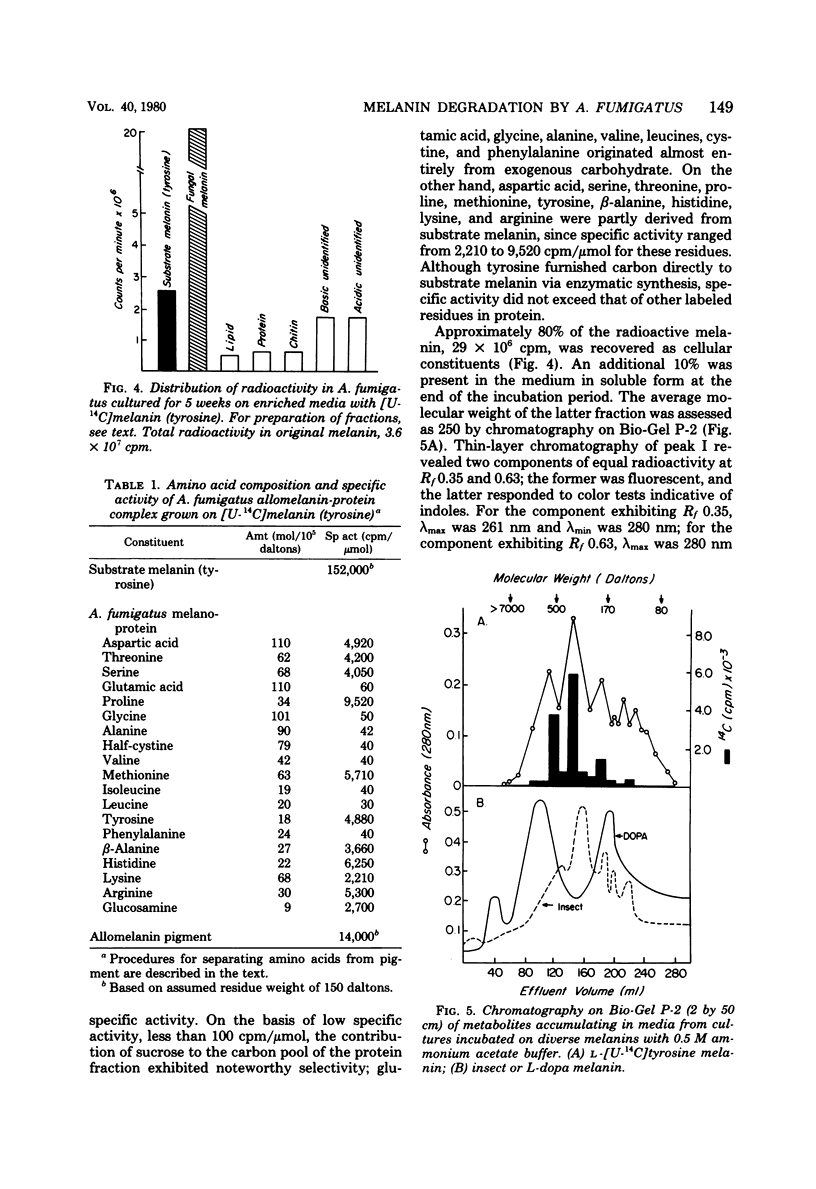
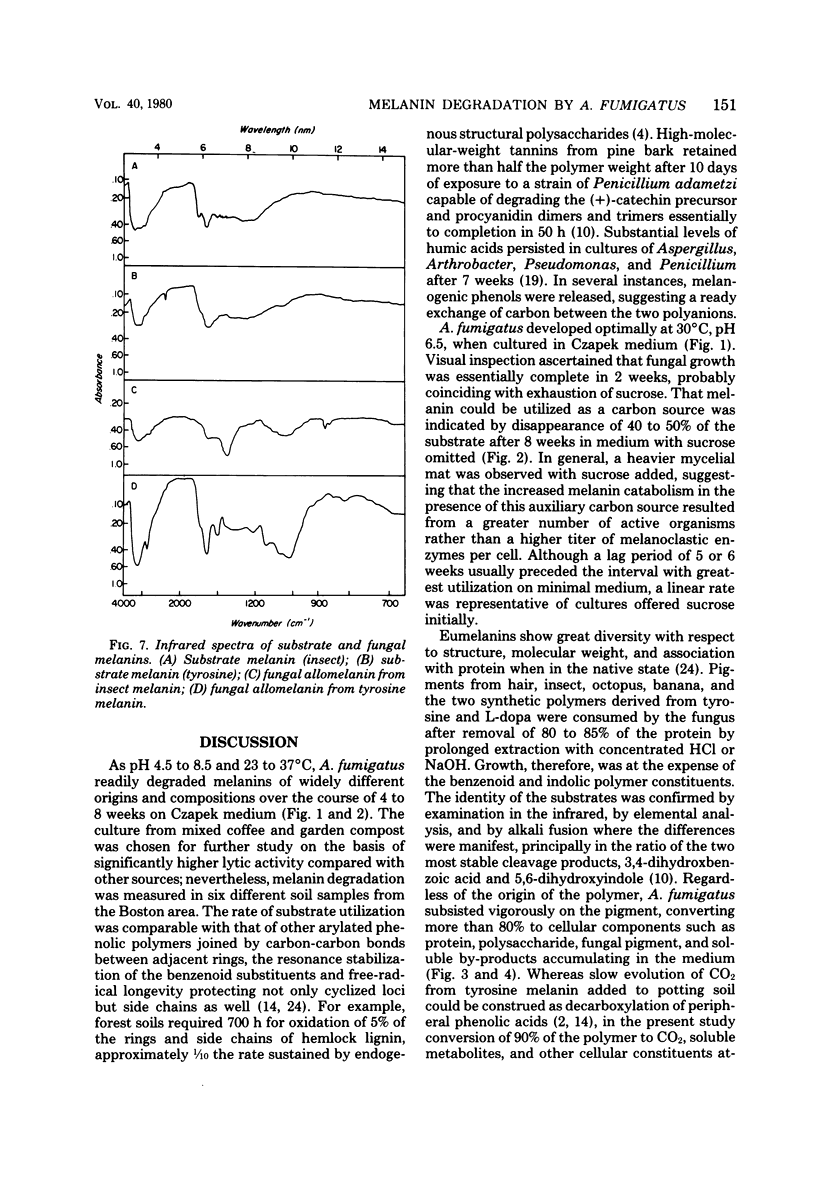
Abstract
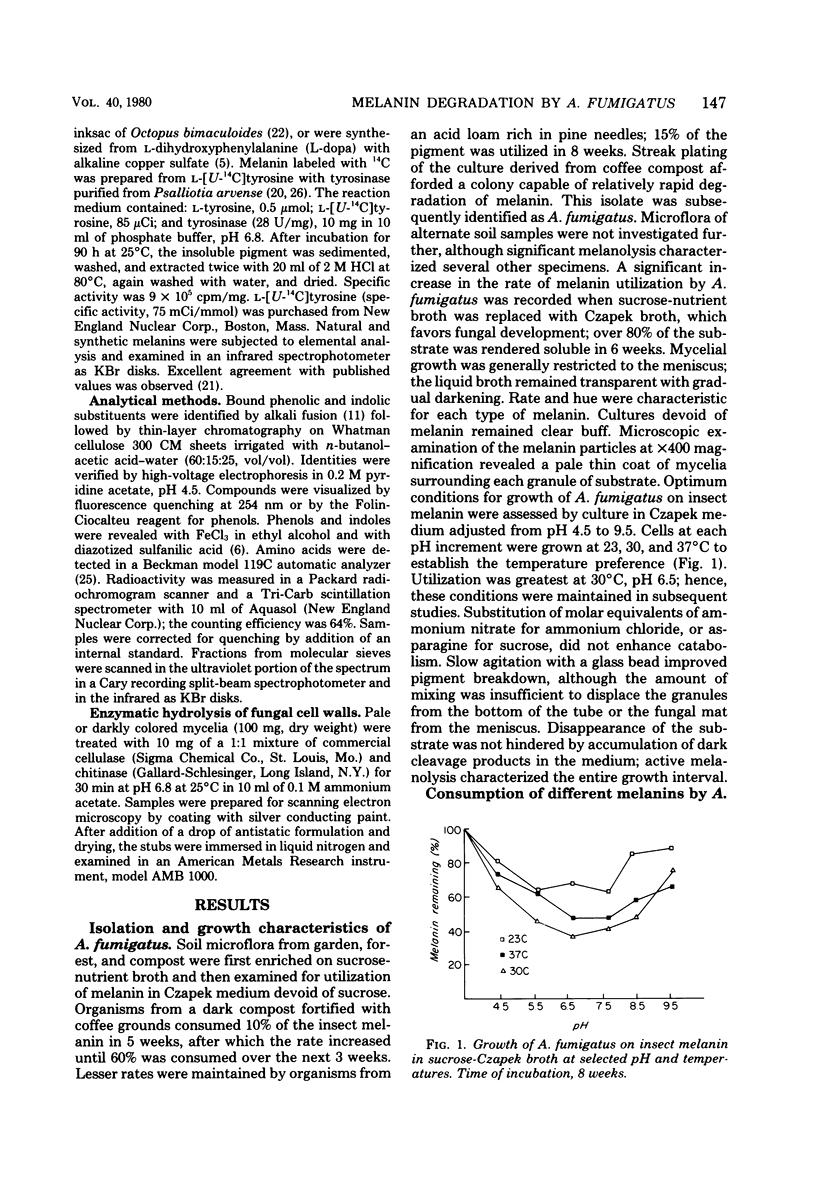
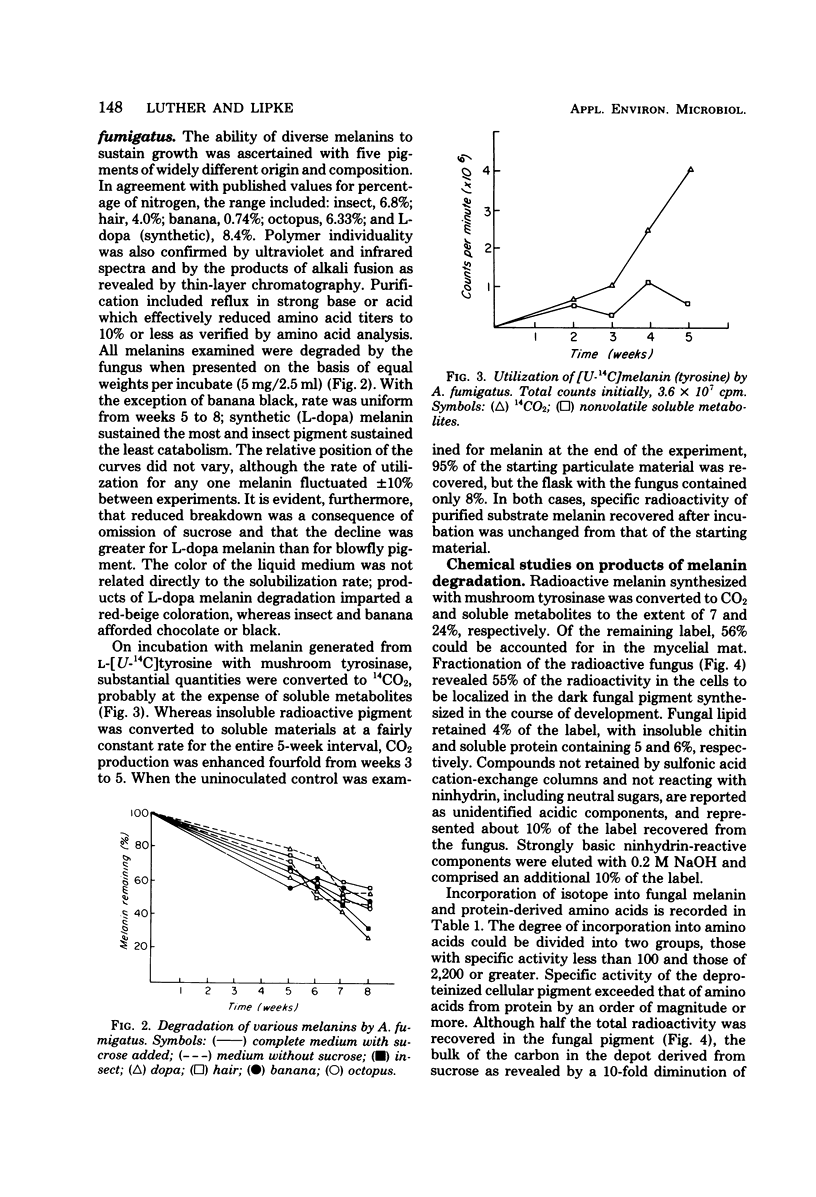
A strain of Aspergillus fumigatus from composted coffee and garden wastes utilized natural deproteinized insect, banana, hair, octopus, and synthetic tyrosine and dopa melanins as sole sources of carbon. With a sucrose supplement, degradation was essentially complete after 50 days in Czapek medium pH 6.5 at 30 degrees C. The catabolic rate differed for each substrate pigment, as did the molecular weight distribution of products accumulating in the medium. After incubation with L-[U-14C]melanin, over 50% was recovered in a dark fungal pigment, the remainder appearing as cell protein, chitin, lipid, CO2, and polar metabolites. When grown on melanin, the normally pale mycelia darkened with the production of a fungal allomelanin, with infrared spectrum and alkali fusion products differing from those of the substrate pigment. Isotope distribution in amino acids for A. fumigatus grown on labeled melanin supplemented with sucrose suggested separate pools for synthesis of cell proteins and