Summary
Gallstone disease is a frequent condition throughout the world and cholesterol stones are the most frequent form in western countries. Current standard treatment of symptomatic gallstone subjects remains laparoscopic cholecystectomy. The selection of patients amenable for non-surgical, medical therapy is of key importance: a careful analysis should consider the natural history of the disease and the overall costs of therapy. Only patients with mild symptoms and small, uncalcified cholesterol gallstones in a functioning gallbladder with a patent cystic duct will be considered for oral litholysis by the hydrophilic ursodeoxycholic acid (UDCA) hopefully leading to cholesterol desaturation of bile and progressive stone dissolution. Recent studies have raised the possibility that cholesterol-lowering agents which inhibit hepatic cholesterol synthesis (statins) or intestinal cholesterol absorption (ezetimibe), or drugs acting on specific nuclear receptors involved in cholesterol and bile acid homeostasis may offer, alone or in combination, additional medical therapeutic tools for treating cholesterol gallstones. Recent perspectives on medical treatment of cholesterol gallstone disease will be discussed in this chapter.
Keywords: bile salts, cholesterol absorption, ezetimibe, gallbladder, nuclear receptors, statins
Introduction
Gallstone disease is one of the most frequent and costly digestive diseases in western countries, as its prevalence in adults ranges from 10% to 15% (1-4). Although frequent, many patients with gallstones remain “silent”, symptoms and/or complications occur in approximately a third of patients. In the United States, medical expenses for the treatment of gallstones exceeded $6 billion in the year 2000. Furthermore, the prevalence of gallstones seems to be rising and approximately one million new cases are discovered each year (5). About 75% of the gallstones in the United States and westernized countries, including Italy are cholesterol gallstones (6-8). The remaining gallstones are pigment stones that contain less than 30% cholesterol by weight, which can be subclassed into two groups: black pigment stones (about 20% of all gallstones, found in the gallbladder and/or bile duct, containing mainly insoluble bilirubin pigment polymer mixed with calcium phosphate and carbonate, and cholesterol) and brown pigment stones (about 5% of all gallstones, found mainly in bile ducts, containing calcium bilirubinate, calcium palmitate, and stearate and cholesterol)(9).
Cholesterol gallstones are associated with well known risk factors, such as obesity, type 2 diabetes, dyslipidaemia, and hyperinsulinaemia (1), which are often components of the metabolic syndrome epidemic (10-14), which prevalence is greater than 35% in the adult pupulation and continues to raise in westernized countries (15;16). Epidemiological surveys have observed that cholesterol cholelithiasis is prevalent in populations consuming a “Western” diet (i.e. enriched in saturated fatty acids, cholesterol, and rapidly absorbed refined carbohydrates), rather than a more “prudent” diet (i.e. enriched in mono- polyunsaturated fats, fruit, vegetables and low in refined carbohydrates) associated with physical activity (17-25). Thus, the prevalence of cholesterol gallstone disease is significantly higher in North and South American as well as European populations than that in Asian and African populations (6). In China, the prevalence of cholesterol gallstones appears to increase with the “westernization” of the traditional Chinese diet (26-28). Even in Japan, the adoption of Western-type dietary habits has resulted in a marked increase of the prevalence of cholesterol cholelithiasis over the past 40 years (29;30). As discussed later, high efficiency of intestinal cholesterol absorption and high dietary cholesterol appear to be two key and independent risk factors for the formation of cholesterol gallstones. The complex pathogenesis of cholesterol gallstones depends on the concurrent existence of hepatic hypersecretion of cholesterol into bile leading to bile supersaturation with cholesterol, accelerated nucleation/crystallization of cholesterol in gallbladder bile, impaired gallbladder motility leading to gallbladder stasis, and increased cholesterol availability from the small intestine, as well as LITH genes and genetic factors (1;31;32). A complex genetic basis plays a key role in determining individual predisposition to develop cholesterol gallstones in response to environmental factors (33-37). Some “gallstone genes” might also play a potential role, including some genes governing the nuclear bile acid receptors such as farnesoid X receptor (FXR). For example, FXR variants seem to affect gallbladder motor-function and intestinal microflora in Mexicans (38), while functional variants in FXR might account for intrahepatic cholestasis of pregnancy in Caucasians, as well as be associated with other cholestatic and dyslipidemic disorders (39)).
From a therapeutic point of view, although gallstone disease is frequent in the general population and the costs of therapeutic interventions are high, the natural history of the disease suggests to restrict the medical treatment of gallstones to a subgroup of symptomatic patients (1;36;40). The selection of patients eligible for medical or surgical therapy, therefore, is of key importance. The onset of biliary pain is the only suggestive marker of “symptomatic” gallstone disease (41;42), although it can be difficult to distinguish between symptomatic and asymptomatic subjects in a random population of gallstone patients (43). The diagnosis can be misleading if patients inadequately describe “typical” symptoms or suffer from highly atypical symptoms (44). Of note, previously symptomatic patients who are symptom-free for five consecutive years should be included in the group of asymptomatic subjects again. After this time, the risk of pain attacks gradually decreases towards values similar to those of patients with asymptomatic gallstones (45). Classical drug therapy for cholesterol gallstones (i.e. oral litholysis by the bile acid ursodeoxycholic acid, UDCA) plays at the moment a limited role, but novel interesting therapeutic options might arise in the near future, when looking at the molecular mechanisms responsible for the formation of cholesterol gallstones (1). Such novel therapeutic approaches might involve subgroups of patients permanently or temporarily at risk for gallstone formation. In this respect, recent studies in both animal models and humans have found that blocking the intestinal absorption of cholesterol with ezetimibe (EZT), the potent inhibitor of the Niemann-Pick C1-like 1 (NPC1L1) protein (46), may provide a novel powerful strategy for the medical treatment of cholesterol gallstones (47). Modification of the expression levels of specific nuclear receptors in the liver might also provide a clue for novel therapeutic approaches for cholesterol gallstones via manipulation of cholesterol and bile acid homeostasis. Current views and perspectives on medical treatment of cholesterol gallstone disease will be discussed in the present paper.
Guidelines for Management of Gallstone Disease
Gallbladder stones are frequently found in asymptomatic subjects during routine abdominal ultrasonography, since in the majority of the cases (60-80%) gallstones do not generate symptoms (43;48;49). Previous observations have shown that average risk of developing symptomatic gallstones is as low as 2.0-2.6% per year (45;50). By contrast, the presence of microstones and sludge in the gallbladder is a major risk factor for the development of biliary pain and complicated gallstone disease, and also plays a main role in the etiology of acute otherwise idiopathic pancreatitis (51-53). Nevertheless, the yearly incidence of complications is very low (0.3%), and the annual risk for gallbladder cancer is as low as 0.02% (54;55). Treatment of “asymptomatic” gallstone patients, therefore, is not routinely recommended, as the overall risk of biliary colic, complications and gallbladder cancer are very low (56-58). Rather, the expectant management is currently considered the appropriate choice in the vast majority of asymptomatic gallstone patients (Grade A). The decision is different in symptomatic gallstone patients and should follow the algorithm depicted in Figure 1, where surgery (namely laparoscopic cholecystectomy) represents the gold standard for treatment while oral litholysis with hydrophilic bile salts plays a limited role (1;36). Other non-surgical (non pharmachological) therapies include direct contact dissolutions of gallstones using the potent cholesterol solvent methyl tert-butyl ether (MTBE)(59), and extracorporeal shock wave lithotripsy (60). Both options, however, have lost their interest because of potential side effects (MTBE) and/or high post-dissolution recurrence rate (1). Available medical treatments for gallstones are discussed in the following paragraphs and include the treatment of biliary colic (all types of stones), oral litholysis by hydrophilic bile acids and novel approaches with statins, EZT, and agonists/antagonists of nuclear receptors (NR) (all for cholesterol gallstones).
Figure 1.
