation to produce an acyl-NAD adduct (Zhao et al., 2007). This adduct is of extreme importance since it is an inhibitor of the enoyl reductase (Mtb InhA), essential for the biosynthesis of acids present in mycobacterial cell walls. Picolinohydrazide, (II), and isonicotinohydrazide, (III), generate the hydrazide-NAD adduct in this system, while nicotinohydrazide, (I), does not. However, the yield of the (II)-NAD adduct is around 35% of that of the (III)-NAD adduct. As a result, (III) is a potent antituberculosis drug, while (I) and (II) are not.
In this context, studies of structural analogues of these biologically active compounds become fundamental and will be useful in elucidating the mechanism of action, which strongly depends on substrate selection and binding stoichiometry to the (III) binding site in KatG, which still has not been completely elucidated.
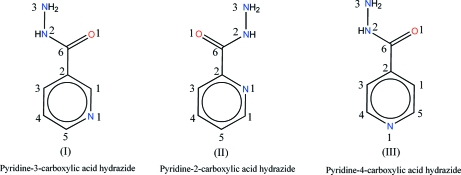
The crystal structures of picolinohydrazide, (II) (Zareef et al., 2006), and isonicotinohydrazide, (III) (Jensen, 1954; Bhat et al., 1974), have been previously reported and the structure of nicotinohydrazide (I) is here described. The three isomeric hydrazides are distinguished by just the position of the N atom in the pyridine ring with respect to hydrazide group (scheme 2). A selection of their structural parameters is shown in Table 2.
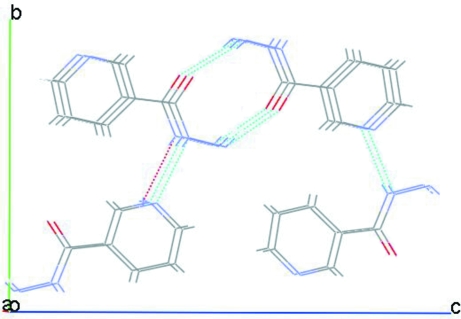
When the structural parameters of isomeric hydrazides are compared, some interesting aspects can be observed, which depend on the structural relation between the N atom in the ring and the hydrazide group. Indeed, while (II) crystallizes in the monoclinic system, isomers (I) and (III) crystallize in the orthorhombic system. The C6?O1 bond length in (I) and also in (II) and (III) are smaller than those usually observed in carboxylic acids (1.365 Å, Zareef et al., 2006). Similarly, the C6—N2 bond distance observed in (I) is consistent with those reported for (II) and (III) hydrazides, suggesting a significant partial double-bond character; the bond lengths are consistent with resonance hybrids between a polar and a neutral form (Bhat et al., 1974). Similar to the results reported (Bhat et al., 1974) for isonicotinohydrazide, the N2—N3 and C2—C6 bonds of (II) have distances similar to their corresponding single bonds. In (I), the pyridine ring bond lengths are very similar to those obtained in related compounds and the ring lies in a plane which forms an angle of 33.79 (9)° with that of the non-H atoms in the hydrazide group. This lack of coplanarity between the hydrazide functionality and the pyridine ring is considerably greater than that observed in isonicotinohydrazide (-17.14°), while picolinohydrazide is almost fully planar, probably because in (II) N2 is in the same side and therefore closer to N1, favoring intramolecular N2—H···N1 hydrogen bond. Conversely, in the crystal structure of (I) N2 and N1 are on opposite sides of the molecule, and in this case only intermolecular hydrogen bonding takes place. The intermolecular hydrogen bonds N3—H···O1 and N2—H···N1 (Table 1), which form a three-dimensional polymeric structure (Fig. 2) are fundamental for the stability of the crystal structure of (I).
Experimental
Nicotinic acid hydrazine was synthesized by the reaction of ethyl nicotinate (43.9 mmol) and hydrazine hydrate 99% (27.5 mmol) in methanol. The reaction mixture was refluxed for 24 h., yielding a yellow solution. Upon cooling to 298 K, the product precipitated and it was washed with methanol and filtered. Colorless needle shaped crystals of (I) suitable for X-ray analysis were grown by recrystallization from a chloroform-methanol (9:1) solution by slow evaporation at room temperature.
Refinement
All non-H atoms were refined with anisotropic displacement parameters. H atoms attached to C atoms were added at their calculated positions, with C—H = 0.93 Å and Uiso(H) = 1.2Ueq(carrier C). H atoms of the hydrazide group were found in a difference map and treated with a riding model and with Uiso(H) = 1.2Ueq(carrier N). In the absence of significant anomalous scattering effects, no Friedel pairs were collected.
Figures
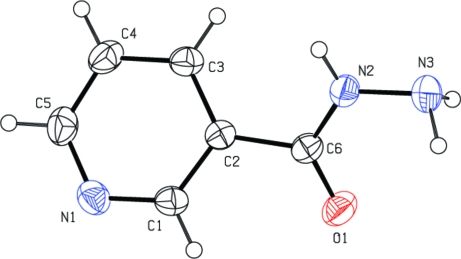
Fig. 1.
