Abstract
Background
Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the intestinal tract. The standard treatment of localized, primary GIST has been surgical resection alone. Most GISTs have a mutation in the KIT proto-oncogene, or less commonly in platelet-derived growth factor receptor alpha (PDGFRα). Imatinib mesylate is a small molecule that inhibits activation of the KIT and PDGFRα proteins and is effective in metastatic GIST. We hypothesized that adjuvant treatment with imatinib would result in improved recurrence-free survival (RFS) compared to placebo treatment following resection of localized, primary GIST.
Methods
We performed a randomized phase 3, double-blind, placebo-controlled, multicenter trial. Eligible patients had complete gross resection of a primary GIST at least 3 cm in size that stained positive for KIT protein. Patients were randomly assigned to receive imatinib 400 mg/day or placebo daily for one year following surgical resection. Patients assigned to placebo were eligible to crossover to imatinib treatment in the event of tumor recurrence. The primary endpoint was RFS and intention to treat analysis was performed. This study is registered at ClinicalTrials.gov, number NCT00041197.
Findings
From July 2002 to April 2007, 359 patients were randomized to imatinib and 354 to placebo. Accrual was stopped early based on the results of a planned interim analysis. Imatinib significantly prolonged RFS compared with placebo (98% vs. 83% at 1 year; overall hazard ratio 0.35; one-sided p<0.0001). Overall survival (OS) was similar (99.2% vs. 99.7% at 1 year; hazard ratio 0.66; p=0.47). Adjuvant imatinib was well-tolerated with a low rate of serious adverse events.
Interpretation
Adjuvant imatinib therapy is safe and compared to placebo treatment appears to prolong RFS following the resection of primary GIST. OS is not different at this time.
INTRODUCTION
Gastrointestinal stromal tumor (GIST) has an estimated annual incidence in the U.S. of approximately 3,000–4,000.1, 2 Only within the last decade has GIST been widely recognized as distinct from intestinal leiomyosarcoma. GIST typically arises in the stomach or small intestine, but can also be found occasionally in the rectum and rarely in the esophagus or colon. Approximately 85% of GISTs contain an activating mutation in the KIT proto-oncogene while 3–5% instead have a mutation in PDGFRα.3–7 The mainstay of treatment for localized, primary GIST has been surgical resection. Postoperative adjuvant chemotherapy has not generally been recommended because conventional cytotoxic agents are ineffective against GIST.8 Unfortunately, the results of surgery alone have been inadequate, with up to 50% of patients developing tumor recurrence within 5 years and eventually dying of disease.9, 10 The most frequent sites of initial tumor recurrence are the peritoneal surface and the liver.
Imatinib mesylate (Gleevec®, Novartis Pharmaceuticals, Basel, Switzerland) is an oral agent that is a selective molecular inhibitor of the KIT, PDGFRα, ABL, and BCR-ABL tyrosine kinases. Imatinib was first utilized for chronic myelogenous leukemia and proved to be safe and achieved a complete hematologic response in nearly 100% of patients by inhibiting the BCR-ABL oncoprotein.11 In the year 2000, imatinib was found to be effective against metastatic GIST in the initial patient tested12 and efficacy was then confirmed in a phase II trial13, 14 and phase III trials (Table 1).15, 16 Given the activity of imatinib, the proclivity for tumor recurrence after resection, and the lack of effective conventional chemotherapeutic agents, there was substantial rationale for testing the benefit of adjuvant imatinib in GIST. We hypothesized that adjuvant treatment with imatinib would improve RFS compared to placebo treatment in patients who underwent resection of localized, primary GIST.
Table 1.
