Abstract
In the title compound (alternative name: pyridine-3-carbohydrazide, C6H7N3O), the asymmetric unit contains a single molecule. In contrast with nicotinic acid and nicotinamide, the C=O bond is found to be oriented cis with respect to the Cipso C N fragment in the pyridine ring. The pyridine ring and the hydrazide group make a dihedral angle of 34.0 (2)°. In the crystal structure, molecules are associated into a three-dimensional framework by a combination of N—H⋯N and three-centre N—H⋯O hydrogen bonds.
Related literature
The structure of the same compound has been determined independently and is reported in the preceding paper (Priebe et al., 2008 ▶). For related literature, see: Bhat et al. (1974 ▶); Kutoglu & Scheringer (1983 ▶); Miwa et al. (1999 ▶); Portalone (2007 ▶); Portalone & Colapietro (2007 ▶). For computation of ring patterns formed by hydrogen bonds in crystal structures, see: Etter et al. (1990 ▶); Bernstein et al. (1995 ▶); Motherwell et al. (1999 ▶).
Experimental
Crystal data
C6H7N3O
M r = 137.15
Orthorhombic,
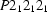
a = 3.8727 (10) Å
b = 10.481 (2) Å
c = 15.855 (2) Å
V = 643.6 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 298 (2) K
0.15 × 0.05 × 0.05 mm
Data collection
Oxford Diffraction Xcalibur S CCD diffractometer
Absorption correction: none
3076 measured reflections
1139 independent reflections
695 reflections with I > 2σ(I)
R int = 0.019
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.131
S = 1.19
1139 reflections
93 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.22 e Å−3
Δρmin = −0.22 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2006 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis RED (Oxford Diffraction, 2006 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536807066561/bh2149sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807066561/bh2149Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H21⋯N1i | 0.89 (4) | 2.09 (4) | 2.964 (4) | 168 (4) |
| N3—H31⋯O1ii | 0.84 (5) | 2.57 (5) | 3.146 (4) | 127 (4) |
| N3—H32⋯O1iii | 1.00 (5) | 2.08 (5) | 3.027 (4) | 157 (4) |
Symmetry codes: (i) 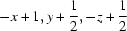 ; (ii)
; (ii) 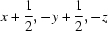 ; (iii)
; (iii) 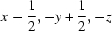 .
.
Acknowledgments
We thank MIUR (Rome) for 2006 financial support of the project ‘X-ray diffractometry and spectrometry’.
supplementary crystallographic information
Comment
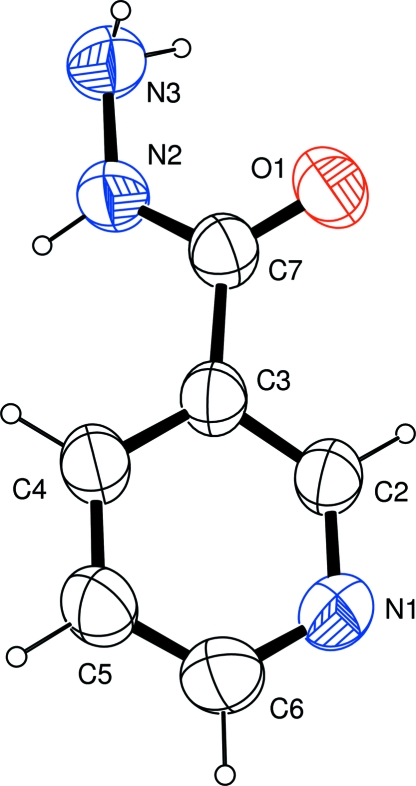
As a part of a more general study of multiple-hydrogen-bonding N -heterocyclic systems as potential supramolecular reagents (Portalone, 2007; Portalone & Colapietro, 2007), we report here the structure of the title compound (I, Fig. 1). The asymmetric unit of (I) comprises one independent molecule, and the angle between the mean planes of the acid hydrazine group and the pyridine ring is 34.0 (2)°. Noteworthy, in contrast to nicotinic acid (Kutoglu & Scheringer, 1983) and nicotinamide (Miwa et al., 1999), the C?O bond is oriented cis with respect to the C2—C3 bond.