The molecular structure of (I) with labeling scheme. Displacement ellipsoids are shown at the 40% probability level.
Fig. 2.
Packing of (I) showing the molecules connected through hydrogen bonds and stacked along [100].
Fig. 3.
The structures of (I)–(III).
Crystal data
| C6H7N3O | F000 = 288 |
| Mr = 137.15 | Dx = 1.401 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 25 reflections |
| a = 3.8855 (7) Å | θ = 5.5–18.7º |
| b = 10.5191 (5) Å | µ = 0.10 mm−1 |
| c = 15.9058 (9) Å | T = 293 (2) K |
| V = 650.10 (13) Å3 | Prismatic, colourless |
| Z = 4 | 0.46 × 0.30 × 0.20 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.015 |
| Radiation source: fine-focus sealed tube | θmax = 29.0º |
| Monochromator: graphite | θmin = 2.3º |
| T = 293(2) K | h = −5→2 |
| ω–2θ scans | k = −14→0 |
| Absorption correction: none | l = −21→0 |
| 1534 measured reflections | 3 standard reflections |
| 1051 independent reflections | every 200 reflections |
| 866 reflections with I > 2σ(I) | intensity decay: <1% |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.031 | w = 1/[σ2(Fo2) + (0.0344P)2 + 0.1144P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.087 | (Δ/σ)max < 0.001 |
| S = 1.09 | Δρmax = 0.18 e Å−3 |
| 1051 reflections | Δρmin = −0.15 e Å−3 |
| 92 parameters | Extinction correction: SHELXL97 (Sheldrick, 1997), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.040 (6) |
| Secondary atom site location: difference Fourier map |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.3825 (5) | 0.84759 (16) | 0.25136 (11) | 0.0373 (4) | |
| H1 | 0.4007 | 0.9092 | 0.2930 | 0.045* | |
| C2 | 0.2298 (5) | 0.73203 (14) | 0.27215 (10) | 0.0319 (4) | |
| C3 | 0.2025 (5) | 0.64013 (16) | 0.20969 (10) | 0.0382 (4) | |
| H3 | 0.1029 | 0.5617 | 0.2212 | 0.046* | |
| C4 | 0.3262 (6) | 0.66735 (19) | 0.12995 (11) | 0.0455 (5) | |
| H4 | 0.3114 | 0.6076 | 0.0870 | 0.055* | |
| C5 | 0.4720 (7) | 0.78517 (19) | 0.11576 (11) | 0.0472 (5) | |
| H5 | 0.5524 | 0.8032 | 0.0620 | 0.057* | |
| C6 | 0.0965 (5) | 0.71648 (17) | 0.36028 (10) | 0.0344 (4) | |
| N1 | 0.5043 (5) | 0.87491 (14) | 0.17489 (10) | 0.0440 (4) | |
| N2 | 0.1167 (5) | 0.59906 (15) | 0.39179 (9) | 0.0405 (4) | |
| H2N | 0.2155 | 0.5365 | 0.3637 | 0.049* | |
| N3 | −0.0001 (5) | 0.56759 (16) | 0.47365 (9) | 0.0472 (4) | |
| H3NA | 0.0877 | 0.6189 | 0.5112 | 0.057* | |
| H3NB | −0.2101 | 0.5876 | 0.4784 | 0.057* | |
| O1 | −0.0227 (5) | 0.80795 (12) | 0.39876 (8) | 0.0487 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0405 (9) | 0.0307 (7) | 0.0405 (9) | 0.0021 (8) | −0.0023 (8) | −0.0007 (6) |
| C2 | 0.0312 (8) | 0.0314 (7) | 0.0330 (7) | 0.0041 (8) | −0.0025 (7) | −0.0001 (6) |
| C3 | 0.0445 (11) | 0.0316 (8) | 0.0384 (8) | 0.0007 (8) | −0.0049 (8) | −0.0009 (7) |
| C4 | 0.0594 (14) | 0.0442 (9) | 0.0328 (8) | 0.0060 (11) | −0.0039 (9) | −0.0057 (7) |
| C5 | 0.0550 (13) | 0.0522 (10) | 0.0345 (8) | 0.0062 (11) | 0.0050 (9) | 0.0069 (8) |
| C6 | 0.0324 (9) | 0.0367 (8) | 0.0339 (7) | 0.0012 (8) | −0.0035 (7) | −0.0040 (7) |
| N1 | 0.0481 (10) | 0.0388 (7) | 0.0451 (8) | 0.0002 (8) | 0.0019 (8) | 0.0078 (6) |
| N2 | 0.0505 (10) | 0.0373 (7) | 0.0337 (7) | 0.0027 (7) | 0.0057 (7) | 0.0007 (6) |