melanoproteins. Deposition of allomelanin increased resistance of conidia, sterigma, and conidiophores to lytic carbohydrases as judged by scanning electron microscopy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield B. J., Alexander M. Melanins and resistance of fungi to lysis. J Bacteriol. 1967 Apr;93(4):1276–1280. doi: 10.1128/jb.93.4.1276-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull A. T. Inhibition of polysaccharases by melanin: enzyme inhibition in relation to mycolysis. Arch Biochem Biophys. 1970 Apr;137(2):345–356. doi: 10.1016/0003-9861(70)90448-0. [DOI] [PubMed] [Google Scholar]
- Crawford R. L., Crawford D. L., Olofsson C., Wikstrom L., Wood J. M. Biodegradation of natural and man-made recalcitrant compounds with particular reference to lignin. J Agric Food Chem. 1977 Jul-Aug;25(4):704–708. doi: 10.1021/jf60212a018. [DOI] [PubMed] [Google Scholar]
- Das K. C., Abramson M. B., Katzman R. A new chemical method for quantifying melanin. J Neurochem. 1976 Apr;26(4):695–699. doi: 10.1111/j.1471-4159.1976.tb04439.x. [DOI] [PubMed] [Google Scholar]
- Gledhill W. E. Screening test for assessment of ultimate biodegradability: linear alkylbenzene sulfonates. Appl Microbiol. 1975 Dec;30(6):922–929. doi: 10.1128/am.30.6.922-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D. Microbial degradation of condensed tannins. Science. 1976 Sep 17;193(4258):1137–1139. doi: 10.1126/science.959828. [DOI] [PubMed] [Google Scholar]
- Hackman R. H., Goldberg M. Microchemical detection of melanins. Anal Biochem. 1971 May;41(1):279–285. doi: 10.1016/0003-2697(71)90214-4. [DOI] [PubMed] [Google Scholar]
- Kuo M. J., Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967 Sep;94(3):624–629. doi: 10.1128/jb.94.3.624-629.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. P., Haider K. Biodegradation of C-labeled model and cornstalk lignins, phenols, model phenolase humic polymers, and fungal melanins as influenced by a readily available carbon source and soil. Appl Environ Microbiol. 1979 Aug;38(2):283–289. doi: 10.1128/aem.38.2.283-289.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaus R. A., Piattelli M., Fattorusso E. The structure of melanins and melanogenesis. IV. On some natural melanins. Tetrahedron. 1964 May;20(5):1163–1172. doi: 10.1016/s0040-4020(01)98983-5. [DOI] [PubMed] [Google Scholar]