Summary of randomized controlled trials testing the benefit of imatinib in metastatic/unresectable GIST.
| Trial | N | Phase | Primary endpoint | Imatinib dose | PFS | OS |
|---|---|---|---|---|---|---|
| B2222 | 147 | II | Response | 400 mg qd 600 mg qd |
24 months median | 57 months median |
|
EORTC 62005 |
946 | III | PFS | 400 mg qd 400 mg bid |
22 months median† 27 months median†* |
69% at 2 yrs 74% at 2 yrs |
|
SWOG S0033 |
746 | III | PFS/OS | 400 mg qd 400 mg bid |
18 months median 20 months median |
55 months median 51 months median |
METHODS
Patient Eligibility
Patients were eligible if they had a histologic diagnosis of localized, primary GIST measuring at least 3 cm that expressed KIT protein (CD117) by immunohistochemistry using the Dako antibody (Denmark). Size measurements were performed by the local institutional pathologist, either before or after formalin fixation. Retrospective central pathologic review was performed by 2 pathologists. Patients were to be registered within 70 days following complete gross tumor resection (regardless of microscopic margins) and start therapy by 84 days. The technique of resection was at the discretion of the individual surgeon. Patients were at least 18 years of age with an Eastern Cooperative Oncology Group (ECOG)/Zubrod performance status of ≤ 2. Within 28 days prior to registration, patients must have been deemed free of tumor by postoperative imaging that included a baseline chest x-ray (or chest CT) and a post-operative abdomen and pelvis CT scan with intravenous and oral contrast or MRI with intravenous contrast. Additional inclusion criteria included adequate renal, hematologic, and hepatic function and a negative serum pregnancy test where applicable. Prior imatinib use or chemotherapy, radiation therapy, or investigational treatment following surgery was not allowed. Also excluded were patients with an active infection requiring antibiotics within 14 days prior to registration, female patients who were breast feeding, patients with New York Heart Association Class 3 or 4 cardiac disease, and patients taking full dose warfarin. The study was approved by the institutional review board (IRB) of each participating institution, and written informed consent was obtained from all patients.
Study Design
Patients were randomly assigned to receive 1 year of adjuvant imatinib at a dose of 400 mg/day or 1 year of placebo in a double-blind fashion. Patients were assigned to take four capsules of 100 mg imatinib or placebo once a day with food. Imatinib and placebo capsules looked alike. Patients were evaluated frequently in the first six months, every 3 months until year 2, and then every 6 months until year 5 with physical examination, CBC with differential, creatinine, bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and assessment of adverse events. Toxicity was graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.17 Attribution was recorded as definite, probable, possible, unlikely, or unrelated to therapy. Dose modifications were made for grade 3 and 4 events (excluding anemias) that were thought to be at least possibly related to treatment. Patients kept a diary to record dose administration and adverse events. CT scans with intravenous and oral contrast (or MRI with intravenous contrast) of the abdomen and pelvis were performed every 3 months for the first 2 years and then every 6 months for the next 3 years. At the time of reported tumor recurrence, the treatment arm was unblinded after central review. Biopsy was mandatory and performed when medically feasible. Patients were not allowed to crossover prior to an observed recurrence. Patients unblinded for tumor recurrence were eligible for imatinib 400 mg/day if they had either been assigned to the imatinib arm and already completed study therapy or assigned to the placebo arm. Imatinib 800 mg/day could be prescribed if the patient was actively taking imatinib during recurrence.
Statistical Analysis
The original primary endpoint was OS and an accrual of 380 patients over 3.8 years with a minimum follow-up period of 3 years was planned. At a 0.05 one-sided level of significance, the log-rank test would have had 90% power to detect a minimum hazard ratio of 0.65, assuming exponential decay in both arms and uniform censoring. Six months prior to the first planned efficacy interim analysis, the primary endpoint was changed to RFS based on discussions with CTEP and the FDA. The trial was designed at the end of the year 2000, when fewer than 150 patients with metastatic GIST had been treated worldwide. During the present trial, it became clear that the actual event (death) rate would be considerably lower than the putative event rate specified in the original statistical design because of the efficacy of imatinib in recurrent GIST and the crossover design that allowed patients who progressed on placebo to receive imatinib. Consequently, the study was vastly underpowered to show a difference in OS between taking imatinib immediately after surgery versus waiting until recurrence occurred. In the revised statistical design, the putative median RFS for the placebo arm was assumed to be 3.5 years based on historical data. From the time of the amendment, the intent was to accrue 600 more patients over 2.5 years (to reach a total of 803) with a minimum follow-up period of 3 years. This would yield 90% power, at a 0.025 one-sided significance level, to detect a 40% improvement in RFS in the imatinib arm, which corresponds to a median RFS of 4.9 years for the imatinib arm and a hazard ratio of 0.71.
