Abstract
The SRC family kinases are the largest family of nonreceptor tyrosine kinases and one of the best-studied targets for cancer therapy. SRC, arguably the oldest oncogene, has been implicated in pathways regulating proliferation, angiogenesis, invasion and metastasis, and bone metabolism. More recently, researchers have proposed that the transforming ability of SRC is linked to its ability to activate key signaling molecules in these pathways, rather than through direct activity. It has been hypothesized that blocking SRC activation may inhibit these pathways, resulting in antitumor activity. However, successfully targeting SRC in a clinical setting remains a challenge, and SRC inhibitors have only recently begun to move through clinical development. Preclinical studies have identified specific molecular “subgroups“ and histologies that may be more sensitive to SRC inhibition. In addition, other studies have demonstrated synergistic interactions between SRC inhibitors and other targeted therapies and cytotoxics. In this review, we summarize SRC biology and how it has been applied to the clinical development of SRC inhibitors. The status of SRC inhibitors, including dasatinib, saracatinib, and bosutinib, which are in phase 1, 2, and 3 trials, is highlighted.
Introduction
The SRC family of tyrosine kinases (SFKs) has nine members: LYN, FYN, LCK, HCK, FGR, BLK, YRK, YES, and c-SRC. Of these, c-SRC is the best studied and most frequently implicated in oncogenesis [1].
Almost 100 years have elapsed since Peyton Rous first described a filterable agent (i.e., virus) that could induce solid tumors in birds. Arguably ahead of his time, Rous' discovery would linger on the fringes of the scientific establishment for more than 50 years. It took the advent of modern molecular biology techniques in the 1960s and 1970s for Rous' filterable agent, now renamed the Rous sarcoma virus, to ignite research that would help elucidate our current understanding of cancer biology. Studies into the molecular biology and genetics of Rous sarcoma virus identified v-SRC as the viral oncogene responsible for cellular transformation. Shortly thereafter, Bishop and Varmus demonstrated that v-SRC had a cellular counterpart, the proto-oncogene c-SRC [2].
c-SRC (henceforth referred to as SRC) encodes a nonreceptor tyrosine kinase that, when activated, is involved in cellular proliferation, survival, migration, and angiogenesis. When deregulated, these processes represent four of the six so-called “hallmarks of cancer“ [1,3]. Furthermore, numerous human malignancies display increased SRC expression and activity, suggesting that SRC may be intimately involved in oncogenesis [4]. Despite this, SRC alone is insufficient in transforming human cells in vitro, and so far, only rare cases of activating SRC mutations have been identified in human cancers [5,6]. Although numerous questions regarding the role of SRC in cancer remain unanswered, SRC's involvement in intracellular signaling pathways and overexpression in many human malignancies has renewed interest in developing SRC inhibitors. In this review, we highlight the rationale for SRC as a therapeutic target in cancer medicine and examine the preclinical and clinical data relevant to SRC inhibitors in development.
SRC Structure and Function
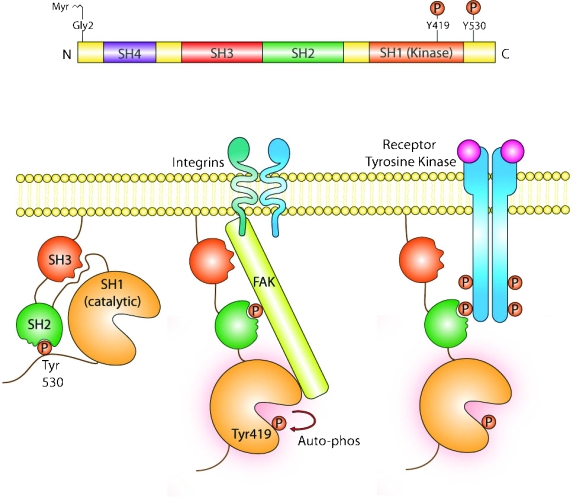
Proteins in the SRC family have a conserved organization consisting of four SRC homology (SH) domains and a C-terminal segment containing a negative regulatory tyrosine residue (Tyr530) (Figure 1). SRC exists in both active and inactive conformations. Negative regulation occurs through phosphorylation of Tyr530, resulting in an intramolecular association between phosphorylated Tyr530 and the SH2 domain of SRC, thereby locking the protein in a closed conformation. Further stabilization of the inactive state occurs through interactions between the SH3 domain and a proline-rich stretch of residues within the kinase domain. Conversely, dephosphorylation of Tyr530 allows SRC to assume an open conformation. Full activity requires additional autophosphorylation of the Tyr419 residue within the catalytic domain. Loss of the negative-regulatory C-terminal segment, as occurs in v-Src, has been shown to result in increased activity and transforming potential [1,7]. However, similar activating mutations are rare in human tumors, with just one published report that found activating SRC mutations in approximately 12% of human colon cancers [5].
Figure 1.
Methods of SRC activation and inactivation. Phosphorylation of Tyr530 at the C-terminus locks the protein in a closed, inactive conformation stabilized through interactions between the SH3 and kinase domains. Dephosphorylation of Tyr530 and autophosphorylation of Tyr419 within the catalytic domain allow SRC to assume an open, active conformation. SRC activity is also regulated by receptor tyrosine kinases and direct binding of FAK to the SH2 domain.
The intramolecular activity of SRC is regulated by a balance between kinases and phosphatases that act at the C-terminal Tyr530 residue. Phosphorylation by C-terminal SRC kinase (CSK) and CSK homology kinase results in increased intramolecular interactions and consequent SRC inactivation. Indeed, CSK overexpression suppresses metastasis in animal models of colon cancer, suggesting a possible tumor suppressor role [8]. By contrast, CSK levels are decreased in hepatocellular carcinoma compared with matched cirrhotic controls [9]. Less evidence exists relating to the involvement of specific phosphatases in SRC activation. Protein tyrosine phosphatase α (PTPα) and the SH-containing phosphatases SHP1/SHP2 are the most-studied examples, showing SRC-specific dephosphorylation activity in vitro and in vivo [1]. Furthermore, the SRC-specific PTP1β is upregulated in certain breast cancers [10].
SRC is also activated by direct binding of focal adhesion kinase (FAK) and CRK-associated substrate (CAS) to the SH2 domain [11]. When bound, these molecules activate SRC by disrupting inhibitory intramolecular interactions. Interestingly, both FAK and CAS are principal regulators of focal adhesion complex formation and actin cytoskeleton dynamics, essential processes for cell adhesion and migration [12]. In addition, SRC activity can be regulated by numerous receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR), HER2, fibroblast growth factor receptor, platelet-derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptor (VEGFR) [13].
