Abstract
We report three rare cases of sclerosing angiomatoid nodular transformation (SANT) in the spleen. We compared the conventional and contrast-enhanced ultrasonographic appearance. The conventional sonographic examinations exhibited solitary lesions without common respects, while contrast-enhanced ultrasonography (CEUS) revealed nodular appearance mimicking its pathologic characteristics. It suggests that CEUS can provide morphologic information for diagnosing SANT.
Keywords: Sclerosing angiomatoid nodular transformation, Contrast-enhanced ultrasonography, Ultrasound
INTRODUCTION
Sclerosing angiomatoid nodular transformation (SANT) is a rare benign lesion. Martel named it for its pathological characteristics[1]. Its particular morphologic appearance, immunophenotype, and benign clinical course indicate that it is a distinctive non-neoplastic vascular lesion in the spleen. We report the ultrasonographic images in three cases of SANT confirmed by splenectomy. Two of them also underwent contrast-enhanced ultrasonography (CEUS). We used a second-generation contrast agent, SonoVue (Bracco, Italy) at a dose of 1.2 mL[2].
CASE REPORT
Case 1
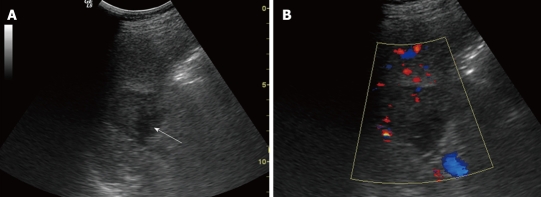
A 36-year-old man was found to have a splenic mass by a routine medical examination without any symptoms. Physical examination found nothing remarkable. No cervical or inguinal lymphadenopathy was found. The laboratory test results were as follows: hemoglobin 130 g/L, Ery 4.1 T/L, WBC 14.5 g/L, neutrophils 12.0 g/L and platelets 174 g/L, and all other data were within normal limits. Abdominal ultrasonography (US) was done using a 3.5C ultrasound transducer attached to a LOGIQ 5 Expert; it demonstrated a 4.2 cm × 3.7 cm well-demarcated, hypoechoic lesion in the spleen (Figure 1). The patient also underwent an MRI examination. On T1- and T2-weighted images, heterogeneous hypo-signal intensity with peripheral and septa enhancement, especially at the delayed phase, was displayed. The patient underwent splenectomy. In the surgery, the mass was found to have an integrated envelope and a heterogeneous cut surface. A few days after the surgery, his WBC and neutrophils became normal.
Figure 1.
Conventional sonographic findings of sclerosing angiomatoid nodular transformation in a 36-year-old man. A: B mode ultrasonography demonstrates a hypoechoic lesion (arrow) in the spleen; B: Color Doppler flow imaging shows a low color-flow signal in the lesion.
Case 2
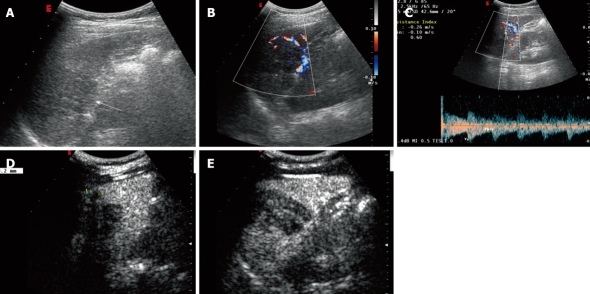
A 37-year-old woman was referred to abdominal ultrasound examination because of pain in the left upper quadrant. Lesions were found both in her liver and spleen. Proliferative disease of metastasis was taken into consideration during the differentiation process. Physical examination found nothing remarkable. No cervical or inguinal lymphadenopathy was observed. The laboratory tests yielded the following results: hemoglobin 120 g/L, Ery 3.88 T/L, WBC 4.6 g/L, neutrophils 3.2 g/L, platelets 214 g/L, CEA 1.16 ng/mL, AFP 2.4 ng/mL and CA19-9 6.79 U/mL. Other data were all within normal limits. Abdominal ultrasonography was done using a CA430E5-2 ultrasound transducer attached to a Technos ESAOTE DU 8; it demonstrated a 5.2 cm × 3.6 cm heterogeneous hypoechoic lesion in the spleen with an unclear margin. Color Doppler flow imaging (CDFI) showed blood-flow signals in the peripheral area of the lesion and resistance index (RI) was 0.66 (Figure 2A-C). The lesion was enhanced in the diffuse pattern from 11 s after injection, and appeared to be homogeneously hyperechoic in comparison with the splenic parenchyma in 21 s. It turned out to be isoechoic in 4 s and hypoechoic in 30 s after the injection (Figure 2E). The lesion showed a persistent enhancement up to about 7 min. Another 0.9 cm × 0.9 cm homologous nodule was found attaching to the lesion which became evident after being enhanced (Figure 2D). In order to reach a final diagnosis, the patient underwent surgical excision of the spleen and part of the liver. In the surgery, a splenic mass was firm with a clear margin. The pathological result is a SANT of the spleen and hemangiomas in the liver.