Interim analyses for superiority and futility were scheduled every six months beginning in December 2005. A truncated O’Brien-Fleming bound was utilized to monitor treatment efficacy.18 Futility was monitored using a 0.0025 fixed level of significance at each interim analysis. This study was monitored by a Data Monitoring Committee that was approved by CTEP and independent of Novartis.
Patients were randomized at the ACOSOG central office via a computer program using a stratified biased coin design with the objective of equal allocation to each arm, and stratified by tumor size (≥3 to <6, ≥6 to <10, or ≥10 cm). Patients and investigators were blinded to the arm that the patient was assigned. Sixty patients were mis-randomized due to a programming error that assigned them to the placebo arm. Patients, physicians, IRBs, and health authorities were notified of the error and the patients were removed from the study. No data were collected on these patients after their removal from the study resulting in the lack of follow-up information for these patients.
RFS was defined as the time from patient registration to the development of tumor recurrence or death due to any cause. OS was defined as the time from patient registration to death from any cause. Patients who were alive and free of recurrent disease on April 12, 2007 were censored for OS and RFS. Intent-to-treat analyses were done for both RFS and OS; patients were analyzed by randomized arm. RFS and OS were estimated with the Kaplan-Meier method. Differences in RFS and OS between the arms were analyzed with a one-sided log-rank test stratified by tumor size. HRs and 95% CI were reported based on a Cox proportion hazards regression model, also stratified by tumor size for RFS. An unstratified Cox model was used for OS due to the small number of observed deaths. The placebo arm was used as the HR denominator so that HRs of less than one are in favor of imatinib. The proportionality assumption of the Cox model was tested using Schoenfeld residuals and was found to be valid for all of the analyses. For the safety analysis, all patients receiving one or more doses of their assigned treatment were included. Chi-square tests were used to compare categorical variables between the two arms. All analyses were done with SAS version 8.2.
Role of the Funding Source
The ACOSOG Z9001 trial was conducted through collaboration between the American College of Surgeons Oncology Group (ACOSOG) and the National Cancer Institute. Novartis employees provided input regarding the study design, but did not participate in the collection, analysis, or interpretation of data. Novartis provided imatinib and the placebo. Data were collected at the local institution and transferred electronically to the ACOSOG central database. The database was audited and updated by members of the Duke Clinical Research Institute, which received funding from Novartis. The results were analyzed by the principal academic investigators. This article was written by the lead author and reviewed by all authors and was submitted to Novartis for comments. The decision to submit the manuscript was independent of Novartis.
RESULTS
Between July 2002 and April 2007, 778 patients were accrued from 230 institutions. Based on the recommendation of the ACOSOG Data and Safety Monitoring Committee, accrual to the study was halted on April 12, 2007 and the NCI issued a press release of the preliminary findings that day because the trial results crossed the interim analysis efficacy boundary for RFS. The interim analysis hazard ratio was 0.33 (95% CI 0.20–0.53) with a p-value < 0.0001. The final analysis includes all data collected through April 12, 2007.