SRC Activation in Normal and Malignant Cells
Cell Adhesion and Invasion
Dynamic turnover of cell-cell (adherens junctions) and cell-matrix (focal adhesions) junctions is crucial for normal cellular adhesion, migration, and division. SRC plays a key role in regulating the assembly and disassembly of these junctions [1]. The subcellular localization of SRC is critical to its function [14]. SRC associates with the plasma membrane through an N-terminal fatty acid moiety and when activated, translocates to sites of membrane-cytoskeletal interface where it acts to promote turnover of adherens junctions and focal adhesions [15].
Adherens junctions are maintained by homotypic interactions between E-cadherin molecules present on neighboring cells. Loss of E-cadherin is a key event in the epithelial-to-mesencymal transition and is associated with enhanced invasive and metastatic potential. Increased SRC signaling correlates with decreased E-cadherin expression and decreased cell-cell adhesion [16,17]. At the cell periphery, activated SRC forms complexes with cytoplasmic proteins such as FAK and CAS [15,18]. In association with FAK, SRC mediates signals from extracellular matrix-integrin complexes to the cell interior, thereby influencing cell motility, survival, and proliferation. The SRC-FAK complex interacts with a multitude of substrates, including CAS, paxillin, and p190RhoGAP, which play critical roles in promoting actin remodeling and cellular migration [19,20]. In cancer, dysregulated focal adhesion signaling has been implicated in increased invasion and metastasis, in addition to decreased patient survival [21].
Receptor-Mediated Activation
Growth factor signaling through RTKs can also activate SRC, most likely by disrupting inhibitory intramolecular forces. Many tumors that overexpress or have constitutively activated RTK signaling also have upregulated SRC expression or activity. Furthermore, experiments using epithelial and fibroblast cell lines suggest that SRC and EGFR act synergistically to increase cellular proliferation and invasion [22,23]. Direct phosphorylation of EGFR by SRC is required for efficient EGF-induced DNA synthesis and signal transducer and activator of transcription 5B (STAT5b) activation [24]. In addition, SRC overexpression increases ERBB2 (HER2) and ERBB3 (HER3) heterodimer formation and potentiates downstream signaling [25]. SRC also associates with PDGFR through its SH2 domain and is required for efficient PDGF-induced mitogenic signaling and DNA synthesis [26]. PDGFR seems to exert an activating effect on SRC through phosphotyrosines at Tyr579 and Tyr581 because replacement of these residues decreases SRC-mediated signaling [27].
Cell Proliferation and Mitogenesis
Increasing evidence suggests that SRC is intimately involved in regulating cell cycle progression and mitogenesis. For example, SRC overexpression abrogates MYC requirement for G0/G1, but not G1/S, phase transition [28]. Furthermore, SRC inhibition is associated with decreased β-catenin binding to cyclin D1 and MYC promoters and decreased expression of these mediators [29]. SRC is transiently activated during G2/M transition and is required for efficient cellular division [30]. Downstream substrates of SRC seem to act largely in parallel to increase cell proliferation and survival because simultaneous inhibition of PI3K and RAS signaling abrogates SRC-induced transformation, but inhibition of either pathway alone does not [2].
Regulation of Angiogenesis
Angiogenesis is frequently dysregulated in cancer, and antiangiogenics are approved for the treatment of several solid tumors. Angiogenesis is regulated by multiple cytokines that trigger a cellular cascade favoring endothelial cell migration and proliferation. SRC activation is associated with increased expression of proangiogenic cytokines such as VEGF and interleukin 8 (IL-8) [31]. In hypoxia-induced models of angiogenesis, SRC activation and antisense SRC inhibition positively and negatively regulate VEGF expression, respectively. Treatment with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), a potent and selective inhibitor of SFKs, inhibits angiogenesis in vivo and blocks endothelial cell differentiation in vitro [32]. SRC is also involved in regulating IL-8 expression, with v-SRC-transformed cells showing enhanced IL-8 expression [33]. Conversely, inhibiting SRC blocks IL-8-mediated VEGFR2 activation and decreases vascular permeability [34]. Furthermore, SFKs are implicated in endothelial cell function, with inhibition of SRC, FYN, and YES decreasing VEGF-induced endothelial cell migration [35].
Metastasis and Bone Remodeling
Bone metastases often occur in patients with lung, prostate, colorectal, or breast carcinoma and often lead to pathologic fracture and bone pain. Metastatic cancer affects bone remodeling, which is normally regulated by the dynamic process of osteoblast-mediated bone formation and osteoclast-mediated bone resorption. SRC is implicated as a central regulator of bone remodeling, demonstrated by SRC-/- mice being highly prone to developing osteopetrosis, a disease characterized by decreased bone resorption [36]. In addition, SRC is increased in functioning osteoclasts, and disrupted SRC signaling prevents osteoclast migration and bone resorption activity [37,38].
Notably, nude mice injected with SRC-overexpressing MDA-231 breast cancer cells preferentially developed osteolytic bone metastases [39]. In a similar model of breast cancer, SRC inhibition decreased metastatic disease burden and overall lethality, reduced osteoclast bone resorption, and impaired function of osteoblasts in vitro [40].
Clinical Development of SRC Inhibitors
Given the critical role of SRC in promoting cell proliferation, invasion, and metastasis and in regulating bone remodeling, molecular inhibitors of SFKs are being developed and evaluated. Evidence discussed previously suggests that inhibiting SRC may slow disease progression and help control the formation of distant metastases, in addition to reducing concomitant lytic bone lesions.
Successful development of targeted therapeutics often depends on identifying reliable molecular and clinical markers associated with clinical benefit. Experience with oncologic agents such as trastuzumab, gefitinib, and cetuximab demonstrates that clinical efficacy may prove elusive if predictive markers of response and/or resistance are not identified. We now recognize that molecular heterogeneity exists even within a particular cancer type, and therefore targeted agents may only benefit select cohorts of patients. Consequently, biomarker identification is a focus of development for many new agents. Preclinical and clinical data for the three most-studied SRC inhibitors (dasatinib, bosutinib, and saracatinib) are reviewed in the next sections.
SRC Inhibitors: Preclinical Data
Dasatinib
Dasatinib (Sprycel; Bristol Myers-Squibb) is an orally available, small-molecule SRC/ABL inhibitor that has robust antitumor and antiproliferative activity against numerous hematologic and solid tumor cell lines [41,42]. In addition to inhibiting SRC and BCR-ABL in the subnanomolar range, dasatinib also variably inhibits other SFKs, c-KIT, PDGFR, and ephrin A2 [41]. The mechanism of SRC inhibition results from a hydrogen bond-mediated association with the ATP binding site, resulting in competitive restriction of ATP binding by SRC [42].