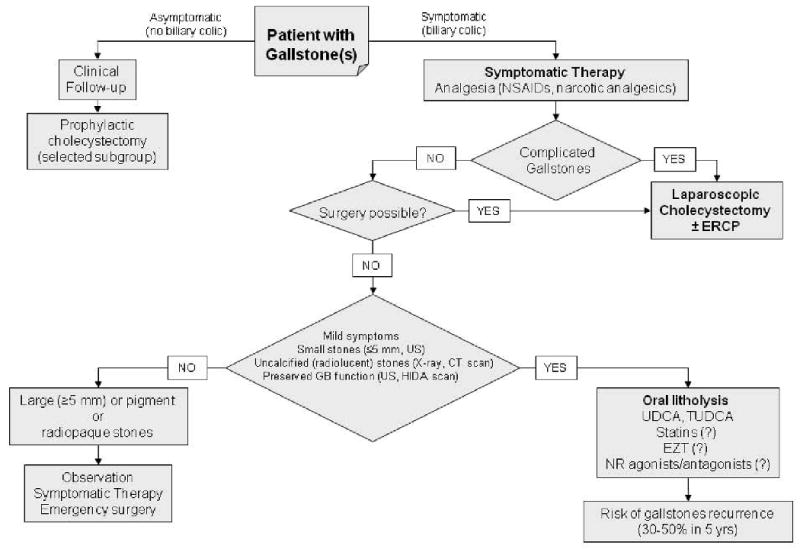
Current therapies of gallstone disease, including cholesterol gallstones (adapted from P. Portincasa et al. (1;36;123)). Novel and potentially effective medical therapies are denoted by the symbol (?). See text for details. Results from meta-analyses indicate surgery as the gold standard for the treating symptomatic gallstones (164-166). Laparoscopic cholecystectomy and small incision cholecystectomy (166), are safe and have similar mortality rate (from 0.1% to 0.7%) (122;165). Both approaches are cost-effective, if compared with open cholecystectomy (165). Compared with open cholecystectomy, both convalescence and hospital stay are shorter and total cost is lower for laparoscopic cholecystectomy (122). Complication rates (including bile duct injuries) are similar between laparoscopic and open cholecystectomy (122;165). When looking at surgical options, a “prophylactic” cholecystectomy can be taken into account in a subgroup of asymptomatic patients bearing a high risk of becoming symptomatic: children (who are exposed to long-term physical presence of stones (167)), morbid obese patients undergoing bariatric surgery (who are at high risk to became symptomatic during rapid weight loss (168)), patients at increased risk for gallbladder cancer (169) (i.e. those with large gallstones, greater than 3 cm) (170;171), a “porcelain” gallbladder (172) or gallbladder polyps rapidly growing or larger than 1 cm). Prophylactic cholecystectomy should also be considered in Native Americans with gallstones, who are at increased risk of gallbladder cancer (3 to 5 percent) (173), and asymptomatic gallstone patients with sickle cell anemia, who form calcium bilirubinate gallstones due to chronic hemolysis and may become symptomatic with recurrent episodes of abdominal pain (174). Prophylactic cholecystectomy has also been proposed in patients with small gallstones and gallbladder dysmotility, since the coexistence of these conditions increases the risk of pancreatitis (51). Abbreviations: CT, computerized tomography; ERCP, endoscopic retrograde cholangiopancreatography; EZT, ezetimibe; HIDA, 99mTc-N-(2,6-dimethylacetanilide)-iminodiacetic acid; GB, gallbladder; GS, gallstones; NR, nuclear receptors; NSAIDs, non-steroidal anti-inflammatory drugs; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; US, abdominal ultrasonography.
Medical Treatments of Gallstone Disease
Treatment of the biliary colic
The presence of gallstone of any type and size may put the patient at risk of biliary pain. As the intensity of pain is usually high (mean visual analogue scale of 9 cm on a 0-10 cm scale), patients require immediate medical attention and analgesia. The pain is not exclusively postprandial, and is typically intermittent. The most frequent localization is the right upper quadrant of the abdomen and/or the epigastrium (representative dermatomes T8/9), and the duration is generally longer than 15-30 minutes. The pain is radiating to the angle of the right scapula and/or shoulder in about 60% of cases. In less than 10% of cases the pain is radiating to the retrosternal area. About two-third of patients experience an urge to walk (44), and often are nauseated or vomit (41;44;49). In biliary colic, the pain is visceral and is caused by the impaction of the stone in cystic duct or sphincter of Oddi. Distension of the gallbladder and/or biliary tract with activation of visceral sensory neurons may follow (41). The pain can last for several hours and be associated with non-specific symptoms of indigestion. The pain could be relieved if the stone returns into the gallbladder lumen, passes through the sphincter into the duodenum or migrates back to the common bile duct (40). The biliary pain is rapidly responsive to narcotic analgesics (meperidine (61)) or non-steroidal anti-inflammatory drugs (NSAIDs) (such as i.m. or i.v. ketorolac or ibuprofen p.o.) which could also reduce the risk of evolution towards acute cholecystitis (62-65). A second-line therapy includes the use of antispasmodic (anticholinergic) agents like hyoscine (scopolamine) which are known to be less effective than NSAIDs (62) (Grade A). The patient with biliary colic should remain fasting to avoid release of endogenous cholecystokinin and further gallbladder contraction. If a complicated biliary pain is suspected (association of leukocytosis, nausea, jaundice, vomiting, and fever), the patient should be quickly admitted to hospital and treated accordingly. Typical complications of gallstone disease are acute pancreatitis, acute cholecystitis, biliary obstruction and cholangitis, gallbladder perforation, abscess formation, mucocele of the gallbladder which may require additional medical therapy with antibiotics or invasive procedures with or without surgery. In case of mild and moderate acute cholecystitis, early laparoscopic cholecystectomy is recommended between 2 and 4 days (66) (Grade A). The risk of biliary pain in asymptomatic carriers are estimated to be approximately 1-2% annually (67;68). Early not randomized or placebo-controlled studies found that UDCA, beside its litholytic effect (see below), might also reduce the risk of biliary colic (69;70). In a non-randomized study, Tomida et al. treated patients referred for symptomatic or asymptomatic gallstones with 600 mg UDCA per day (71) and used those who refused as a control group. The incidence of biliary pain was apparently reduced by UDCA in asymptomatic patients, although a bias might include a misclassification of symptoms. However, in a large randomized, double-blind, and placebo-controlled trial on the effects of UDCA in highly symptomatic gallstone patients scheduled for cholecystectomy, UDCA did not exert a beneficial effect on biliary colic. The likelihood of remaining colic-free was comparable in patients with strong or weak baseline gallbladder contraction as determined by ultrasonography after a standard mixed meal (72).
Dissolution of cholesterol gallstones by oral bile acids
About two-thirds of the gallstones in western countries are composed mainly of cholesterol. However, dissolution therapy by oral administration of the hydrophilic bile acid UDCA, the 7β-epimer of chenodeoxycholate is suitable only for a small subgroup (about 15%) of symptomatic patients (1;40). Similar results are reached with the taurine-conjugated UDCA, i.e. tauroursodeoxycholic acid (TUDCA). Chances of dissolution are much higher if gallstones are small (less than 0.5 cm in size), not calcified (radiolucent on X-ray, including CT scan), cholesterol-enriched (i.e. more than 80%) and contained within a functioning gallbladder with a patent cystic duct (73). Complete dissolution of gallstones by bile acids was first documented by Rewbridge in 1937 (74), although initial reports were published in 1873 and 1876 (75;76). The bile acid chenodeoxycholic acid (CDCA) was first used in the 1970s (77) but was associated with a dose-dependent increase in serum aminotransferases, serum low-density lipoprotein (LDL) cholesterol levels, and diarrhea. In 1975 Makino et al. identified UDCA as a more hydrophilic bile acid which could replace CDCA without side effects (78). Dissolution of cholesterol gallstones by UDCA following fragmentation by extracorporeal shock wave lithotripsy was introduced first by Paumgartner et al. in Munich in 1986 (60). The bile acid UDCA is currently employed for oral dissolution at a dosage of 10-14 mg/kg body weight per day. Bedtime administration is suggested because it maintains hepatic bile acid secretion rate overnight, thus reducing secretion of supersaturated bile and increasing the dissolution rate (79;80) (Grade A). Oral UDCA (at least 10 mg/kg/day) results in increased proportion of biliary UDCA in bile (from less than 8-10% of biliary bile acid pool to about 40%). Increasing biliary UDCA, in turn, results in a decreased hepatic secretion of biliary cholesterol and the formation of unsaturated gallbladder bile (i.e. containing less cholesterol in solution) with a cholesterol saturation index of less than 1 (81-83) (Figure 2). This step represents a key factor in initiating the process of dissolution of cholesterol crystals and gallstones. During UDCA treatment, cholesterol crystallization can be prevented since more cholesterol can be transported within vesicles which contain mainly phospholipids and cholesterol and little bile acids (84). Also, oral therapy with UDCA is associated with the reduction of intestinal absorption of cholesterol (85-87), as well as with a better contractility of the stimulated gallbladder smooth muscle, as shown by in vitro studies in animals and gallstone patients (88;89). By decreasing cholesterol saturation of bile, UDCA might counteract the impaired contractility due to incorporation of excessive luminal cholesterol into the plasmalemma of gallbladder smooth muscles (88-91). UDCA might also counteract the detrimental effects of the hydrophobic bile acid deoxycholate on the gallbladder smooth muscle contractility (91;92), and have effect on local oxidative stress (89;93) and risk of acute cholecystitis (71). Excess biliary cholesterol might provide the basis for stimulation of inflammatory cells in the gallbladder, since cholesterol monohydrate crystals induce expression of T-cell-dependent proinflammatory cytokines in murine model of cholesterol cholelithogenesis (94).
Figure 2.