| N3 | 0.0557 (11) | 0.0519 (9) | 0.0338 (7) | −0.0021 (10) | 0.0028 (8) | 0.0045 (6) |
| O1 | 0.0604 (10) | 0.0447 (7) | 0.0409 (6) | 0.0120 (7) | 0.0060 (7) | −0.0060 (5) |
Geometric parameters (Å, °)
| C1—N1 | 1.336 (2) | C5—N1 | 1.338 (2) |
| C1—C2 | 1.392 (2) | C5—H5 | 0.9300 |
| C1—H1 | 0.9300 | C6—O1 | 1.231 (2) |
| C2—C3 | 1.390 (2) | C6—N2 | 1.335 (2) |
| C2—C6 | 1.503 (2) | N2—N3 | 1.418 (2) |
| C3—C4 | 1.386 (2) | N2—H2N | 0.8830 |
| C3—H3 | 0.9300 | N3—H3NA | 0.8746 |
| C4—C5 | 1.381 (3) | N3—H3NB | 0.8461 |
| C4—H4 | 0.9300 | ||
| N1—C1—C2 | 123.70 (16) | N1—C5—H5 | 118.1 |
| N1—C1—H1 | 118.2 | C4—C5—H5 | 118.1 |
| C2—C1—H1 | 118.2 | O1—C6—N2 | 123.96 (16) |
| C3—C2—C1 | 118.02 (16) | O1—C6—C2 | 120.55 (16) |
| C3—C2—C6 | 124.35 (16) | N2—C6—C2 | 115.49 (15) |
| C1—C2—C6 | 117.60 (15) | C1—N1—C5 | 117.04 (16) |
| C4—C3—C2 | 118.93 (17) | C6—N2—N3 | 122.80 (15) |
| C4—C3—H3 | 120.5 | C6—N2—H2N | 121.7 |
| C2—C3—H3 | 120.5 | N3—N2—H2N | 115.4 |
| C5—C4—C3 | 118.49 (17) | N2—N3—H3NA | 111.0 |
| C5—C4—H4 | 120.8 | N2—N3—H3NB | 109.4 |
| C3—C4—H4 | 120.8 | H3NA—N3—H3NB | 99.3 |
| N1—C5—C4 | 123.81 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2N···N1i | 0.88 | 2.11 | 2.975 (2) | 166 |
| N3—H3NA···O1ii | 0.87 | 2.22 | 3.045 (2) | 157 |
| N3—H3NB···O1iii | 0.85 | 2.55 | 3.155 (2) | 130 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x+1/2, −y+3/2, −z+1; (iii) x−1/2, −y+3/2, −z+1.
Table 2 Bond lengths and angles (Å, °) of nicotinohydrazide (I), picolinic acid hydrazide (II) and isonicotinohydrazide (III)
| (I) | (II) | (III) | |
| N2—N3 | 1.418 (2) | 1.422 | 1.429 |
| C6—N2 | 1.335 (2) | 1.334 | 1.346 |
| C6—O1 | 1.231 (2) | 1.235 | 1.235 |
| C6—C2 | 1.503 (2) | 1.507 | 1.513 |
| N3—N2—C6 | 122.80 (15) | 121.45 | 121.06 |
| N2—C6—O1 | 123.96 (16) | 123.04 | 122.07 |
| N2—C6—C2 | 115.49 (15) | 116.08 | 115.90 |
| O1—C6—C2 | 120.55 (16) | 120.87 | 122.00 |
| N3—N2—C6—O1 | 0.17 (32) | 177.39 | 175.13 |
| C2—N2—C6—N3 | 179.56 (19) | 177.72 | 173.03 |
| N2—C6—C2—C3 | 34.62 (27) | 177.06 | 162.86 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2145).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Bhat, T. N., Singh, T. P. & Vijayan, M. (1974). Acta Cryst. B30, 2921–2922.
- Enraf–Nonius (1994). CAD-4 EXPRESS Version 5.1/1.2. Enraf–Nonius, Delft, The Netherlands.
- Jensen, L. H. (1954). J. Am. Chem. Soc.76, 4663–4667.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Ouelleta, M., Aitkenb, S. M., Englishc, A. M. & Percivala, M. D. (2004). Arch. Biochem. Biophys.431, 107–118.
- Portalone, G. & Colapietro, M. (2008). Acta Cryst. E64, o304. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1997). SHELXL97 University of Göttingen, Germany.
- Spek, A. L. (1996). HELENA University of Utrecht, The Netherlands.
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Zareef, M., Iqbla, R., Zaidi, J. H., Qadeer, G., Wong, W. Y. & Akhtar, H. (2006). Z. Kristallogr. New Cryst. Struct.221, 307–308.
- Zhao, X., Yu, S. & Magliozzo, R. S. (2007). Biochemistry, 46, 3161–3170. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680706655X/bh2145sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680706655X/bh2145Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report