In preclinical studies, dasatinib was active in numerous cancer cell lines and in vivo tumor models. Studies of dasatinib in prostate [43] and colon cancer cell lines [44] showed inhibition of cellular adhesion, migration, and invasion. Breast cancer cell lines belonging to the basal/“triple-negative“ subtype were particularly sensitive to dasatinib. Breast cancers within this subgroup express basal cell cytokeratins (CK5 and CK17), do not express estrogen (ER) or progesterone receptors (PRs) or HER2 [45,46], and are notorious for poor prognosis [47]. Interestingly, in EGFR-overexpressing breast cancer cell lines, dasatinib inhibited cell growth, invasion, and angiogenesis, and stimulated apoptosis by activating caspase 8 and 9 [48]. Similarly, in lung cancer cells, dasatinib seemed to inhibit EGFR-dependent cell lines preferentially, whereas having a minimal effect on their wild-type EGFR-expressing counterparts. Moreover, dasatinib inhibited cell growth by promoting G1/S cell cycle arrest, with associated changes in the levels of cyclin D and p27 [49].
Dasatinib can also reduce metastatic disease and osteoclast-mediated bone resorption. In animal models of pancreatic and prostate cancer, dasatinib significantly reduced tumor size and incidence of metastases [50,51]. In addition, recent data showed that dasatinib inhibits osteoclast activity in vitro, in part by inhibiting the macrophage colony-stimulating factor receptor (c-FMS), which may act in concert with SRC to potentiate osteoclast activation [52,53]. Signaling through c-FMS is critical for osteoclast survival and activity, with disruption resulting in an osteopetrotic phenotype, much like that observed in SRC-/- deficient mice [54]. In a recent study using osteoclast precursors obtained from ovarian tumor ascites, dasatinib inhibited osteoclast production at concentrations less than 1 nM; this effect may be mediated by the concerted inhibition of c-FMS and SRC because imatinib (a known c-FMS, but not SRC, inhibitor) produced inhibition, albeit at much higher concentrations [55].
Bosutinib
Bosutinib (previously SKI-606; Wyeth) is a dual SRC/ABL kinase inhibitor that inhibits SRC with an 50% inhibitory concentration (IC50) of 1.2 nM and SRC-dependent fibroblasts in suspension with an IC50 of 100 nM. Bosutinib does not inhibit RTKs (KIT or PDGFR) at any appreciable level, but it does have activity against other SFKs [56,57].
In cellular assays, bosutinib treatment resulted in a dose-dependent reduction in proliferation, invasion, and migration of breast cancer cells [58,59]. Furthermore, in a murine model of breast carcinoma, bosutinib inhibited tumor growth and significantly reduced the number of liver, spleen, and lung metastases. These effects correlated with reduced phosphorylation of AKT, FAK, and MAPK and with an increase in apoptosis and E-cadherin expression [58]. In addition, bosutinib inhibited colorectal cancer cell adhesion and motility. Interestingly, this effect seemed to result from reduced SRC-dependent β-catenin activation, with small interfering RNA-driven knockdown of β-catenin abrogating the effects of bosutinib on cell-cell adhesion [60]. Furthermore, bosutinib showed modest activity in xenograft models of colon cancer and had an oral bioavailability of 18% and a plasma half-life of 8.6 hours [61].
Recent work has shown that SFKs are activated in 33% of non-small cell lung cancers (NSCLCs), with up-regulation correlating with male gender, active smoker status, and squamous cell histology. Treatment of NSCLC cell lines with bosutinib had an antiproliferative and proapoptotic effect, particularly in cell lines with increased Tyr419 SRC autophosphorylation at baseline [62]. Recent work has also showed that some human-derived pancreatic tumor xenografts were sensitive to bosutinib and sensitivity correlated with caveolin 1 expression, previously identified as a predictor of response to dasatinib in breast cancer cell lines [45,63].
Saracatinib
Saracatinib (formerly AZD0530; AstraZeneca) is another ATP-competitive inhibitor of SRC and SFKs, with activity against ABL and activated mutant forms of EGFR (L858R and L861Q) [64,65]. In a panel of 13 human cancer cell lines treated with saracatinib, there was submicromolar growth inhibition in four cell lines (derived from colon, prostate, and lung tumors) and inhibitory effects on migration and invasion [29,66]. In vivo, saracatinib inhibited the growth of 3 of 16 human-derived pancreatic cancer xenografts, with associated decreases in FAK, paxillin, and STAT3 activation. The authors also identified and validated a gene expression profile, based on the expression of LRRC19 and IGFBP2, which achieved 100% sensitivity and 83% specificity at predicting growth inhibition in an independent sample of eight xenografts [67]. In addition, saracatinib showed activity in in vitro and in vivo models of castration-resistant prostate cancer (CRPC) [68].
SRC Inhibitors: Preclinical Data Evaluating Novel Combinations
Dysregulated SRC signaling has been implicated in the development of resistance to numerous anticancer agents, including cetuximab, oxaliplatin, and gemcitabine [69–71]. Given these findings, and the involvement of SRC in modulating multiple signaling pathways, there is considerable interest in studying SRC inhibitors in conjunction with chemotherapeutic and biologic agents.
Combination with Antiestrogen Therapies
Current antihormonal treatments for ER-positive breast cancer include selective ER modulators (e.g., tamoxifen) and aromatase inhibitors (e.g., anastrozole), which decrease ER signaling and estrogen production, respectively. SRC potentiates ER signaling by phosphorylating the ER on Tyr537, and when complexed with estrogen, the ER associates with SRC to promote cellular proliferation [72,73]. This cross talk suggests possible synergy between antiestrogens and SRCinhibitors, with recent data supporting this supposition. In ER-overexpressing breast cancer cell lines, saracatinib and tamoxifen synergistically inhibited cell growth [74] and prevented the development of tamoxifen resistance [75]. Similarly, saracatinib and anastrozole in combination reduced both the development of drug resistance and tumor growth in vivo [76]. Furthermore, treating tamoxifen-resistant cells with PP2 restores tamoxifen sensitivity [77].
Combination with Cytotoxic Therapies
In an in vitro study of 5-fluorouracil (5-FU)-resistant pancreatic cancer cells, PP2 reversed 5-FU chemoresistance and restored 5-FU- induced apoptosis. Furthermore, 5-FU and PP2 in combination decreased in vivo tumor growth and metastatic disease [78]. In pancreatic adenocarcinoma cell lines, the level of SRC expression correlated with increased resistance to gemcitabine, and small interfering RNA-mediated SRC inhibition potentiated gemcitabine-induced caspase-mediatedapoptosis [69]. In ovarian and colon carcinoma cells, dasatinib restored paclitaxel sensitivity and acted synergistically with oxaliplatin, respectively [70,79].