Figure 2.
Conventional ultrasonography and contrast-enhanced ultrasonography findings of sclerosing angiomatoid nodular transformation in a 37-year-old woman with pain in the left upper quadrant. A-C: A heterogeneous hypoechoic lesion (arrow) in the spleen, with blood-flow signals in the peripheral area; D: There was a 0.9 cm homologous nodule attaching to the side of the lesion that became evident after being enhanced (2 min 48 s after injection); E: The lesion was enhanced homogeneously.
Case 3
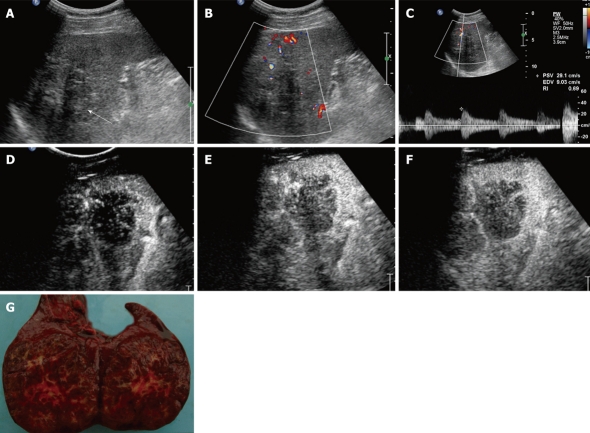
A 39-year-old man was admitted to the hospital because of a left upper quadrant mass of unknown cause. Splenomegaly was suspected by physical examination. No cervical or inguinal lymphadenopathy was found. The laboratory test results were: hemoglobin 123 g/L, Ery 4.23 T/L, WBC 6.7 g/L, neutrophils 4.6 g/L, platelets 167 g/L, CEA 0.56 ng/mL, AFP 2.4 ng/mL and CA19-9 3.6 U/mL. All other laboratory tests were within normal limits. Abdominal ultrasonography was done using a C5-2 ultrasound transducer attached to a Philips IU22; it demonstrated a 7.0 cm × 6.2 cm well-demarcated, hypoechoic lesion in the spleen. CDFI showed branch-shaped and wire-like blood-flow signals inside and spot-shaped blood-flow signals in the periphery of the lesion. RI was 0.50-0.66 (Figure 3A-C). The lesion was enhanced in the branch-shaped diffuse pattern from 12 s after injection (Figure 3D). It presented heterogeneous lobular appearance when reaching its peak enhancement in 23 s (Figure 3E). We also detected lots of septa inside the lesion (Figure 3F). It became hypoechoic in 37 s in comparison with the splenic parenchyma. The lesion showed a persistent enhancement up to about 3 min. Its enhancement intensity was less than that of the spleen during the whole enhancement process. Its structure and margin were clearer than those of conventional ultrasonographic images. The patient also received CT examination, which revealed a low-density mass with mildly septa enhancement. The final diagnosis of SANT was confirmed by operation (Figure 3G).
Figure 3.
Conventional ultrasonography and contrast-enhanced ultrasonography findings of sclerosing angiomatoid nodular transformation in a 39-year-old man. A: A hypoechoic lesion (arrow) in the spleen; B and C: Color Doppler flow imaging shows branch-shaped and wire-like blood-flow signals inside and spot-shaped blood-flow signals in the periphery of the lesion; D: The lesion was enhanced in the branch-shaped diffuse pattern (15 s after injection); E: It presented heterogeneous lobular appearance when reaching its peak enhancement; F: Lots of septa inside the lesion; G: Surface of the lesion found during the operation.
DISCUSSION
SANT is a rare mild vascular lesion in the spleen. The age of our patients ranged from 36 to 39 years. Two of them were asymptomatic (Case 1 and Case 3). The lesions were usually found incidentally. Few patients presented with abdominal discomfort or splenomegaly. Its etiology is still unclear. Concurrent diseases included chronic lymphocytic leukemia, lung squamous cell carcinoma, colonic carcinoma and some other diseases[1]. We cannot confirm whether hepatic hemangioma as shown in Case 2 is related to SANT. The lesion presented as a solitary mass measuring 3-17 cm in greatest diameter as reported before[1]. And usually it has a clear margin with a normal splenic parenchyma. The gross section shows splenic angiomatoid nodules as altered red pulp tissue is entrapped by a non-neoplastic stromal proliferative process[3]. SANT is composed of small blood vessels of three typically distinct immunophenotypes: cord capillaries (CD34+/CD8-/CD31+), sinusoids (CD34-/CD8+/CD31+), and small veins (CD34-/CD8-/CD31+). It can be differentiated from other vascular tumors or tumor-like lesions, including hemangioma, littoral cell angioma (LCA), inflammatory pseudotumor (IPT) and follicular dendritic cell tumor (FDC), by immunohistochemical examinations.