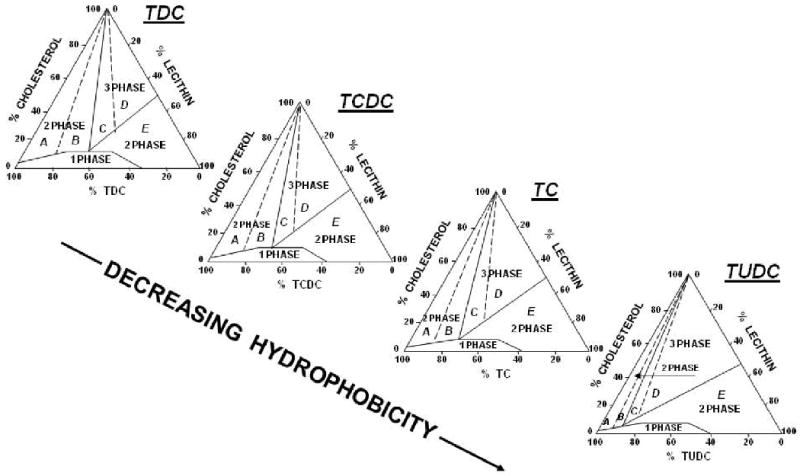
Effects of UDCA on bile composition and cholesterol solubility are explained by using the ternary phase diagram (175). A group of the equilibrium phase diagram of cholesterol-lecithin-taurine-conjugated bile saltacid systems (37°C, 0.15 M NaCl, pH 7.0, total lipid concentration 7.5 g/dL) are drawn to display varied positions and configuration of crystallization regions due to decreasing bile salt hydrophobicity. The lipid components are expressed in moles percent. The one-phase micellar zone at bottom is enclosed by a solid curved line. Above it, two solid lines divide the two-phase zones from a central three-phase zone. Based upon the solid and liquid crystallization sequences present in the bile, the left two-phase and the central three-phase regions are divided by dashed lines into regions A to D. The number of phases given represents the equilibrium state. They are cholesterol monohydrate crystals and saturated micelles for crystallization regions A and B. Cholesterol monohydrate crystals, saturated micelles and liquid crystals for regions C and D, and liquid crystals of variable compositions and saturated micelles for region E (175). As the bile acid hydrophobicity decreased, the maximum micellar cholesterol solubility is reduced and crystallization pathways A-E move to the left. This change results in an enlarged region E that extends to the left and overlaps pathophysiological compositions as exemplified in the tauroursodeoxycholate (TUDC)-lecithin-cholesterol system. This event induces a greatly reduced chance for the formation of solid plate-like cholesterol monohydrate crystals in bile. Adapted and reproduced with permission from (175) and (123).
Patients suitable for medical dissolution of cholesterol gallstones need to be carefully selected. Well-selected patients are those who have the higher chance for successful oral litholysis alone or after extracorporeal shock wave lithotripsy for stone fragmentation (95-99). Ultrasonography of the right upper quadrant is still the best and more convenient diagnostic tool for detecting gallstones (58;100), as well as for assessing both gallstone size and burden (101). “Functional” ultrasonography, i.e. the study of time-dependent changes of both fasting and postprandial gallbladder volumes following a standard test meal, although not routinely employed, provides additional information about gallbladder size, emptying, and bile duct patency (36;95-98;102-105). Abdominal plain radiography (not routinely used) and the computerized tomographic (CT) scan (106;107) detect only calcified stones (as radiopaque bodies) in the right upper quadrant. Such stones are obviously unfit for dissolution because they are either calcified cholesterol stones or stones made of pigment calcium bilirubinate (9). Oral cholecystography might disclose the presence of floating (cholesterol) stones in the gallbladder and a preserved cystic duct patency. The expected dissolution rate following UDCA at the standard dosage is estimated to about a 1 mm decrement in stone diameter per month (108). In patients with a gallstone diameter less than 5 mm, the complete disappearance of stones assessed by ultrasonography is expected to be reached in about 90% of cases by 6 months of UDCA administration (109). A series of conditions might impede the dissolution of cholesterol gallstones by UDCA or TUDCA. In patients with larger and/or multiple stones the dissolution rate approaches 40-50% after one year of the treatment (54;110), while the appearance of a surface calcification on cholesterol gallstones during oral dissolution therapy with UDCA, CDCA, or TUDCA has been reported in about 10-12% of patients. This event would obviously impede any further dissolution of the calcified stone (111;112). Another issue is the possibility that gallstones will recur sometime after dissolution with bile acids, and this is a major limitation of oral dissolution therapy. Overall, recurrence might be as high as 10% per year, i.e. about 30-50% of cases 5 years after bile acid therapy or lithotripsy (96;113-117). Recurrence rate is higher particularly in subjects with multiple gallstones (116). Whereas recurrent gallstones respond well to re-treatment (107;118), it is logical to speculate that such an high recurrence rate is dependent on persistent pathogenetic conditions (1). Oral dissolution therapy of cholesterol gallstones with bile acids might still represent the option in patients who are at minimal risk of gallstone recurrence or have transient risk factors including rapid weight loss (i.e. obese patients following bariatric surgery), pregnancy, and convalescence from abdominal surgery, (1;119-121)). Major limitations for oral litholysis with bile acids, by contrast, are the small number of suitable patients and the high rate of gallstone recurrence (37;122;123).
Novel Medical Treatments
The presence of a lithogenic bile is primarily due to a sustained hypersecretion of biliary cholesterol which has two key components: hepatic and intestinal (31). In principle, drugs influencing hepatic synthesis and/or secretion of cholesterol (i.e. statins) and/or intestinal absorption of cholesterol (i.e. EZT), are potentially able to influence the formation of cholesterol gallstones and to promote dissolution of gallstones.
Inhibition of hepatic cholesterol synthesis by statins
Statins are competitive inhibitors of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, the rate-limiting step in cholesterol biosynthesis. They occupy a portion of the binding site of HMG-CoA, blocking access of this substrate to the active site on the enzyme (124). Currently available statins in the United States include lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, and rosuvastatin. Statins appear also to reduce cholesterol secretion and concentration in bile independently of their ability to block hepatic cholesterol synthesis (125-128). Such combined effects of statins on cholesterol homeostasis in the liver and bile might be potentially able to lower the risk of cholesterol gallstones (129-131). Indeed, beneficial effects of statins in preventing gallstone formation have been reported in animal studies (132;133). In humans the effect of statins on gallstone disease has been controversial: reduced gallstone formation, decreased cholesterol concentration in bile, and gallstone dissolution following therapy with statins have been reported by some (134-137), but not all studies (130;138;139). Another two small studies have also been conflicting with either no association between statin use and the risk of gallstones (140) or with an effect of statin on gallstones, although the statistical power was small (141). More recently, two studies have re-assessed the problem of statin use and risk of gallstone disease, and opened new perspectives. In a cohort of US women self-reporting long-term use statins, the risk of cholecystectomy was found to decrease slightly (142). In a case-control analysis using the UK-based General Practice Research Database and evaluating incident patients between 1994 and 2004, long-term use of statins (1 to 1.5 yrs) was associated with a decreased risk of gallstones followed by cholecystectomy, compared with patients without statin use (143). Whether statin use will be part of the medical therapeutic armamentarium in a subgroup of patients with gallstone disease or to prevent gallstone disease in selected patients at risk, needs to be investigated further by appropriate clinical studies.
Inhibition of intestinal cholesterol absorption by ezetimibe
The importance of intestinal factors in the pathogenesis of cholesterol gallstones has recently raised the interest of several research groups (1;144). Animal studies have shown that when no dietary cholesterol is available, all biliary cholesterol is mainly derived from hepatic de novo synthesis with a limited contribution (less than 15%) to biliary cholesterol secretion. Rather, the small intestine is the site which solely provides the absorption of dietary cholesterol, as well as re-absorption of biliary cholesterol (144). The importance of intestinal absorption of cholesterol for gallstone pathogenesis is supported by the fact that a positive correlation exists between the efficiency of intestinal cholesterol absorption and the prevalence of cholesterol gallstone formation in several strains of inbred mice (34). The protein Niemann-Pick C1-like 1 (NPC1L1) is highly expressed in the small intestine and localized along the brush border of the enterocytes in both humans and mice (145;146). There is also a significant amount of NPC1L1 in the human liver but not in the mouse liver (146;147). Of note, cholesterol is the most effective substrate of NPC1L1 (148) which governs intestinal absorption of cholesterol (146) by recycling between endocytic recycling compartment and plasma membrane (148) (Figure 3). Thus, inhibition of cholesterol absorption in the intestine or hepatic uptake of chylomicron remnants has become an attractive possibility to decrease biliary cholesterol secretion and saturation [100]. More interestingly, similar to humans, the abundance of Niemann-Pick C1-like 1 (NPC1L1) in the small intestine far exceeded that in other regions of the gastrointestinal tract, liver, and gallbladder in the Golden Syrian hamster (149). Ezetimibe-induced reduction in intestinal cholesterol absorption is coupled with a decrease in the absolute and relative cholesterol levels in bile in hamsters fed a high cholesterol and high fat diet. These results are consistent with the recent finding that ezetimibe treatment significantly reduced biliary cholesterol saturation in patients with gallstones (47). EZT belongs to the new class of 2-azetidinones approved as a novel hypocholesterolemic drug (150) with a potent inhibitory effect on intestinal cholesterol absorption by specifically suppressing the NPC1L1. EZT might therefore play a primary role in the medical treatment or prevention of cholesterol gallstones, as suggested by studies from our group (Figure 4). In mice, EZT reduces cholesterol and partly phospholipid but not bile salt content in gallbladder bile; all crystallization pathways and phase boundaries in the bile phase diagram remain similar, with or without EZT (47). If EZT is increased, the relative lipid compositions of pooled gallbladder bile samples are progressively shifted down and to the left of the phase diagram, and enter the one-phase (protective) micellar zone (which contains an abundance of unsaturated micelles but never solid cholesterol crystals or liquid crystals). Thus, the micellar cholesterol solubility is increased in gallbladder bile with more cholesterol molecules transferred from the cholesterol monohydrate surface into unsaturated micelles. In this environment, gallstones can dissolve (47;151). EZT also protected gallbladder motility function by desaturating bile (47). It must be noted that the physical-chemical mechanisms underlying the beneficial effects of EZT on supersaturated bile, cholesterol crystals, cholesterol stones differ from those of hydrophilic bile acids such as UDCA, TUDCA and β-muricholic acid. These hydrophilic bile acids enhance dissolution of cholesterol gallstones by promoting the formation of a vesicle-enriched liquid crystalline mesophase (152). Translational studies have shown that EZT (20 mg p.o./day for one month) significantly reduced cholesterol concentration and cholesterol saturation index and retarded cholesterol crystallization in gallstone patients (47).