Combination with Anti-EGFR Therapies
Recent work using an in vitro model of colorectal cancer showed that combination of a monoclonal antibody to EGFR and a SRC inhibitor synergistically inhibited cell proliferation and colony formation [80]. Similarly, recent findings indicate that both dasatinib and saracatinib can restore the sensitivity of resistant head and neck squamous cell carcinoma cell lines to the EGFR inhibitors cetuximab and gefitinib [71,81].
SRC Inhibitors: Preliminary Clinical Activity
In light of promising preclinical studies, dasatinib, bosutinib, and saracatinib have entered clinical trials. Preliminary data suggest that the agents are well tolerated at doses that achieve clinically meaningful plasma drug concentrations. Clinical studies of SRC inhibitors as single agents or in combination are shown in Tables 1 and 2.
Table 1.
Clinical Studies of SRC Inhibitors as Single Agents.
| Drug | Tumor Type | ClinicalTrials.gov Identifier | Phase | Dose and Schedule | Completion Date | Enrolment Status | Expected Enrolment (n) |
| Dasatinib | Advanced solid tumors | NCT00099606 | 1 | 35–120 mg twice daily | Jul 2007 | Completed | 60 |
| Hormone-sensitive breast cancer | NCT00371345 | 2 | 70 mg twice daily | Mar 2009 | Completed | 70 | |
| Triple-negative breast cancer | NCT00371254 | 2 | 70 mg twice daily | Sep 2008 | Completed | 44 | |
| Head and neck squamous cell carcinoma | NCT00507767 | 2 | 100 mg twice daily | Jul 2010 | Active, not recruiting | 35 | |
| Castration-resistant prostate cancer | NCT00385580 | 2 | 70 mg twice daily | Dec 2008 | Active, not recruiting | 100 | |
| Multiple myeloma | NCT00429949 | 2 | NR | NR | Completed | NR | |
| Pancreatic cancer | NCT00544908 | 2 | (dose NR) twice daily | Dec 2009 | Active, not recruiting | 41 | |
| Colorectal cancer | NCT00504153 | 2 | (dose NR) twice daily | Nov 2008 | Active, not recruiting | 54 | |
| Small cell lung cancer | NCT00470054 | 2 | (dose NR) twice daily | Oct 2008 | Active, not recruiting | 56 | |
| NSCLC | NCT00787267 | 2 | 70 mg twice daily | Sep 2011 | Recruiting | 100 | |
| Breast cancer (patient selection by genomic status) | NCT00780676 | 2 | 100 mg once daily | Oct 2024 | Recruiting | 532 | |
| Transitional cell carcinoma of the bladder (adjuvant treatment before surgery) | NCT00706641 | Pilot study | 100 mg once daily | Dec 2010 | Recruiting | 25 | |
| Hepatocellular carcinoma | NCT00459108 | 2 | (dose NR) twice daily | Jun 2009 | Recruiting | 41 | |
| Sarcomas | NCT00464620 | 2 | (dose NR) twice daily | Dec 2013 | Recruiting | 502 | |
| Biomarker analysis of EGFR status | NCT00903734 | 1 | NA | May 2013 | Recruiting | 102 | |
| Bosutinib | Advanced solid tumors | NCT00195260 | 1 | 50–600 mg once daily | Dec 2009 | Active, not recruiting | 151 |
| Breast cancer | NCT00319254 | 2 | 400 mg once daily | Jun 2008 | Completed | 75 | |
| Saracatinib | Advanced solid tumors | NCT00704366 | 1 | (variable dose) once daily | Feb 2010 | Active, not recruiting | 24 |
| Osteosarcoma (localized to lung) | NCT00923286 | 2 | 175 mg once daily | Feb 2015 | Recruiting | 88 | |
| Hormone receptor-negative breast cancer | NCT00559507 | 2 | NR | Jul 2010 | Recruiting | 41 | |
| Soft tissue sarcoma | NCT00659360 | 2 | NR | Feb 2009 | Active, not recruiting | 37 | |
| Melanoma | NCT00669019 | 2 | NR | Feb 2008 | Recruiting | 40 | |
| Castration-resistant prostate cancer | NCT00513071 | 2 | (dose NR) once daily | Oct 2008 | Completed | 28 | |
| Thymoma or thymic cancer | NCT00718809 | 2 | (dose NR) once daily | Jan 2011 | Recruiting | 39 | |
| Stomach or gastroesophageal junction cancer | NCT00607594 | 2 | NR | Sep 2009 | Recruiting | 35 | |
| Colorectal cancer | NCT00397878 | 2 | (dose NR) once daily | Apr 2008 | Active, not recruiting | 35 | |
| Head and neck cancer | NCT00513435 | 2 | (dose NR) once daily | Sep 2010 | Active, not recruiting | 28 | |
| Small cell lung cancer | NCT00528645 | 2 | (dose NR) once daily | Apr 2009 | Active, not recruiting | 44 |
NA indicates not applicable; NR, not reported.
Table 2.
Clinical Studies of SRC Inhibitors Combination with Other Agents.
| Drug | Combination Agent(s) | Tumor Type | ClinicalTrials.gov Identifier | Phase | SRC Inhibitor Dose and Schedule | Completion Date | Enrolment Status | Expected Enrolment (n) |
| Dasatinib | Erlotinib | NSCLC | NCT00444015 | 1 | NR | Jan 2010 | Active, not recruiting | 20 |
| Erlotinib | Glioma | NCT00609999 | 1 | 100 mg once daily | Jan 2009 | Recruiting | 48 | |
| Capecitabine | Breast cancer | NCT00452673 | 1 | 50–100 mg twice daily | Dec 2009 | Active, not recruiting | 50 | |
| Paclitaxel | Ovarian, peritoneal, or tubal cancer | NCT00672295 | 1 | 50–250 mg once daily | Mar 2010 | Recruiting | 24 | |
| Carboplatin | ||||||||
| Capecitabine | Colorectal cancer | NCT00920868 | 1 | 50 mg twice daily | May 2011 | Recruiting | 56 | |
| Oxaliplatin | ||||||||
| Bevacizumab | ||||||||
| Bevacizumab | Advanced solid tumors | NCT00792545 | 1 | (dose NR) once daily | Jul 2010 | Recruiting | 48 | |
| Paclitaxel | Breast cancer | NCT00820170 | 1/2 | (dose NR) once daily | Jan 2012 | Recruiting | 60 | |
| Docetaxel | Castration-resistant prostate cancer | NCT00439270 | 1/2 | 50–150 mg once daily | Oct 2009 | Active, not recruiting | 66 | |
| Zoledronic acid | Breast cancer with bone metastasis | NCT00566618 | 1/2 | 100 mg once daily | Mar 2010 | Recruiting | 55 | |
| Dacarbazine | Melanoma | NCT00597038 | 1/2 | 50–70 mg twice daily | Feb 2010 | Recruiting | 47 | |
| Letrozole | Hormone receptor-positive/HER2-negative breast cancer | NCT00696072 | 2 | 100 mg once daily | Jun 2012 | Recruiting | 120 | |
| Docetaxel | Castration-resistant prostate cancer | NCT00744497 | 3 | 100 mg once daily | Sep 2012 | Recruiting | 1380 | |
| Prednisone | ||||||||
| Bosutinib | Capecitabine | Solid tumors and HER2-advanced breast cancer | NCT00959946 | 1/2 | NR | Dec 2011 | Recruiting | 152 |
| Letrozole | Hormone-sensitive breast cancer | NCT00880009 | 2 | NR | Dec 2013 | Recruiting | 250 | |
| Exemestane | Hormone-sensitive breast cancer | NCT00793546 | 2 | NR | Jul 2011 | Recruiting | 224 | |
| Saracatinib | Carboplatin | Advanced solid tumors | NCT00496028 | 1 | NR | Oct 2009 | Active, not recruiting | 234 |
| Paclitaxel | ||||||||
| Cediranib | Advanced solid tumors | NCT00475956 | 1 | 125 or 175 mg once daily | Mar 2009 | Active, not recruiting | 56 | |
| Gemcitabine | Pancreatic cancer | NCT00265876 | 1/2 | (dose NR) once daily | Jun 2009 | Suspended | 60 | |
| Carboplatin | Ovarian cancer | NCT00610714 | 2 | (dose NR) once daily | May 2010 | Active, not recruiting | 241 | |
| Paclitaxel | ||||||||
| Zoledronic acid | Prostate or breast cancer with bone metastasis | NCT00558272 | 2 | NR | Aug 2010 | Recruiting | 132 |
NR indicates not reported.