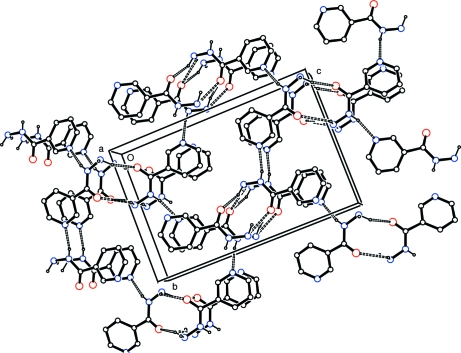
Analysis of the crystal packing of (I) shows that, at variance with isonicotinohydrazide (Bhat et al., 1974), for which the crystal structure is stabilized by a network of N—H···N hydrogen bonds, in compound (I) two of the three independent N—H bonds act as donor in three-centre N—H···O systems (Table 1, entries 2 and 3), and the third is involved in a N—H···N interaction (Table 1, entry 1). These hydrogen bonds delineate patterns in which rings are the most prominent features (Fig. 2). Two small rings with descriptor R22(10) (Etter et al., 1990; Bernstein et al., 1995; Motherwell et al., 1999) are then formed by NH2 functionalities and two symmetry-related carbonyl O atoms [O1ii and O1iii, symmetry codes: (ii) x + 1/2, -y + 1/2, -z; (iii) x - 1/2, -y + 1/2, -z]. The formation of the N—H···N hydrogen bonds between the N—H groups and the pyridyl N atoms [N1i, symmetry code: (i) -x + 1, y + 1/2, -z + 1/2] leads to the formation of larger R66(30) rings.
Experimental
1 mmol of the title compound (purchased from Sigma-Aldrich at 97% purity) was dissolved in a mixture benzene/ethanol (8:1, 50 ml) and refluxed for 1 h. After cooling the solution to ambient temperature, a colorless precipitate was formed, which was collected by filtration and washed with benzene/ethanol (8:1). Crystals suitable for single-crystal X-ray diffraction were grown from a benzene solution, by slow evaporation of the solvent.
Refinement
Diffraction from the very small crystals was weak; nevertheless, these data gave good structural results, albeit with a lower data/parameter ratio than usual. All H atoms were detected in a difference map, after the first cycles of the isotropic refinement. The final full-matrix least-squares refinement was carried out on F2 with anisotropic non-H atoms and isotropic H atoms. C-bonded H atoms were positioned with idealized geometry and refined using a riding model, with C—H bond lengths fixed to 0.95 Å and Uiso(H) = 1.2Ueq(carrier C). H atoms bonded to N atoms were refined freely with Uiso(H) = 1.2Ueq(carrier N). In the absence of significant anomalous scattering in this light-atom study, measured Friedel pairs were merged.
Figures
Fig. 1.
The molecular structure of (I), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Crystal packing diagram for (I) viewed down [100]. All atoms are shown as small spheres of arbitrary radii. For the sake of clarity, H atoms not involved in hydrogen bonding have been omitted. Hydrogen bonding is indicated by dashed lines.