Figure 3.
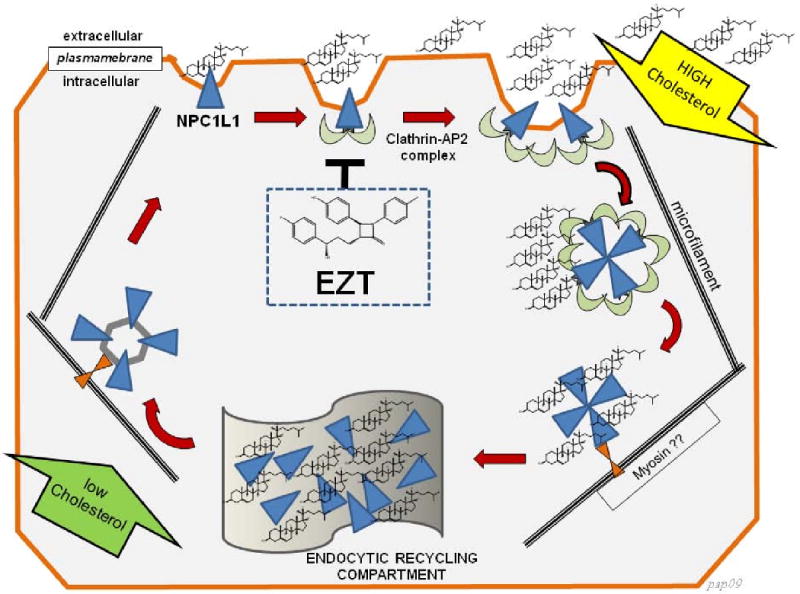
Mechanisms for cholesterol uptake mediated by the NPC1L1 according to the model proposed by Ge et al. (148). Adapted and reproduced with permission, Copyright Bentham Science Publisher, 2009 (123). The NPC1L1 protein recycles between the plasma membrane facing the extracellular space and the endocytic recycling compartment. If extracellular cholesterol concentration is high, cholesterol is incorporated into the plasma membrane and is sensed by cell surface-localized NPC1L1. Both NPC1L1 and cholesterol are then internalized together through clathrin/AP2-mediated endocytosis. The clathrin-coated globular vesicles are transported along microfilaments to the endocytic recycling compartment. The role of myosin in this process is unclear. Large quantities of cholesterol and NPC1L1 are subsequently stored within the endocytic recycling compartment. If the intracellular cholesterol level is low, endocytic recycling compartment-localized NPC1L1 moves back to the plasma membrane along microfilaments and new cholesterol is absorbed. The key role of the NPC1L1 inhibitor ezetimibe (EZT) is shown at the center of the cell. EZT prevents NPC1L1 from entering the AP2-mediated clathrin-coated vesicles. At this stage, the endocytosis of NPC1L1 is inhibited and cholesterol absorption is decreased.
Figure 4.
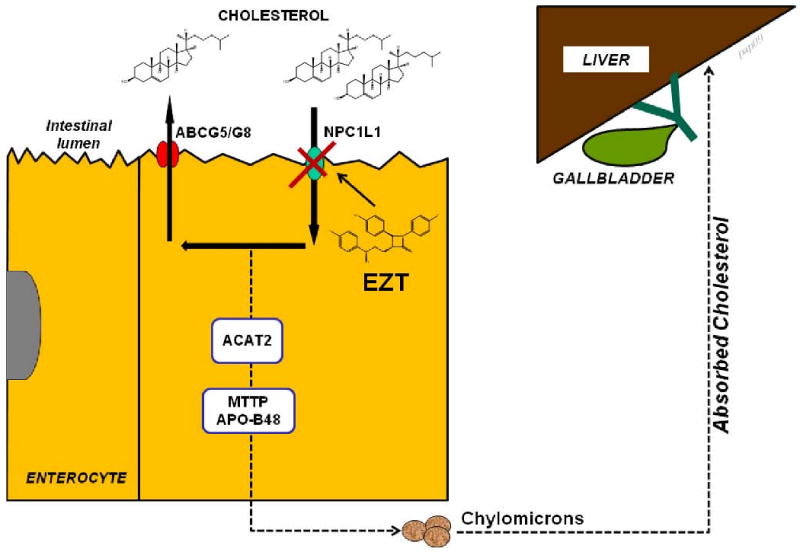
Pathways underlying absorption of cholesterol from the intestinal lumen and its delivery to the liver. High dietary cholesterol through the chylomicron pathway could provide an important source of excess cholesterol molecules for secretion into bile, thereby inducing cholesterol-supersaturated bile and enhancing cholesterol gallstone formation (31;47). Ezetimibe (EZT) significantly suppresses cholesterol absorption from the small intestine via the Niemann-Pick C1-like 1 (NPC1L1) pathway (47). This effect should diminish the cholesterol content of the liver, which in turn decreases bioavailability of cholesterol for biliary secretion. Abbreviations: ABCG5/G8, ATP-binding cassette (transporter); ACAT2, acyl-CoA:cholesterol acyltransferase isoform 2; APO-B48, apolipoprotein B48; MTTP, microsomal triglyceride transfer protein. See text for details.
In conclusions, both animal and preliminary human studies show that EZT by inhibiting NPC1L1-mediated intestinal cholesterol absorption, also lowers biliary cholesterol secretion, desaturates bile, and preserves gallbladder motility function, even under conditions of high dietary cholesterol loads (47;151;153). Whether EZT will become a novel and effective cholelitholytic agent (alone or combined with statins and/or hydrophilic bile acids), is a matter of research in future well-designed, controlled, long-term clinical studies.
Agents effective on nuclear receptors
Coherent and coordinate activation of sets of genes involved in multiple cellular activities depend on nuclear receptors (NRs), which are ligand-activated transcription factors (154). Lipid sensing NRs govern lipid homeostasis in the hepatobiliary and gastrointestinal systems. Both hepatic and biliary lipid metabolism are involved in lipid secretion by the hepatocytes, and are controlled by the oxysterol receptor LXR (the intracellular “sensor” of cholesterol (155)) and the bile acid receptor farnesoid X receptor (FXR) (the intracellular “sensor” of bile acids (156;157)). The subtle cellular mechanisms governing lipid homeostasis imply that cells synthesize oxysterols under conditions of cholesterol overload, and oxysterols in turn bind and activate LXR which acts to reduce systemic cholesterol burden (156-158). In the enterohepatic system FXR is highly expressed and regulates the expression of genes involved in the maintenance of cholesterol, bile acid and triglyceride homeostasis (159). Of note, liver FXR might become an interesting pharmacological target for the treatment of cholestasis and cholelithiasis (160), since this NR up-regulates the expression of bile acid transporters in the canalicular membrane and of enzymes responsible for bile acid detoxification. Interestingly, manipulation of NRs in the animal model has disclosed novel potential approaches to the problem of cholesterol gallstone disease. FXR is a promising therapeutic target for treating or preventing cholesterol gallstone disease. FXR null mice are susceptible to cholesterol gallstone formation; activating FXR with the compound GW4064, a specific synthetic ligand, by contrast, increase biliary bile salt and phospholipid concentrations (161). The effect is tightly dependent on FXR-induced regulation of the energy dependent ATP-Binding Cassette (ABC) transporters ABCB11 (for bile salts) and ABCB4 (for phospholipid) (162) and is associated with better solubilization of cholesterol, and prevention of solid plate-like crystals and stones. Looking at the other side, increased propensity to cholesterol crystallization and stone formation in bile has been described in mice following the activation of hepatic LXR and direct up-regulation of the major hepatocyte cholesterol canalicular transporters ABCG5 and ABCG8 which together for an heterodimer (163). Again, future studies need to test if liver-specific FXR agonists and LXR antagonists might be safe and effective in human gallstone disease, as is the case for dyslipidemia, type II diabetes and several cancers.
Conclusions
The advent of laparoscopic cholecystectomy has moved the interest away from the pharmachological treatment of gallstones. Currently, medical therapy is restricted to a scant group of symptomatic (colicky pain) well-selected patients, in which both the unfavorable cost-benefit analyses and a high rate of gallstone recurrence play a negative role. Following early cholelitholytic therapies with the oral bile bile acid UDCA, recent studies indicate that the research agenda should include studies on the role of gallstone (LITH) genes, as well as the mechanisms of intestinal absorption of cholesterol and pathways of liver synthesis-secretion of cholesterol. Promising agents might include, alone or in combination, statins (competitive inhibitors of HMG-CoA reductase, the rate-limiting step in cholesterol biosynthesis), ezetimibe (specific inhibitor of the intestinal cholesterol transporter protein NPC1L1), and liver-specific agonists/antagonists of the nuclear receptors FXR/LXR involved in biliary lipid secretion.
Acknowledgments
This work was supported in part by research grants from the Italian Ministry of University and Research (FIRB 2003 RBAU01RANB002), the Italian National Research Council (CNR) (short-term mobility grant 2005), the University of Bari (grants ORBA09XZZT, ORBA08YHKX) (P.P.), and from the National Institutes of Health (US Public Health Service) (research grants DK54012 and DK73917) (D.Q.-H.W.). We are grateful to Paola De Benedictis, Rosa De Venuto and Michele Persichella for their skillful technical assistance.