Single-Agent Studies with Dasatinib
Currently, dasatinib is approved for the second-line treatment of chronic myeloid leukemia and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Dasatinib is currently being studied in numerous solid malignancies. In a phase 1 dose-escalation study, Demetri et al. [82] reported on the safety, tolerability, and pharmacologic profile of dasatinib in 67 patients with refractory solid tumors. Patients received oral dasatinib either every 12 hours for five consecutive days followed by two nontreatment days (5D2) or as continuous twice-daily dosing. Maximum tolerated dosages (MTDs) were established as 120 mg twice daily for dosing for five consecutive days followed by two nontreatment days and 70 mg for continuous twice-daily dosing. Dose-limiting toxicities (DLTs) included grade 2 rash, grade 3 lethargy, grade 3 proteinuria, and grade 3 hypocalcemia. Previous studies of dasatinib in Ph+ leukemias showed high rates of treatmentassociated neutropenia (45%), thrombocytopenia (35%), and pleural effusion (35%) [83]. Interestingly, in solid tumors at least, most treatment-related toxicities were nonhematologic (nausea, fatigue, lethargy, anorexia, proteinuria, and diarrhea), suggesting that hematologic adverse effects may be related to antileukemic activity. Pleural effusions were infrequent (three patients), although subsequent phase 2 studies showed a higher incidence [84]. The reasons for these differences are unclear, although they may relate to patient selection and underlying malignancies. Whereas no objective responses were reported, 16% of patients had stable disease and 25% had metabolic partial response (as judged by positron emission tomography scan).
Recent phase 2 studies suggest that dasatinib is well tolerated with modest single-agent activity in breast cancer. In a phase 2 study, Finn et al. [84] enrolled 44 patients with recurrent ormetastatic triple-negative breast carcinomas. Initial dosing at 100 mg twice daily was modified to a 70-mg twice-daily protocol after serious adverse events (AEs) in 22% of patients at the higher dose. The lower dose was well tolerated, with partial responses confirmed in two patients and stable disease achieved in 11 patients (two for >16 weeks). In a phase 2 study of 68 patients with advanced hormone receptor-positive breast cancers (ER+ and/or PR+ and/or HER2 amplified), there were three partial responses and six instances of stable disease (range, 24–33 weeks) [85]. All nine of these patients had ER- and PR-positive tumors, with two tumors also having amplified HER2.
In an analysis of pretreatment and posttreatment prostate tumor samples from patients with CRPC, SRC activity was increased in 28% of patients and was associated with decreased survival and increased metastatic disease [86]. Two recent phase 2 studies have evaluated the efficacy of dasatinib in CRPC [87,88]. Both studies enrolled men who were chemotherapy naive and had progressive metastatic CRPC; the first study used dasatinib 100 mg or 70 mg twice daily and the second used 100 mg once daily. Response rates were similar for the two dosing regimens. However, once-daily dosing was better tolerated, with 13% of patients reporting grade 3/4 AEs compared with 32% on the twice-daily regimen. Of 48 patients treated with 100 mg once daily, 1 patient had a confirmed prostate-specific antigen response (>50% decrease from baseline), 1 patient had a partial tumor response, and 8 patients had stable disease after 12 weeks. Levels of urinary N-telopeptide (a marker of osteoclast activity and bone resorption) decreased by more than 40% in 21 of 43 evaluable patients. Similarly, serum bone-specific alkaline phosphatase (marker of osteoblast activity) was decreased in 25 of the 44 patients with data, suggesting that dasatinib is effective at stabilizing metastatic disease and decreasing bone turnover.
Single-Agent Studies with Bosutinib and Saracatinib
At this time, few clinical studies have assessed the safety and efficacy of saracatinib and bosutinib. A recent two-part, phase 1 study of 81 patients with advanced solid tumors sought to establish the MTD of saracatinib and its effect on downstream targets of SRC [89]. In the first part of the study, 30 patients received saracatinib at doses ranging from 50 to 250 mg daily. The MTD was established as 175 mg with once-daily dosing. DLTs were leukopenia (grade 3), asthenia (grade 3), febrile neutropenia (grade 3), and respiratory failure (grade 5). One patient had renal failure (grade 4) with concomitant septic shock (resulting in death), although the relationship of this event to saracatinib was unclear. Other AEs were relatively mild and included nausea, asthenia, anorexia, vomiting, and diarrhea. In the second part of the study, 51 patients were randomized to receive 50, 125, or 175 mg of saracatinib. Dose-dependent reductions in levels of phospho-FAK and phospho-paxillin were noted in posttreatment samples, and patients with high baseline levels had proportionally larger reductions in these substrates after treatment. A modulatory effect of saracatinib on bone turnover was also observed, with the authors reporting a dose-dependent decrease in C-terminal telopeptide (a bone resorption marker) levels after treatment. There were no objective tumor responses, although 16% of patients continued treatment for more than 12 weeks. Thus, at the doses tested, saracatinib seems to be well tolerated and able to inhibit SRC kinase activity. On the basis of these results, follow-up phase 2 studies used saracatinib as a monotherapy in patients with advanced CRPC (n = 28) and advanced colorectal cancer (n = 10). Although saracatinib was generally well tolerated, there was no meaningful single-agent clinical activity [90,91].