Sonographic findings of our cases are described as follows: All the lesions were solitary and heterogeneous. Their greatest diameters were 3-7 cm. Two of them were hypoechoic and the other lesion was hyperechoic. Linear and slit-like hyperechoic septa was presented in one lesion (Case 3). The ratio of having a clear and unclear margin is 2:1. The color-Doppler showed arterial flow signals in all the lesions and their RIs were low. The contrast-enhanced images in the two lesions had something in common. The contrast media entered the lesions 1-2 s later than the splenic parenchyma. And they came out of the lesions earlier than that of the spleen. The lesions were both enhanced in diffuse pattern. One lesion (Case 3) presented a heterogeneous lobule with lots of septa inside at the peak enhancement. An extra 0.9 cm × 0.9 cm nodule attaching to the previously-found lesion was detected in Case 2 during the enhancement process.
Usually, we consider hyperechoic lesions in the spleen as benign ones on US images such as hemangioma. Malignant lesions are mostly hypoechoic and occur most frequently in lymphomas (80% of focal lesions)[4]. On conventional US images, the three lesions presented with different echogenicity. They were lack of consistency in their boundaries and shapes. An assessment of conventional US images does not provide any differential diagnostic clues. Additionally, color-Doppler technique is only capable of detecting some obvious color flow signals in big solid masses in the spleen. The images of conventional US examination are not characteristic.
We found few cases reporting the behavior of SANT with ultrasonography in the literature. Gutzeit reported a case of SANT[5], which had a hypoechoic halo, predominantly vascular on conventional US images and a “spoke wheel” pattern on CEUS images. In our cases, the heterogeneous lobule and lots of septa inside at the peak enhancement in Case 3 are similar to the “spoke wheel” pattern, but no vascular halo was present. The angiomatoid nodules are frequently delimited by concentric bands of collagen fibers, and there is always an inflamed fibrocellular to sclerotic stroma between the nodules at low magnification. Some exhibited fibrin deposition in the peripheral zone of the nodules. CEUS has shown to be useful in observing nodules whose contrast to the spleen parenchyma was enhanced after injection of SonoVue. Because of its multinodular angiomatoid appearance, SANT was described by Rosai[6] as the term “multinodular hemangioma”. In conclusion, CEUS is a new imaging technique that provides direct visualization of vessel structure and morphologic characteristics of the lesions.
Pathologically, SANT has been thought to be a variant of IPT. SANT has fewer inflammatory cells and myofibroblasts than those in IPT. Martel et al[1] reported one case of fever and three cases of high erythrocyte sedimentation among 25 cases of SANT. One patient (Case 1) also had high WBC and neutro phils before the operation. Consequently, the US appearances of the lesions reported above are between those of IPT and hemangioma. It can explain the diversity of its echogenicity, morphology and boundary.
Footnotes
Peer reviewer: Dr. Serdar Karakose, Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol. 2004;28:1268–1279. doi: 10.1097/01.pas.0000138004.54274.d3. [DOI] [PubMed] [Google Scholar]
- 2.Lim AK, Patel N, Eckersley RJ, Taylor-Robinson SD, Cosgrove DO, Blomley MJ. Evidence for spleen-specific uptake of a microbubble contrast agent: a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Lien HC, Hsiao CH. Coexisting sclerosing angiomatoid nodular transformation of the spleen with multiple calcifying fibrous pseudotumors in a patient. J Formos Med Assoc. 2007;106:234–239. doi: 10.1016/S0929-6646(09)60245-X. [DOI] [PubMed] [Google Scholar]
- 4.Woszczyk D, Kołodziej-Jaskuła A, Mykała-Cieśla J, Bal A, Łabuzek K, Gasińska T. Focal lesions in the ultrasonographic examination of the spleen as a symptom of various disease statuses: literature survey and case descriptions. Med Sci Monit. 2006;12:CS67–CS73. [PubMed] [Google Scholar]
- 5.Gutzeit A, Stuckmann G, Dommann-Scherrer C. Sclerosing angiomatoid nodular transformation (SANT) of the spleen: sonographic finding. J Clin Ultrasound. 2009;37:308–311. doi: 10.1002/jcu.20549. [DOI] [PubMed] [Google Scholar]
- 6.Rosai J. Spleen. In: Rosai and Ackerman’s Surgical Pathology., editor. 9th ed. Edinburgh: Mosby; 2004. pp. 20–35. [Google Scholar]