- Swan G. A. Structure, chemistry, and biosynthesis of the melanins. Fortschr Chem Org Naturst. 1974;31(0):521–582. doi: 10.1007/978-3-7091-7094-6_9. [DOI] [PubMed] [Google Scholar]
- THOMSON A. R., MILES B. J. ION-EXCHANGE CHROMATOGRAPHY OF AMINO-ACIDS: IMPROVEMENTS IN THE SINGLE COLUMN SYSTEM. Nature. 1964 Aug 1;203:483–484. doi: 10.1038/203483a0. [DOI] [PubMed] [Google Scholar]
- Van Woert M. H., Prasad K. N., Borg D. C. Spectroscopic studies of substantia nigra pigment in human subjects. J Neurochem. 1967 Jul;14(7):707–716. doi: 10.1111/j.1471-4159.1967.tb10304.x. [DOI] [PubMed] [Google Scholar]
- Wheeler M. H., Tolmsoff W. J., Bell A. A., Mollenhauer H. H. Ultrastructural and chemical distinction of melanins formed by Verticillium dahliae from (+)-scytalone, 1,8-dihydroxynaphthalene, catechol, and L-3,4-dihydroxyphenylalanine. Can J Microbiol. 1978 Mar;24(3):289–297. doi: 10.1139/m78-049. [DOI] [PubMed] [Google Scholar]
- Whittaker J. R. Melanoprotein: absence of a direct melanin-protein relationship in chick embryo melanocytes. Biochim Biophys Acta. 1979 Mar 22;583(3):378–387. doi: 10.1016/0304-4165(79)90462-8. [DOI] [PubMed] [Google Scholar]