Crystal data
| C6H7N3O | F000 = 288 |
| Mr = 137.15 | Dx = 1.415 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation λ = 0.71069 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 6060 reflections |
| a = 3.8727 (10) Å | θ = 2.3–30.0º |
| b = 10.481 (2) Å | µ = 0.10 mm−1 |
| c = 15.855 (2) Å | T = 298 (2) K |
| V = 643.6 (2) Å3 | Plate, colourless |
| Z = 4 | 0.15 × 0.05 × 0.05 mm |
Data collection
| Oxford Diffraction Xcalibur S CCD diffractometer | 1139 independent reflections |
| Radiation source: Enhance (Mo) X-ray source | 695 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.019 |
| Detector resolution: 16.0696 pixels mm-1 | θmax = 30.0º |
| T = 298(2) K | θmin = 2.3º |
| ω and φ scans | h = −5→5 |
| Absorption correction: none | k = −14→14 |
| 3076 measured reflections | l = −22→22 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.042 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.131 | w = 1/[σ2(Fo2) + (0.022P)2 + 0.3733P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.19 | (Δ/σ)max < 0.001 |
| 1139 reflections | Δρmax = 0.22 e Å−3 |
| 93 parameters | Δρmin = −0.22 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 1.0228 (9) | 0.1920 (2) | 0.10123 (15) | 0.0690 (8) | |
| N1 | 0.4952 (10) | 0.1249 (3) | 0.32513 (17) | 0.0622 (8) | |
| N2 | 0.8838 (10) | 0.4009 (3) | 0.10818 (16) | 0.0613 (9) | |
| H21 | 0.777 (5) | 0.464 (3) | 0.1359 (12) | 0.074* | |
| N3 | 0.9984 (11) | 0.4321 (3) | 0.02617 (17) | 0.0670 (9) | |
| H31 | 1.210 (6) | 0.4151 (6) | 0.0228 (2) | 0.080* | |
| H32 | 0.889 (3) | 0.3722 (18) | −0.0149 (13) | 0.080* | |
| C2 | 0.6160 (10) | 0.1529 (3) | 0.2485 (2) | 0.0565 (9) | |
| H2 | 0.5944 | 0.0902 | 0.2056 | 0.068* | |
| C3 | 0.7709 (10) | 0.2678 (3) | 0.22785 (19) | 0.0517 (8) | |
| C4 | 0.7961 (10) | 0.3597 (3) | 0.2905 (2) | 0.0574 (9) | |
| H4 | 0.8954 | 0.4406 | 0.2786 | 0.069* | |
| C5 | 0.6755 (12) | 0.3321 (3) | 0.3700 (2) | 0.0648 (11) | |
| H5 | 0.6931 | 0.3932 | 0.4140 | 0.078* | |
| C6 | 0.5291 (13) | 0.2149 (4) | 0.3845 (2) | 0.0673 (10) | |
| H6 | 0.4474 | 0.1966 | 0.4397 | 0.081* | |
| C7 | 0.9030 (11) | 0.2833 (3) | 0.14019 (19) | 0.0545 (8) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.089 (2) | 0.0590 (14) | 0.0593 (13) | 0.0139 (16) | 0.0077 (16) | −0.0061 (11) |
| N1 | 0.075 (2) | 0.0528 (15) | 0.0586 (15) | −0.0012 (19) | 0.0015 (17) | 0.0083 (13) |
| N2 | 0.083 (2) | 0.0536 (15) | 0.0468 (13) | 0.0037 (17) | 0.0062 (16) | 0.0023 (12) |
| N3 | 0.083 (2) | 0.0649 (17) | 0.0526 (14) | −0.004 (2) | 0.0038 (18) | 0.0066 (13) |
| C2 | 0.071 (2) | 0.0457 (15) | 0.0528 (16) | 0.0009 (18) | −0.0027 (18) | 0.0009 (13) |
| C3 | 0.062 (2) | 0.0440 (14) | 0.0496 (15) | 0.0019 (17) | −0.0038 (16) | −0.0007 (13) |
| C4 | 0.071 (2) | 0.0475 (16) | 0.0535 (16) | 0.0034 (19) | −0.0040 (18) | −0.0040 (14) |
| C5 | 0.089 (3) | 0.0574 (18) | 0.0480 (16) | 0.004 (2) | −0.004 (2) | −0.0041 (14) |