List of abbreviations
- CDCA
chenodeoxycholic acid
- EZT
ezetimibe
- FXR
farnesoid X receptor
- LXR
liver X receptor
- NPC1L1
Niemann-Pick C1-like 1
- NR
nuclear receptors
- NSAIDs
non-steroidal anti-inflammatory drugs
- TUDCA
tauroursodeoxycholic acid
- UDCA
ursodeoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Agostino Di Ciaula, Email: agostinodiciaula@tiscali.it.
David Q.-H. Wang, Email: dqwang@caregroup.harvard.edu.
Helen H. Wang, Email: hjiang@caregroup.harvard.edu.
Leonilde Bonfrate, Email: microscopia@semeiotica.uniba.it.
References
- 1.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368(9531):230–9. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang DQH, Afdhal NH. Genetic analysis of cholesterol gallstone formation: searching for Lith (gallstone) genes. Curr Gastroenterol Rep. 2004;6(2):140–50. doi: 10.1007/s11894-004-0042-1. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Khare M, Hill M, et al. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117(3):632–9. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122(5):1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 5.Liver Disease Subcommittee of the Digestive Disease Interagency Coordinating Committee. Action Plan for Liver Disease Research. Bethesda: NIH; 2004. pp. 145–50. [Google Scholar]
- 6.Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20(1):1–19. [PubMed] [Google Scholar]
- 7.Attili AF, Carulli N, Roda E, et al. Epidemiology of gallstone disease in Italy: prevalence data of the multicenter italian study on cholelithiasis (M.I.C.O.L.) Am J Epidemiology. 1995;141:158–65. doi: 10.1093/oxfordjournals.aje.a117403. [DOI] [PubMed] [Google Scholar]
- 8.Attili AF, Capocaccia R, Carulli N, et al. Factors associated with gallstone disease in the MICOL experience. Hepatology. 1997;26:809–18. doi: 10.1002/hep.510260401. [DOI] [PubMed] [Google Scholar]
- 9.Sherlock S, Dooley J. Diseases of the liver and biliary system. Oxford: Blackwell Science; 2002. [Google Scholar]
- 10.Grundy SM, Barnett JP. Metabolic and health complications of obesity. Dis Mon. 1990;36:641–731. [PubMed] [Google Scholar]
- 11.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25(11):2243–4. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CJ, Leitzmann MF, Willett WC, et al. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80(1):38–44. doi: 10.1093/ajcn/80.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27(10):2444–9. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–9. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CJ, Leitzmann MF, Willett WC, et al. Dietary protein and the risk of cholecystectomy in a cohort of US women: the Nurses' Health Study. Am J Epidemiol. 2004;160(1):11–8. doi: 10.1093/aje/kwh170. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CJ, Leitzmann MF, Hu FB, et al. Frequent nut consumption and decreased risk of cholecystectomy in women. Am J Clin Nutr. 2004;80(1):76–81. doi: 10.1093/ajcn/80.1.76. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CJ, Leitzmann MF, Willett WC, et al. Fruit and vegetable consumption and risk of cholecystectomy in women. Am J Med. 2006;119(9):760–7. doi: 10.1016/j.amjmed.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CJ, Leitzmann MF, Willett WC, et al. Long-term intake of dietary fiber and decreased risk of cholecystectomy in women. Am J Gastroenterol. 2004;99(7):1364–70. doi: 10.1111/j.1572-0241.2004.30153.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsai CJ, Leitzmann MF, Willett WC, et al. Long-term intake of trans-fatty acids and risk of gallstone disease in men. Arch Intern Med. 2005;165(9):1011–5. doi: 10.1001/archinte.165.9.1011. [DOI] [PubMed] [Google Scholar]
- 22.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341(11):777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CJ, Leitzmann MF, Willett WC, et al. The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med. 2004;141(7):514–22. doi: 10.7326/0003-4819-141-7-200410050-00007. [DOI] [PubMed] [Google Scholar]
- 24.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128(6):417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20(6):981–96. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, Zhang S, Huang Z. The trend of the gallstone disease in China over the past decade. Zhonghua Wai Ke Za Zhi. 1995;33(11):652–8. [PubMed] [Google Scholar]
- 27.Huang YC, Zhang XW, Yang RX. Changes in cholelithiasis in Tianjin in the past 30 years. Chin Med J (Engl) 1984;97(2):133–5. [PubMed] [Google Scholar]
- 28.Sun H, Tang H, Jiang S, et al. Gender and metabolic differences of gallstone diseases. World J Gastroenterol. 2009;15(15):1886–91. doi: 10.3748/wjg.15.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama F, Miyake H. Changing state of gallstone disease in Japan. Composition of the stones and treatment of the condition. Am J Surg. 1970;120(6):794–9. doi: 10.1016/s0002-9610(70)80052-6. [DOI] [PubMed] [Google Scholar]
- 30.Nagase M, Tanimura H, Setoyama M, et al. Present features of gallstones in Japan. A collective review of 2,144 cases. Am J Surg. 1978;135(6):788–90. doi: 10.1016/0002-9610(78)90165-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang HH, Portincasa P, Wang DQ. Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci. 2008;13:401–23. doi: 10.2741/2688. [DOI] [PubMed] [Google Scholar]
- 32.Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47(6):2112–26. doi: 10.1002/hep.22204. [DOI] [PubMed] [Google Scholar]
- 33.Wittenburg H, Lammert F. Genetic predisposition to gallbladder stones. Semin Liver Dis. 2007;27(1):109–21. doi: 10.1055/s-2006-960174. [DOI] [PubMed] [Google Scholar]
- 34.Wang DQH, Zhang L, Wang HH. High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim Biophys Acta. 2005;1733(1):90–9. doi: 10.1016/j.bbalip.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Lammert F, Sauerbruch T. Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat Clin Pract Gastroenterol Hepatol. 2005;2(9):423–33. doi: 10.1038/ncpgasthep0257. [DOI] [PubMed] [Google Scholar]
- 36.Portincasa P, Moschetta A, Puglisi F, Wang DQH. Medical treatment of gallstone disease. In: Borzellino G, Cordiano C, editors. Biliary lithiasis Basic Science, Current Diagnosis and Management. Milano: Springer Italia S.r.l.; 2008. pp. 149–157. [Google Scholar]
- 37.Lammert F, Miquel JF. Gallstone disease: from genes to evidence-based therapy. J Hepatol. 2008;48 1:S124–S135. doi: 10.1016/j.jhep.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs P, Kress R, Rocha J, et al. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48(1):116–24. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 39.Van Mil SW, Milona A, Dixon PH, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133(2):507–16. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Portincasa P, Moschetta A, Petruzzelli M, et al. Gallstone disease: Symptoms and diagnosis of gallbladder stones. Best Pract Res Clin Gastroenterol. 2006;20(6):1017–29. doi: 10.1016/j.bpg.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Diehl AK, Sugarek NJ, Todd KH. Clinical evaluation for gallstone disease: usefulness of symptoms and signs in diagnosis. Am J Med. 1990;89(1):29–33. doi: 10.1016/0002-9343(90)90094-t. [DOI] [PubMed] [Google Scholar]
- 42.Paumgartner G, Carr-Locke DL, Roda E, et al. Biliary stones: non-surgical therapeutic approach. Gastroenterology International. 1988;1(1):17–24. [Google Scholar]
- 43.Jorgensen T. Abdominal symptoms and gallstone disease: an epidemiological investigation. Hepatology. 1989;9:856–60. doi: 10.1002/hep.1840090611. [DOI] [PubMed] [Google Scholar]
- 44.Berhane T, Vetrhus M, Hausken T, et al. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol. 2006;41(1):93–101. doi: 10.1080/00365520510023990. [DOI] [PubMed] [Google Scholar]
- 45.Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol. 1989;42(2):127–36. doi: 10.1016/0895-4356(89)90086-3. [DOI] [PubMed] [Google Scholar]
- 46.Hou R, Goldberg AC. Lowering low-density lipoprotein cholesterol: statins, ezetimibe, bile acid sequestrants, and combinations: comparative efficacy and safety. Endocrinol Metab Clin North Am. 2009;38(1):79–97. doi: 10.1016/j.ecl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Wang HH, Portincasa P, Mendez-Sanchez N, et al. Effect of Ezetimibe on the Prevention and Dissolution of Cholesterol Gallstones. Gastroenterology. 2008;134:2101–10. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibney EJ. Asymptomatic gallstones. Br J Surg. 1990;77(4):368–72. doi: 10.1002/bjs.1800770405. [DOI] [PubMed] [Google Scholar]
- 49.Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL) Hepatology. 1999;30(4):839–46. doi: 10.1002/hep.510300401. [DOI] [PubMed] [Google Scholar]