In a phase 1 dose-escalation study of bosutinib in 51 patients with advanced solid tumors, bosutinib was generally well tolerated with an MTD of 400 mg for once-daily dosing. DLTs included grade 3 diarrhea (two patients) and grade 3 rash (one patient). Drug-related AEs were mainly gastrointestinal and included nausea, diarrhea, anorexia, vomiting, and asthenia, with diarrhea being the only grade 3 AE occurring in more than 5% of patients (14%). There were no objective responses, although six patients had stable disease lasting longer than 15 weeks and one patient had stable disease lasting longer than 52 weeks (pancreatic cancer) [92]. In a follow-up phase 2 study of women with stage IIIB, IIIC, or metastatic breast cancer, 73 women received bosutinib 400 mg daily. The drug was generally well tolerated, with only eight patients requiring dose reduction, mainly secondary to gastrointestinal adverse effects (diarrhea, nausea, and vomiting). Of 62 evaluable patients, four had partial responses and 13 and 25 had stable disease lasting 24 weeks or longer and less than 24 weeks, respectively [93].
Combination Studies
On the basis of promising results from preclinical studies, SRC inhibitors are being tested in combination with chemotherapies and other targeted agents. In a phase 1/2 study, dasatinib was administered with docetaxel to 46 patients with progressive CRPC. There was a prostate-specific antigen response in 13 of 32 patients and a Response Evaluation Criteria In Solid Tumors partial response in 12 of 21 patients. In addition, nine patients had stable disease (four at >21 weeks and five at >6 weeks). There was also indirect evidence of decreased bone resorption and formation. Of patients with measurable serum levels of urinary N-telopeptide and bone-specific alkaline phosphatase, there was more than 35% decrease in 12 of 26 and in 17 of 24 patients, respectively [94].
Dasatinib was also well tolerated in combination with 5-FU, leucovorin, oxaliplatin, and cetuximab in patients with metastatic colorectal cancer [95]. Of seven patients enrolled, two had radiographic evidence of response, including one confirmed partial response. This study is continuing at using higher dasatinib doses.
Additional studies are assessing SRC inhibitors in combination with anti-VEGF therapies. In a recent study, the effects of saracatinib (175 mg once daily) were examined in patients receiving daily oral cediranib (a small-molecule VEGFR inhibitor) at 20-, 30-, or 45-mg doses. All dose cohorts tolerated the treatment well, with no DLTs reported 28 days into the study. In the 11 patients for whom data were available, nine had stable disease (35–197 days in duration) [96].
Conclusions
There is no single oncogene better studied than SRC. Despite nearly a century of data suggesting a role in promoting malignancy, it is only recently, with the discovery of a class of highly selective and specific molecules, that we can effectively block SRC kinase activity. These clinical grade SRC inhibitors are currently being evaluated in the clinic. With such a central role in regulating so many cellular pathways, perhaps the most challenging task will be selecting the patients most likely to benefit from SRC inhibition. On the basis of SRC's role in tumor biology, these molecules may work best in early stage disease and in combination with other agents. Preliminary clinical data suggest a role for SRC inhibition in human disease. Ongoing studies evaluating the molecular effects of SRC inhibition in clinical tissue and combinations of SRC inhibitors with cytotoxics and other biologic agents are ongoing. Data from these studies are eagerly awaited, and these will help guide the next phase of development of this class of novel agents.
Abbreviations
- AE
adverse event
- CAS
CRK-associated substrate
- c-FMS
macrophage colony-stimulating factor receptor
- CRPC
castration-resistant prostate cancer
- CSK
C-terminal SRC kinase
- DLTs
dose-limiting toxicities
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- FAK
focal adhesion kinase
- 5-FU
5-fluorouracil
- IC50
50% inhibitory concentration
- IL-8
interleukin 8
- MTD
maximum tolerated dose
- PDGFR
platelet-derived growth factor receptor
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- RTK
receptor tyrosine kinase
- SFK
SRC family of tyrosine kinases
- SH
SRChomology
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Both authors take full responsibility for the preparation and content of the article and confirm that it reflects their viewpoint and medical expertise. StemScientific, funded by Bristol-Myers Squibb, provided only editorial support. Neither did Bristol-Myers Squibb influence the content of the article nor did the authors receive financial compensation for authoring the article.
References
- 1.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez RH, Kantarjian HM, Cortes JE. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–1929. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- 5.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 6.Shalloway D, Coussens PM, Yaciuk P. Overexpression of the c-Src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci USA. 1984;81:7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Ann Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- 8.Rengifo-Cam W, Konishi A, Morishita N, Matsuoka H, Yamori T, Nada S, Okada M. CSK defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene. 2004;23:289–297. doi: 10.1038/sj.onc.1207041. [DOI] [PubMed] [Google Scholar]
- 9.Masaki T, Okada M, Tokuda M, Shiratori Y, Hatase O, Shirai M, Nishioka M, Omata M. Reduced C-terminal Src kinase (CSK) activities in hepatocellular carcinoma. Hepatology. 1999;29:379–384. doi: 10.1002/hep.510290239. [DOI] [PubMed] [Google Scholar]
- 10.Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. 2000;275:41439–41446. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- 11.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol. 2004;24:8113–8133. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 14.Biscardi JS, Tice DA, Parsons SJ. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 15.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–5635. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- 16.Irby RB, Yeatman TJ. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 17.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–2436. [PubMed] [Google Scholar]