| C6 | 0.083 (3) | 0.066 (2) | 0.0526 (17) | 0.005 (2) | 0.002 (2) | 0.0060 (16) |
| C7 | 0.063 (2) | 0.0497 (15) | 0.0505 (15) | 0.0023 (18) | −0.0032 (17) | −0.0014 (14) |
Geometric parameters (Å, °)
| O1—C7 | 1.230 (4) | C2—H2 | 0.9500 |
| N1—C2 | 1.334 (4) | C3—C4 | 1.387 (4) |
| N1—C6 | 1.340 (4) | C3—C7 | 1.490 (4) |
| N2—C7 | 1.335 (4) | C4—C5 | 1.375 (4) |
| N2—N3 | 1.412 (4) | C4—H4 | 0.9500 |
| N2—H21 | 0.89 (4) | C5—C6 | 1.373 (5) |
| N3—H31 | 0.84 (5) | C5—H5 | 0.9500 |
| N3—H32 | 1.00 (5) | C6—H6 | 0.9500 |
| C2—C3 | 1.385 (4) | ||
| C2—N1—C6 | 116.8 (3) | C5—C4—C3 | 119.1 (3) |
| C7—N2—N3 | 123.1 (3) | C5—C4—H4 | 120.5 |
| C7—N2—H21 | 121.2 | C3—C4—H4 | 120.5 |
| N3—N2—H21 | 115.4 | C6—C5—C4 | 118.9 (3) |
| N2—N3—H31 | 108.4 | C6—C5—H5 | 120.6 |
| N2—N3—H32 | 108.7 | C4—C5—H5 | 120.6 |
| H31—N3—H32 | 103.9 | N1—C6—C5 | 123.5 (3) |
| N1—C2—C3 | 124.0 (3) | N1—C6—H6 | 118.2 |
| N1—C2—H2 | 118.0 | C5—C6—H6 | 118.2 |
| C3—C2—H2 | 118.0 | O1—C7—N2 | 123.2 (3) |
| C2—C3—C4 | 117.7 (3) | O1—C7—C3 | 120.9 (3) |
| C2—C3—C7 | 117.6 (3) | N2—C7—C3 | 115.8 (3) |
| C4—C3—C7 | 124.6 (3) | ||
| C6—N1—C2—C3 | 0.0 (6) | C4—C5—C6—N1 | −0.3 (7) |
| N1—C2—C3—C4 | −1.1 (6) | N3—N2—C7—O1 | 0.2 (7) |
| N1—C2—C3—C7 | 178.1 (4) | N3—N2—C7—C3 | 179.8 (4) |
| C2—C3—C4—C5 | 1.5 (6) | C2—C3—C7—O1 | −33.7 (6) |
| C7—C3—C4—C5 | −177.6 (4) | C4—C3—C7—O1 | 145.5 (4) |
| C3—C4—C5—C6 | −0.9 (6) | C2—C3—C7—N2 | 146.7 (4) |
| C2—N1—C6—C5 | 0.7 (7) | C4—C3—C7—N2 | −34.2 (6) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H21···N1i | 0.89 (4) | 2.09 (4) | 2.964 (4) | 168 (4) |
| N3—H31···O1ii | 0.84 (5) | 2.57 (5) | 3.146 (4) | 127 (4) |
| N3—H32···O1iii | 1.00 (5) | 2.08 (5) | 3.027 (4) | 157 (4) |
Symmetry codes: (i) −x+1, y+1/2, −z+1/2; (ii) x+1/2, −y+1/2, −z; (iii) x−1/2, −y+1/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2149).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bhat, T. N., Singh, T. P. & Vijayan, M. (1974). Acta Cryst. B30, 2921–2922.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Kutoglu, A. & Scheringer, C. (1983). Acta Cryst. C39, 232–234.
- Miwa, Y., Mizuno, T., Tsuchida, K., Taga, T. & Iwata, Y. (1999). Acta Cryst. B55, 78–84. [DOI] [PubMed]
- Motherwell, W. D. S., Shields, G. P. & Allen, F. H. (1999). Acta Cryst. B55, 1044–1056. [DOI] [PubMed]
- Oxford Diffraction (2006). CrysAlis CCD and CrysAlis RED Versions 1.171.32.3. Oxford Diffraction Ltd, Abingdon, Oxfordshire, England.
- Portalone, G. (2007). Acta Cryst. E63, o3232.
- Portalone, G. & Colapietro, M. (2007). Acta Cryst. C63, o655–o658. [DOI] [PubMed]
- Priebe, J. P., Mello, R. S., Nome, F. & Bortoluzzi, A. J. (2008). Acta Cryst. E64, o302–o303. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1997). SHELXL97 University of Göttingen, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536807066561/bh2149sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807066561/bh2149Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report