- 50.Thistle JL, Cleary PA, Lachin JM, et al. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med. 1984;101(2):171–5. doi: 10.7326/0003-4819-101-2-171. [DOI] [PubMed] [Google Scholar]
- 51.Venneman NG, Renooij W, Rehfeld JF, et al. Small gallstones, preserved gallbladder motility, and fast crystallization are associated with pancreatitis. Hepatology. 2005;41(4):738–46. doi: 10.1002/hep.20616. [DOI] [PubMed] [Google Scholar]
- 52.Venneman NG, Buskens E, Besselink MG, et al. Small gallstones are associated with increased risk of acute pancreatitis: potential benefits of prophylactic cholecystectomy? Am J Gastroenterol. 2005;100(11):2540–50. doi: 10.1111/j.1572-0241.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326(9):589–93. doi: 10.1056/NEJM199202273260902. [DOI] [PubMed] [Google Scholar]
- 54.Paumgartner G, Carr-Locke DL, Dubois F, et al. Strategies in the treatment of gallstone disease. Working team report. Gastroenterology International. 1993;6:65–75. [Google Scholar]
- 55.Brugge WR. The Silent Gallstone. In: Afdhal NH, editor. Gallbladder and Biliary Tract Diseases. New York, Basel: Marcel Dekker, Inc.; 2000. pp. 447–453. [Google Scholar]
- 56.Tait N, Little JM. The treatment of gall stones. BMJ. 1995;311(6997):99–105. doi: 10.1136/bmj.311.6997.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmann AF, Amelsberg A, Vansonnenberg E. Pathogenesis and treatment of gallstones. N Engl J Med. 1993;328(25):1854–5. [PubMed] [Google Scholar]
- 58.Johnston DE, Kaplan MM. Pathogenesis and treatment of gallstones. N Engl J Med. 1993;328:412–21. doi: 10.1056/NEJM199302113280608. [DOI] [PubMed] [Google Scholar]
- 59.Thistle JL, May GR, Bender CE, et al. Dissolution of cholesterol gallbladder stones by methyl tert- butyl ether administered by percutaneous transhepatic catheter. N Engl J Med. 1989;320:633–9. doi: 10.1056/NEJM198903093201004. [DOI] [PubMed] [Google Scholar]
- 60.Sauerbruch T, Delius M, Paumgartner G, et al. Fragmentation of gallstones by extracorporeal shock waves. N Engl J Med. 1986;314(13):818–22. doi: 10.1056/NEJM198603273141304. [DOI] [PubMed] [Google Scholar]
- 61.Elta GH, Barnett JL. Meperidine need not be proscribed during sphincter of Oddi manometry. Gastrointest Endosc. 1994;40(1):7–9. doi: 10.1016/s0016-5107(94)70002-8. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A, Deed JS, Bhasin B, et al. Comparison of the effect of diclofenac with hyoscine-N-butylbromide in the symptomatic treatment of acute biliary colic. ANZ J Surg. 2004;74(7):573–6. doi: 10.1111/j.1445-2197.2004.03058.x. [DOI] [PubMed] [Google Scholar]
- 63.Al-Waili N, Saloom KY. The analgesic effect of intravenous tenoxicam in symptomatic treatment of biliary colic: a comparison with hyoscine N-butylbromide. Eur J Med Res. 1998;3(10):475–9. [PubMed] [Google Scholar]
- 64.Akriviadis EA, Hatzigavriel M, Kapnias D, et al. Treatment of biliary colic with diclofenac: a randomized, double-blind, placebo-controlled study. Gastroenterology. 1997;113(1):225–31. doi: 10.1016/s0016-5085(97)70099-4. [DOI] [PubMed] [Google Scholar]
- 65.Goldman G, Kahn PJ, Alon R, et al. Biliary colic treatment and acute cholecystitis prevention by prostaglandin inhibitor. Dig Dis Sci. 1989;34(6):809–11. doi: 10.1007/BF01540262. [DOI] [PubMed] [Google Scholar]
- 66.Gurusamy KS, Samraj K. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Cochrane Database Syst Rev. 2006;(4):CD005440. doi: 10.1002/14651858.CD005440.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Gracie WA, Ransohoff DF. The natural history of silent gallstones: the “innocent” gallstone” is not a myth. N Engl J Med. 1982;307:798–800. doi: 10.1056/NEJM198209233071305. [DOI] [PubMed] [Google Scholar]
- 68.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165(4):399–404. doi: 10.1016/s0002-9610(05)80930-4. [DOI] [PubMed] [Google Scholar]
- 69.Tint GS, Salen G, Colalillo A, et al. Ursodeoxycholic acid: a safe and effective agent for dissolving cholesterol gallstones. Ann Intern Med. 1982;97(3):351–6. doi: 10.7326/0003-4819-97-3-351. [DOI] [PubMed] [Google Scholar]
- 70.Meredith TJ, Williams GV, Maton PN, et al. Retrospective comparison of ‘Cheno’ and ‘Urso’ in the medical treatment of gallstones. Gut. 1982;23(5):382–9. doi: 10.1136/gut.23.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomida S, Abei M, Yamaguchi T, et al. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: a cohort analysis. Hepatology. 1999;30:6–13. doi: 10.1002/hep.510300108. [DOI] [PubMed] [Google Scholar]
- 72.Venneman NG, Besselink MG, Keulemans YC, et al. Ursodeoxycholic acid exerts no beneficial effect in patients with symptomatic gallstones awaiting cholecystectomy. Hepatology. 2006;43(6):1276–83. doi: 10.1002/hep.21182. [DOI] [PubMed] [Google Scholar]
- 73.Paumgartner G, Pauletzki J, Sackmann M. Ursodeoxycholic acid treatment of cholesterol gallstone disease. Scand J Gastroenterol Suppl. 1994;204:27–31. doi: 10.3109/00365529409103622. [DOI] [PubMed] [Google Scholar]
- 74.Rewbridge AG. The disappearance of gallstone shadows following the prolonged admnistration of bile acids. Surgery. 1937;1:395–400. [Google Scholar]
- 75.Schiff M. Il coleinato di soda nella cura dei calcoli biliari. L'Imparziale. 1873;13:97–8. [Google Scholar]
- 76.Dabney WC. The use of choleate of soda to prevent the formation of gallstones. Am J Med Sci. 1876;71:410. [Google Scholar]
- 77.Danzinger RG, Hofmann AF, Schoenfield LJ. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972;286:1–8. doi: 10.1056/NEJM197201062860101. [DOI] [PubMed] [Google Scholar]
- 78.Makino I, Shinozaki K, Yoshino K, et al. Dissolution of cholesterol gallstones by long-term administration of ursodeoxycholic acid. Nippon Shokakibyo Gakkai Zasshi. 1975;72:690–702. [PubMed] [Google Scholar]
- 79.Kupfer RM, Maudgal DP, Northfield TC. Gallstone dissolution rate during chenic acid therapy. Effect of bedtime administration plus low cholesterol diet. Dig Dis Sci. 1982;27(11):1025–9. doi: 10.1007/BF01391750. [DOI] [PubMed] [Google Scholar]
- 80.Lanzini A, Facchinetti D, Northfield TC. Maintenance of hepatic bile acid secretion rate during overnight fasting by bedtime bile acid administration. Gastroenterology. 1988;95(4):1029–35. doi: 10.1016/0016-5085(88)90179-5. [DOI] [PubMed] [Google Scholar]
- 81.van Erpecum KJ, Portincasa P, Stolk MFJ, et al. Effects of bile salt and phospholipid hydrophobicity on lithogenicity of human gallbladder bile. Eur J Clin Invest. 1994;24:744–50. doi: 10.1111/j.1365-2362.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 82.van Erpecum KJ, Portincasa P, Gadellaa M, et al. Effects of bile salt hydrophobicity on nucleation behaviour of cholesterol crystals in model bile. Eur J Clin Invest. 1996;26:602–8. doi: 10.1046/j.1365-2362.1996.1910532.x. [DOI] [PubMed] [Google Scholar]
- 83.Portincasa P, van Erpecum KJ, Jansen A, et al. Behavior of various cholesterol crystals in bile from gallstone patients. Hepatology. 1996;23:738–48. doi: 10.1002/hep.510230414. [DOI] [PubMed] [Google Scholar]
- 84.Moschetta A, vanBerge-Henegouwen GP, Portincasa P, et al. Cholesterol crystallization in model biles. Effects of bile salt and phospholipid species composition. J Lipid Res. 2001;42(8):1273–81. [PubMed] [Google Scholar]
- 85.Hardison WG, Grundy SM. Effect of ursodeoxycholate and its taurine conjugate on bile acid synthesis and cholesterol absorption. Gastroenterology. 1984;87(1):130–5. [PubMed] [Google Scholar]
- 86.Uchida K, Akiyoshi T, Igimi H, et al. Differential effects of ursodeoxycholic acid and ursocholic acid on the formation of biliary cholesterol crystals in mice. Lipids. 1991;26:526–30. doi: 10.1007/BF02536598. [DOI] [PubMed] [Google Scholar]
- 87.Wang DQH, Tazuma S, Cohen DE, et al. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G494–G502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 88.van de Heijning BJM, van de Meeberg P, Portincasa P, et al. Effects of ursodeoxycholic acid therapy on in vitro gallbladder contractility in patients with cholesterol gallstones. Dig Dis Sci. 1999;44:190–6. doi: 10.1023/a:1026635124115. [DOI] [PubMed] [Google Scholar]
- 89.Guarino MP, Cong P, Cicala M, et al. Ursodeoxycholic acid improves muscle contractility and inflammation in symptomatic gallbladders with cholesterol gallstones. Gut. 2007;56(6):815–20. doi: 10.1136/gut.2006.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Portincasa P, Di Ciaula A, vanBerge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep. 2004;6(2):151–62. doi: 10.1007/s11894-004-0043-0. [DOI] [PubMed] [Google Scholar]
- 91.Xiao ZL, Rho AK, Biancani P, et al. Effects of bile acids on the muscle functions of guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G87–G94. doi: 10.1152/ajpgi.00536.2001. [DOI] [PubMed] [Google Scholar]