- 18.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 20.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 21.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 22.Boyer B, Roche S, Denoyelle M, Thiery JP. Src and Ras are involved in separate pathways in epithelial cell scattering. EMBO J. 1997;16:5904–5913. doi: 10.1093/emboj/16.19.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizawar R, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene. 2007;26:3503–3510. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- 26.Twamley-Stein GM, Pepperkok R, Ansorge W, Courtneidge SA. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci USA. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori S, Ronnstrand L, Yokote K, Engstrom A, Courtneidge SA, Claesson-Welsh L, Heldin CH. Identification of two juxtamembrane autophosphorylation sites in the PDGF β-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993;12:2257–2264. doi: 10.1002/j.1460-2075.1993.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prathapam T, Tegen S, Oskarsson T, Trumpp A, Martin GS. Activated Src abrogates the Myc requirement for the G0/G1 transition but not for the G1/S transition. Proc Natl Acad Sci USA. 2006;103:2695–2700. doi: 10.1073/pnas.0511186103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008;27:6365–6375. doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche S, Fumagalli S, Courtneidge SA. Requirement for Src family protein tyrosine kinases in G2 for fibroblast cell division. Science. 1995;269:1567–1569. doi: 10.1126/science.7545311. [DOI] [PubMed] [Google Scholar]
- 31.Kanda S, Miyata Y, Kanetake H, Smithgall TE. Non-receptor protein-tyrosine kinases as molecular targets for antiangiogenic therapy [review] Int J Mol Med. 2007;20:113–121. [PubMed] [Google Scholar]
- 32.Kilarski WW, Jura N, Gerwins P. Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp Cell Res. 2003;291:70–82. doi: 10.1016/s0014-4827(03)00374-4. [DOI] [PubMed] [Google Scholar]
- 33.Yeh M, Gharavi NM, Choi J, Hsieh X, Reed E, Mouillesseaux KP, Cole AL, Reddy ST, Berliner JA. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-Src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem. 2004;279:30175–30181. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]
- 34.Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18:5014–5023. doi: 10.1091/mbc.E07-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werdich XQ, Penn JS. Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis. 2005;8:315–326. doi: 10.1007/s10456-005-9021-x. [DOI] [PubMed] [Google Scholar]
- 36.Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci USA. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horne WC, Neff L, Chatterjee D, Lomri A, Levy JB, Baron R. Osteoclasts express high levels of pp60c-Src in association with intracellular membranes. J Cell Biol. 1992;119:1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki T, Tanaka S, Sanjay A, Baron R. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16:68–74. doi: 10.1007/s10165-006-0460-z. [DOI] [PubMed] [Google Scholar]
- 39.Myoui A, Nishimura R, Williams PJ, Hiraga T, Tamura D, Michigami T, Mundy GR, Yoneda T. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003;63:5028–5033. [PubMed] [Google Scholar]
- 40.Rucci N, Recchia I, Angelucci A, Alamanou M, Del FA, Fortunati D, Susa M, Fabbro D, Bologna M, Teti A. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther. 2006;318:161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- 41.Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074–1082. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 43.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 44.Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, Ashton GH, Frame MC, Brunton VG. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 45.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative“ breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 46.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 47.Kaklamani VG, Gradishar WJ. Gene expression in breast cancer. Curr Treat Options Oncol. 2006;7:123–128. doi: 10.1007/s11864-006-0047-0. [DOI] [PubMed] [Google Scholar]
- 48.Nautiyal J, Majumder P, Patel BB, Lee FY, Majumdar AP. Src inhibitor dasatinib inhibits growth of breast cancer cells by modulating EGFR signaling. Cancer Lett. 2009;283:143–151. doi: 10.1016/j.canlet.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 49.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–5548. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 50.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 51.Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE. Inhibition of Src expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol. 2006;168:962–972. doi: 10.2353/ajpath.2006.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 53.Vandyke K, Dewar AL, Farrugia AN, Fitter S, Bik TL, Hughes TP, Zannettino AC. Therapeutic concentrations of dasatinib inhibit in vitro osteoclastogenesis. Leukemia. 2009;23:994–997. doi: 10.1038/leu.2008.356. [DOI] [PubMed] [Google Scholar]
- 54.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-Src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 55.Brownlow N, Mol C, Hayford C, Ghaem-Maghami S, Dibb NJ. Dasatinib is a potent inhibitor of tumour-associated macrophages, osteoclasts and the FMS receptor. Leukemia. 2009;23:590–594. doi: 10.1038/leu.2008.237. [DOI] [PubMed] [Google Scholar]
- 56.Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, et al. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem. 2001;44:3965–3977. doi: 10.1021/jm0102250. [DOI] [PubMed] [Google Scholar]
- 57.Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, Boschelli F. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res. 2003;63:375–381. [PubMed] [Google Scholar]
- 58.Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. 2007;67:1580–1588. doi: 10.1158/0008-5472.CAN-06-2027. [DOI] [PubMed] [Google Scholar]
- 59.Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7:1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coluccia AM, Benati D, Dekhil H, De FA, Lan C, Gambacorti-Passerini C. SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of β-catenin and its nuclear signaling. Cancer Res. 2006;66:2279–2286. doi: 10.1158/0008-5472.CAN-05-2057. [DOI] [PubMed] [Google Scholar]
- 61.Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, Boghaert E, Arndt K, Ye F, Boschelli DH, et al. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 2005;65:5358–5364. doi: 10.1158/0008-5472.CAN-04-2484. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, Wei W, Ma L, Wistuba II, Johnson FM, Kurie JM. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Messersmith WA, Rajeshkumar NV, Tan AC, Wang XF, Diesl V, Choe SE, Follettie M, Coughlin C, Boschelli F, Garcia-Garcia E, et al. Efficacy and pharmacodynamic effects of bosutinib (SKI-606), a Src/Abl inhibitor, in freshly generated human pancreas cancer xenografts. Mol Cancer Ther. 2009;8:1484–1493. doi: 10.1158/1535-7163.MCT-09-0075. [DOI] [PubMed] [Google Scholar]
- 64.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW, et al. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. 2009;3:248–261. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, Lambert-van der Brempt C, Morgentin R, Norman RA, Olivier A, et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 66.Purnell PR, Mack PC, Tepper CG, Evans CP, Green TP, Gumerlock PH, Lara PN, Gandara DR, Kung HJ, Gautschi O. The Src inhibitor AZD0530 blocks invasion and may act as a radiosensitizer in lung cancer cells. J Thorac Oncol. 2009;4:448–454. doi: 10.1097/JTO.0b013e31819c78fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajeshkumar NV, Tan AC, De OE, Womack C, Wombwell H, Morgan S, Warren MV, Walker J, Green TP, Jimeno A, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15:4138–4146. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- 68.Yang JC, Ok JH, Busby JE, Borowsky AD, Kung HJ, Evans CP. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. 2009;69:151–160. doi: 10.1158/0008-5472.CAN-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J Am Coll Surg. 2004;198:953–959. doi: 10.1016/j.jamcollsurg.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 70.George JA, Chen T, Taylor CC. SRC tyrosine kinase and multidrug resistance protein-1 inhibitions act independently but cooperatively to restore paclitaxel sensitivity to paclitaxel-resistant ovarian cancer cells. Cancer Res. 2005;65:10381–10388. doi: 10.1158/0008-5472.CAN-05-1822. [DOI] [PubMed] [Google Scholar]