- 92.Stolk MFJ, van de Heijning BJM, van Erpecum KJ, et al. Effect of bile salts on in vitro gallbladder motility: preliminary study. Ital J Gastroenterol Hepatol. 1996;28:105–10. [PubMed] [Google Scholar]
- 93.Xiao ZL, Biancani P, Carey MC, et al. Hydrophilic but not hydrophobic bile acids prevent gallbladder muscle dysfunction in acute cholecystitis. Hepatology. 2003;37(6):1442–50. doi: 10.1053/jhep.2003.50243. [DOI] [PubMed] [Google Scholar]
- 94.Maurer KJ, Rao VP, Ge Z, et al. T-cell function is critical for murine cholesterol gallstone formation. Gastroenterology. 2007;133(4):1304–15. doi: 10.1053/j.gastro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pauletzki JG, Althaus R, Holl J, et al. Gallbladder emptying and gallstone formation: a prospective study on gallstone recurrence. Gastroenterology. 1996;111:765–71. doi: 10.1053/gast.1996.v111.pm8780583. [DOI] [PubMed] [Google Scholar]
- 96.Portincasa P, van Erpecum KJ, van de Meeberg PC, et al. Apolipoprotein (Apo) E4 genotype and galbladder motility influence speed of gallstone clearance and risk of recurrence after extracorporeal shock-wave lithotripsy. Hepatology. 1996;24:580–7. doi: 10.1002/hep.510240320. [DOI] [PubMed] [Google Scholar]
- 97.Venneman NG, vanBerge-Henegouwen GP, Portincasa P, et al. Absence of apolipoprotein E4 genotype, good gallbladder motility and presence of solitary stones delay rather than prevent gallstone recurrence after extracorporeal shock wave lithotripsy. J Hepatol. 2001;35(1):10–6. doi: 10.1016/s0168-8278(01)00093-9. [DOI] [PubMed] [Google Scholar]
- 98.Festi D, Frabboni R, Bazzoli F, et al. Gallbladder motility in cholesterol gallstone disease. Effect of ursodeoxycholic acid administration and gallstone dissolution. Gastroenterology. 1990;99:1779–85. doi: 10.1016/0016-5085(90)90487-l. [DOI] [PubMed] [Google Scholar]
- 99.Sackmann M, Eder H, Spengler U, et al. Gallbladder emptying is an important factor in fragment disappearance after shock wave lithotripsy. J Hepatol. 1993;17:62–6. doi: 10.1016/s0168-8278(05)80522-7. [DOI] [PubMed] [Google Scholar]
- 100.Leopold GR, Amberg J, Gosink BB, et al. Gray scale ultrasonic cholecystography: a comparison with conventional radiographic techniques. Radiology. 1976;121(2):445–8. doi: 10.1148/121.2.445. [DOI] [PubMed] [Google Scholar]
- 101.Portincasa P, Di Ciaula A, Palmieri VO, et al. Sonographic evaluation of gallstone burden in humans. Ital J Gastroenterol Hepatol. 1994;26(3):141–4. [PubMed] [Google Scholar]
- 102.Everson GT, Braverman DZ, Johnson ML, et al. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology. 1980;79:40–6. [PubMed] [Google Scholar]
- 103.Portincasa P, Moschetta A, Colecchia A, et al. Measurement of gallbladder motor function by ultrasonography: towards for standardization. Dig Liver Dis (già Ital J Gastroenterol Hepatol) 2003;35(Suppl.3):S56–S61. doi: 10.1016/s1590-8658(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 104.Portincasa P, Di Ciaula A, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21:430–40. doi: 10.1016/s0168-8278(05)80324-1. [DOI] [PubMed] [Google Scholar]
- 105.Portincasa P, Moschetta A, Di-Ciaula A, et al. Pathophysiology of cholesterol gallstone disease. In: Borzellino G, Cordiano C, editors. Biliary lithiasis. Basic Science, Current Diagnosis and Management. Milano: Springer Italia S.r.l.; 2008. pp. 19–49. [Google Scholar]
- 106.Pereira SP, Veysey MJ, Kennedy C, et al. Gallstone dissolution with oral bile acid therapy - Importance of pretreatment CT scanning and reasons for nonresponse. Dig Dis Sci. 1997;42(8):1775–82. doi: 10.1023/a:1018834103873. [DOI] [PubMed] [Google Scholar]
- 107.Pereira SP, Hussaini SH, Kennedy C, et al. Gallbladder stone recurrence after medical treatment. Do gallstones recur true to type? Dig Dis Sci. 1995;40:2568–75. doi: 10.1007/BF02220443. [DOI] [PubMed] [Google Scholar]
- 108.Senior JR, Johnson MF, DeTurck DM, et al. In vivo kinetics of radiolucent gallstone dissolution by oral dihydroxy bile acids. Gastroenterology. 1990;99(1):243–51. doi: 10.1016/0016-5085(90)91254-4. [DOI] [PubMed] [Google Scholar]
- 109.Jazrawi RP, Pigozzi MG, Galatola G, et al. Optimum bile acid treatment for rapid gall stone dissolution. Gut. 1992;33:381–6. doi: 10.1136/gut.33.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paumgartner G. Advances in Basic and Clinical Bile Acid Research. Dordrecht: Kluwer Academic Publishers; 1996. Therapeutic options and choice of appropriate treatment Bile Acids - Cholestasis - Gallstones; pp. 205–210. [Google Scholar]
- 111.Bateson MC, Bouchier IA, Trash DB, et al. Calcification of radiolucent gall stone during treatment with ursodeoxycholic acid. Br Med J (Clin Res Ed) 1981;283(6292):645–6. doi: 10.1136/bmj.283.6292.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bazzoli F, Festi D, Mazzella G, et al. Acquired gallstone opacification during cholelitholytic treatment with chenodeoxyholic, ursodeoxycholic, and tauroursodeoxycholic acids. Am J Gastroenterol. 1995;90(6):978–81. [PubMed] [Google Scholar]
- 113.Lanzini A, Jazrawi RP, Kupfer RM, et al. Gallstone recurrence after medical dissolution. An overestimated threat? J Hepatol. 1986;3:241–6. doi: 10.1016/s0168-8278(86)80033-2. [DOI] [PubMed] [Google Scholar]
- 114.Rabenstein T, Radespiel-Troger M, Hopfner L, et al. Ten years experience with piezoelectric extracorporeal shockwave lithotripsy of gallbladder stones. Eur J Gastroenterol Hepatol. 2005;17(6):629–39. doi: 10.1097/00042737-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 115.Sackmann M, Ippisch E, Sauerbruch T, et al. Early gallstone recurrence rate after successful shock-wave therapy. Gastroenterology. 1990;98:392–6. doi: 10.1016/0016-5085(90)90830-t. [DOI] [PubMed] [Google Scholar]
- 116.Villanova N, Bazzoli F, Taroni F, et al. Gallstone recurrence after successful oral bile acid treatment: a 12 year follow-up study and evaluation of long term postdissolution treatment. Gastroenterology. 1989;97:726–31. doi: 10.1016/0016-5085(89)90644-6. [DOI] [PubMed] [Google Scholar]
- 117.Petroni ML, Jazrawi RP, Pazzi P, et al. Risk factors for the development of gallstone recurrence following medical dissolution. The British-Italian Gallstone Study Group. Eur J Gastroenterol Hepatol. 2000;12(6):695–700. doi: 10.1097/00042737-200012060-00020. [DOI] [PubMed] [Google Scholar]
- 118.Petroni ML, Jazrawi RP, Lanzini A, et al. Repeated bile acid therapy for the long-term management of cholesterol gallstones. J Hepatol. 1996;25:719–24. doi: 10.1016/s0168-8278(96)80244-3. [DOI] [PubMed] [Google Scholar]
- 119.O'Leary DP, Johnson AG. Future directions for conservative treatment of gallbladder calculi. Br J Surg. 1993;80(2):143–7. doi: 10.1002/bjs.1800800206. [DOI] [PubMed] [Google Scholar]
- 120.Gilat T, Konikoff F. Pregnancy and the biliary tract. Can J Gastroenterol. 2000;14 D:55D–9D. doi: 10.1155/2000/932147. [DOI] [PubMed] [Google Scholar]
- 121.Sugerman HJ, Brewer WH, Shiffman ML, et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg. 1995;169(1):91–6. doi: 10.1016/s0002-9610(99)80115-9. [DOI] [PubMed] [Google Scholar]
- 122.Lammert F, Neubrand MW, Bittner R, et al. S3-guidelines for diagnosis and treatment of gallstones. German Society for Digestive and Metabolic Diseases and German Society for Surgery of the Alimentary Tract. Z Gastroenterol. 2007;45(9):971–1001. doi: 10.1055/s-2007-963437. [DOI] [PubMed] [Google Scholar]
- 123.Portincasa P, Di Ciaula A, Wang HH, et al. Medicinal treatments of cholesterol gallstones: old, current and new perspectives. Curr Med Chem. 2009;16(12):1531–42. doi: 10.2174/092986709787909631. [DOI] [PubMed] [Google Scholar]
- 124.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–4. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 125.Kallien G, Lange K, Stange EF, et al. The pravastatin-induced decrease of biliary cholesterol secretion is not directly related to an inhibition of cholesterol synthesis in humans. Hepatology. 1999;30(1):14–20. doi: 10.1002/hep.510300119. [DOI] [PubMed] [Google Scholar]
- 126.Duane WC, Hunninghake DB, Freeman ML, et al. Simvastatin, a competitive inhibitor of HMG-CoA reductase, lowers cholesterol saturation index of gallbladder bile. Hepatology. 1988;8:1150–47. doi: 10.1002/hep.1840080531. [DOI] [PubMed] [Google Scholar]
- 127.Loria P, Bertolotti M, Cassinadri MT, et al. Short-term effects of simvastatin on bile acid synthesis and bile lipid secretion in human subjects. Hepatology. 1994;19:882–8. [PubMed] [Google Scholar]
- 128.Hanson DS, Duane WC. Effects of lovastatin and chenodiol on bile acid synthesis, bile lipid composition, and biliary lipid secretion in healthy human subjects. J Lipid Res. 1994;35(8):1462–8. [PubMed] [Google Scholar]