- 71.Wheeler DL, Iida M, Kruser TJ, Nechrebecki MM, Dunn EF, Armstrong EA, Huang S, Harari PM. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009;8:696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnold SF, Vorojeikina DP, Notides AC. Phosphorylation of tyrosine 537 on the human estrogen receptor is required for binding to an estrogen response element. J Biol Chem. 1995;270:30205–30212. doi: 10.1074/jbc.270.50.30205. [DOI] [PubMed] [Google Scholar]
- 73.Castoria G, Migliaccio A, Bilancio A, Di DM, De FA, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herynk MH, Beyer AR, Cui Y, Weiss H, Anderson E, Green TP, Fuqua SA. Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol Cancer Ther. 2006;5:3023–3031. doi: 10.1158/1535-7163.MCT-06-0394. [DOI] [PubMed] [Google Scholar]
- 75.Hiscox S, Jordan NJ, Smith C, James M, Morgan L, Taylor KM, Green TP, Nicholson RI. Dual targeting of Src and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res Treat. 2009;115:57–67. doi: 10.1007/s10549-008-0058-6. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Guggisberg N, Jorda M, Gonzalez-Angulo A, Hennessy B, Mills GB, Tan CK, Slingerland JM. Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin Cancer Res. 2009;15:3396–3405. doi: 10.1158/1078-0432.CCR-08-3127. [DOI] [PubMed] [Google Scholar]
- 77.Yue W, Fan P, Wang J, Li Y, Santen RJ. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ischenko I, Camaj P, Seeliger H, Kleespies A, Guba M, De Toni EN, Schwarz B, Graeb C, Eichhorn ME, Jauch KW, et al. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27:7212–7222. doi: 10.1038/onc.2008.326. [DOI] [PubMed] [Google Scholar]
- 79.Kopetz S, Lesslie DP, Dallas NA, Park SI, Johnson M, Parikh NU, Kim MP, Abbruzzese JL, Ellis LM, Chandra J, et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopetz S, Wu J, Johnson F, Donato N. Anti-tumor effects of combination therapy with anti-EGFR and anti-Src therapy in colorectal cancer; First AACR International Conference on Molecular Diagnostics in Cancer Therapeutic Development; September 2006; Washington, DC. 2006. Abstract B51. [Google Scholar]
- 81.Andersen P, Villingshoj M, Poulsen HS, Stockhausen MT. Improved response by co-targeting EGFR/EGFRvIII and Src family kinases in human cancer cells. Cancer Invest. 2009;27:178–183. doi: 10.1080/07357900802570759. [DOI] [PubMed] [Google Scholar]
- 82.Demetri GD, Lo Russo P, Macpherson IR, Wang D, Morgan JA, Brunton VG, Paliwal P, Agrawal S, Voi M, Evans TR. Phase I dose-escalation and pharmacokinetic study of dasatinib (BMS-354825), a Src and multi-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:6232–6240. doi: 10.1158/1078-0432.CCR-09-0224. [DOI] [PubMed] [Google Scholar]
- 83.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 84.Finn RS, Bengala C, Ibrahim N, Strauss LC, Fairchild J, Sy O, Roche H, Sparano J, Goldstein LJ. Phase II trial of dasatinib in triple-negative breast cancer: results of study CA180059. Cancer Res. 2008;69:242S–242S. doi: 10.1158/1078-0432.CCR-11-0288. Abstract 3118. [DOI] [PubMed] [Google Scholar]
- 85.Mayer E, Baurain J, Sparano J, Strauss L, Campone M, Fumoleau P, Rugo H, Awada A, Sy O, Llombart A. Dasatinib in advanced HER2/neu amplified and ER/PR-positive breast cancer: phase II study CA180088. J Clin Oncol. 2009;27:43S–43S. Abstract 1011. [Google Scholar]
- 86.Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:3540–3549. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- 87.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, Hudes GR, Cheng S, Paliwal P, Sternberg CN. Dasatinib in patients with hormone-refractory progressive prostate cancer: a phase II study. J Clin Oncol. 2008;26:288S–288S. Abstract 5156. [Google Scholar]
- 88.Yu EY, Massard C, Gross M, Wilding G, Posadas E, Culine S, Carducci M, Trudel G, Paliwal P, Sternberg C. A phase II study of once-daily dasatinib for patients with castration-resistant prostate cancer (CA180085) J Clin Oncol. 2009;27:270S–270S. Abstract 5147. [Google Scholar]
- 89.Tabernero J, Cervantes A, Hoekman K, Hurwitz HI, Jodrell DI, Hamberg P, Stuart M, Green TP, Iacona RB, Baselga J. Phase I study of AZD0530, an oral potent inhibitor of Src kinase: first demonstration of inhibition of Src activity in human cancers. J Clin Oncol. 2007;25:143S–143S. Abstract 3520. [Google Scholar]
- 90.Eng C, Kopetz S, Morris J, Malik Z, Stewart DJ, Chang H-Y, Ohinata A, Abbruzzese JL, Gallick GE. Phase II study of the novel oral Src-kinase inhibitor, AZD0530, in previously treated advanced colorectal cancer patients; AACR 99th Annual Meeting; April 12–16, 2008; San Diego, CA. 2008. Abstract LB-76. [Google Scholar]
- 91.Lara PN, Jr, Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, Posadas E, Stadler W, Gandara DR. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009;20:179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Messersmith WA, Krishnamurthi S, Hewes BA, Zacharchuk CM, Abbas R, Martins P, Dowling E, Volkert A, Martin A, Daud AI. Bosutinib (SKI-606), a dual Src/Abl tyrosine kinase inhibitor: preliminary results from a phase 1 study in patients with advanced malignant solid tumors. J Clin Oncol. 2007;25:150S–150S. Abstract 3552. [Google Scholar]
- 93.Campone M, Bondarenko I, Brincat S, Epstein RJ, Munster PN, Dubois E, Martin EC, Turnbull K, Zacharchuk C. Preliminary results of a phase 2 study of bosutinib (SKI-606), a dual Src/Abl kinase inhibitor, in patients with advanced breast cancer. Breast Cancer Res Treat. 2007:106–106. Abstract 6062. [Google Scholar]
- 94.Araujo J, Armstrong AJ, Braud EL, Posadas E, Lonberg M, Gallick GE, Trudel GC, Paliwal P, Agrawal S, Logothetis CJ. Dasatinib and docetaxel combination treatment for patients with castration-resistant progressive prostate cancer: a phase I/II study (CA180086) J Clin Oncol. 2009;27:249S–249S. Abstract 5061. [Google Scholar]
- 95.Kopetz S, Wolff RA, Glover K, Henry L, Eng C, Chang DZ, Overman M, Gallick G, Abruzzese J. Phase I study of Src inhibition with dasatinib in combination with 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX) and cetuximab in metastatic colorectal cancer; 2008 Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. 2008. Abstract 325. [Google Scholar]
- 96.Trarbach T, Drevs J, Strumberg D, Gauler TC, Schneider V, Eberhardt WE, Marotti M, Puchalski TA, Swaisland AJ. A phase I, open-label, multicenter study of cediranib and AZD0530 in patients with advanced solid tumors. J Clin Oncol. 2008;26:175S–175S. Abstract 3592. [Google Scholar]