- 129.Saunders KD, Cates JA, Abedin MZ, et al. Lovastatin and gallstone dissolution: a preliminary study. Surgery. 1993;113:28–35. [PubMed] [Google Scholar]
- 130.Smit JWA, van Erpecum KJ, Renooij W, et al. The effects of the 3-hydroxy, 3-methylglutaryl coenzyme A reductase inhibitor pravastatin on bile composition and nucleation of cholesterol crystals in cholesterol gallstone disease. Hepatology. 1995;21(6):1523–9. [PubMed] [Google Scholar]
- 131.Tazuma S, Hatsushika S, Aihara N, et al. Inhibitory effects of pravastatin, a competitive inhibitor of hydroxymethylglutaryl coenzyme A reductase, on cholesterol gallstone formation in prairie dogs. Digestion. 1992;51:179–84. doi: 10.1159/000200894. [DOI] [PubMed] [Google Scholar]
- 132.Abedin MZ, Narins SC, Park EH, et al. Lovastatin alters biliary lipid composition and dissolves gallstones: a long-term study in prairie dogs. Dig Dis Sci. 2002;47(10):2192–210. doi: 10.1023/a:1020174908650. [DOI] [PubMed] [Google Scholar]
- 133.Davis KG, Wertin TM, Schriver JP. The use of simvastatin for the prevention of gallstones in the lithogenic prairie dog model. Obes Surg. 2003;13(6):865–8. doi: 10.1381/096089203322618678. [DOI] [PubMed] [Google Scholar]
- 134.Chapman BA, Burt MJ, Chisholm RJ, et al. Dissolution of gallstones with simvastatin, an HMG CoA reductase inhibitor. Dig Dis Sci. 1998;43(2):349–53. doi: 10.1023/a:1018862507469. [DOI] [PubMed] [Google Scholar]
- 135.Porsch-Ozcurumez M, Hardt PD, Schnell-Kretschmer H, et al. Effects of fluvastatin on biliary lipids in subjects with an elevated cholesterol saturation index. Eur J Clin Pharmacol. 2001;56(12):873–9. doi: 10.1007/s002280000254. [DOI] [PubMed] [Google Scholar]
- 136.Smith JL, Roach PD, Wittenberg LN, et al. Effects of simvastatin on hepatic cholesterol metabolism, bile lithogenicity and bile acid hydrophobicity in patients with gallstones. J Gastroenterol Hepatol. 2000;15(8):871–9. doi: 10.1046/j.1440-1746.2000.02231.x. [DOI] [PubMed] [Google Scholar]
- 137.Wilson IR, Hurrell MA, Pattinson NR, et al. The effect of simvastatin and bezafibrate on bile composition and gall-bladder emptying in female non-insulin-dependent diabetics. J Gastroenterol Hepatol. 1994;9(5):447–51. doi: 10.1111/j.1440-1746.1994.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 138.Miettinen TE, Kiviluoto T, Taavitsainen M, et al. Cholesterol metabolism and serum and biliary noncholesterol sterols in gallstone patients during simvastatin and ursodeoxycholic acid treatments. Hepatology. 1998;27(3):649–55. doi: 10.1002/hep.510270302. [DOI] [PubMed] [Google Scholar]
- 139.Sharma BC, Agarwal DK, Baijal SS, et al. Pravastatin has no effect on bile lipid composition, nucleation time, and gallbladder motility in persons with normal levels of cholesterol. J Clin Gastroenterol. 1997;25(2):433–6. doi: 10.1097/00004836-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 140.Caroli-Bosc FX, Le GP, Pugliese P, et al. Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation: epidemiological study in an unselected population. Dig Dis Sci. 2001;46(3):540–4. doi: 10.1023/a:1005643014395. [DOI] [PubMed] [Google Scholar]
- 141.Gonzalez-Perez A, Garcia-Rodriguez LA. Gallbladder disease in the general population: association with cardiovascular morbidity and therapy. Pharmacoepidemiol Drug Saf. 2007;16(5):524–31. doi: 10.1002/pds.1346. [DOI] [PubMed] [Google Scholar]
- 142.Tsai CJ, Leitzmann MF, Willett WC, et al. Statin use and the risk of cholecystectomy in women. Gastroenterology. 2009;136(5):1593–600. doi: 10.1053/j.gastro.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bodmer M, Brauchli YB, Krahenbuhl S, et al. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA. 2009;302(18):2001–7. doi: 10.1001/jama.2009.1601. [DOI] [PubMed] [Google Scholar]
- 144.Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–48. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 145.Davis HR, Jr, Zhu LJ, Hoos LM, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279(32):33586–92. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 146.Altmann SW, Davis HR, Jr, Zhu L, et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 147.Davies JP, Scott C, Oishi K, et al. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280(13):12710–20. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 148.Ge L, Wang J, Qi W, et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7(6):508–19. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 149.Valasek MA, Repa JJ, Quan G, et al. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14(3):99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 151.Zuniga S, Molina H, Azocar L, et al. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28(7):935–47. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 152.Wang DQH, Tazuma S. Effect of beta-muricholic acid on the prevention and dissolution of cholesterol gallstones in C57L/J mice. J Lipid Res. 2002;43(11):1960–8. doi: 10.1194/jlr.m200297-jlr200. [DOI] [PubMed] [Google Scholar]
- 153.Mathur A, Walker JJ, Al-Azzawi HH, et al. Ezetimibe ameliorates cholecystosteatosis. Surgery. 2007;142(2):228–33. doi: 10.1016/j.surg.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 154.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Janowski BA, Willy PJ, Devi TR, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–31. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 156.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 157.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 158.Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: Potential new players in atherosclerosis. Nat Med. 2002;8(11):1243–8. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 159.Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–91. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 160.Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: Are we there yet? FEBS Lett. 2006;580(23):5492–9. doi: 10.1016/j.febslet.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 161.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10(12):1352–8. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Binz J, Numerick MJ, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112(11):1678–87. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uppal H, Zhai Y, Gangopadhyay A, et al. Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization. Hepatology. 2008;47(4):1331–42. doi: 10.1002/hep.22175. [DOI] [PubMed] [Google Scholar]
- 164.Keus F, de Jong JA, Gooszen HG, et al. Small-incision versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;(4):CD004788. doi: 10.1002/14651858.CD004788.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Keus F, de Jong JA, Gooszen HG, et al. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;(4):CD006231. doi: 10.1002/14651858.CD006231. [DOI] [PubMed] [Google Scholar]
- 166.Keus F, de Jong JA, Gooszen HG, et al. Laparoscopic versus small-incision cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;(4):CD006229. doi: 10.1002/14651858.CD006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pokorny WJ, Saleem M, O'Gorman RB, et al. Cholelithiasis and cholecystitis in childhood. Am J Surg. 1984;148(6):742–4. doi: 10.1016/0002-9610(84)90428-8. [DOI] [PubMed] [Google Scholar]
- 168.Amaral JF, Thompson WR. Gallbladder disease in the morbidly obese. Am J Surg. 1985;149(4):551–7. doi: 10.1016/s0002-9610(85)80055-6. [DOI] [PubMed] [Google Scholar]
- 169.Sleisenger MH, Fordtran JS. Gastrointestinal Disease: pathophysiology, diagnosis, management 8th ed. Philadelphia: W.B. Saunders; 2006. [Google Scholar]
- 170.Lowenfels AB, Walker AM, Althaus DP, et al. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 1989;18:50–4. doi: 10.1093/ije/18.1.50. [DOI] [PubMed] [Google Scholar]
- 171.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118(7):1591–602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 172.Ashur H, Siegal B, Oland Y, et al. Calcified ballbladder (porcelain gallbladder) Arch Surg. 1978;113(5):594–6. doi: 10.1001/archsurg.1978.01370170056010. [DOI] [PubMed] [Google Scholar]
- 173.Lowenfels AB, Lindstrom CG, Conway MJ, et al. Gallstones and risk of gallbladder cancer. J Natl Cancer Inst. 1985;75(1):77–80. [PubMed] [Google Scholar]
- 174.Bonatsos G, Birbas K, Toutouzas K, et al. Laparoscopic cholecystectomy in adults with sickle cell disease. Surg Endosc. 2001;15(8):816–9. doi: 10.1007/s004640000383. [DOI] [PubMed] [Google Scholar]
- 175.Wang DQH, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing system. J Lipid Res. 1996;37:606–30. [PubMed] [Google Scholar]