Patients
The intent to treat analysis consisted of 713 total patients (Fig. 1); 60 of the 778 patients were excluded from the study due to a randomization error and another 5 patients were excluded because they were registered after the study closure date. The intent to treat population included 65 (9.1%) patients who did not meet all eligibility requirements. Retrospective central pathology review was performed in 631 (88.5%) patients, of whom 16 (2.5%) were found to have another type of sarcoma. The other major reasons for ineligibility were improper timing (n=26) of baseline tests (laboratory or radiologic) or surgery, incomplete baseline laboratory tests (n=6), and incomplete baseline radiologic imaging (n=10). Clinicopathologic features were similar between the study arms (Table 2).
Figure 1.
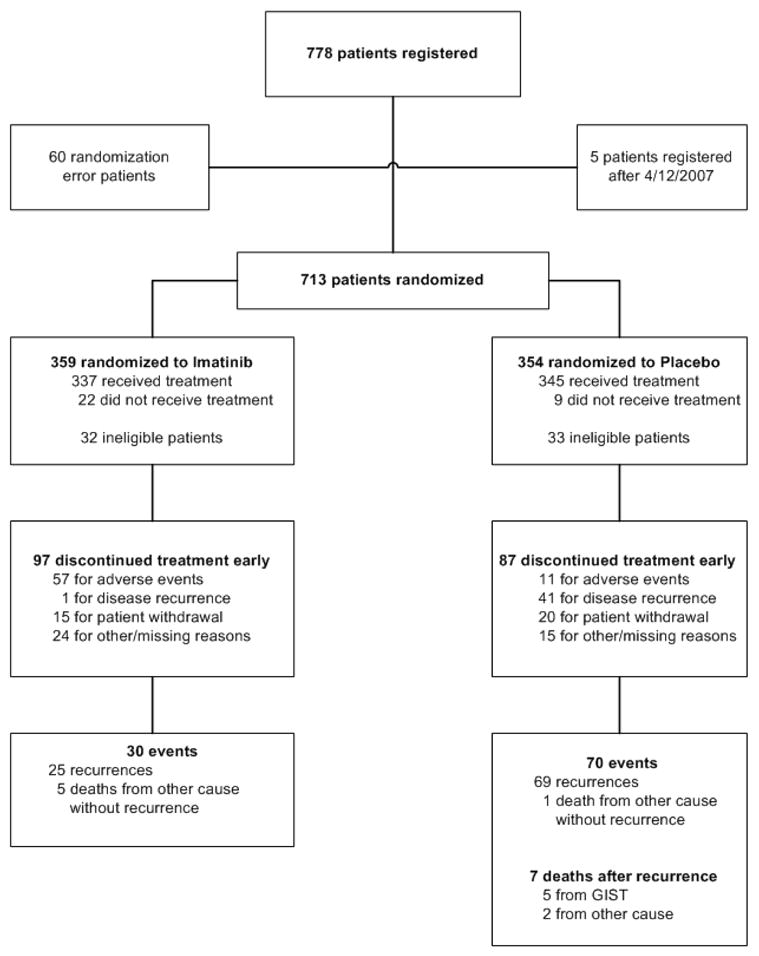
CONSORT diagram.
Table 2.
Clinicopathologic features.
| Placebo (n=354) | Imatinib (n=359) | |
|---|---|---|
| Age, median (min-max) | 58 (18–91) | 59 (18–88) |
| Gender, n (%) | ||
| Female | 163 (46.0%) | 189 (52.6%) |
| Male | 191 (54.0%) | 170 (47.4%) |
| Performance Status, n (%) | ||
| 0 | 265 (74.9%) | 281 (78.3%) |
| 1 | 81 (22.9%) | 74 (20.6%) |
| 2 | 8 (2.3%) | 4 (1.1%) |
| Days between resection and randomization; median (min-max) | 59 (15–96) | 57 (20–74) |
| Tumor size, n (%) | ||
| ≥3 and <6 cm | 149 (42.1%) | 143 (39.8%) |
| ≥6 and <10 cm | 119 (33.6%) | 123 (34.3%) |
| ≥10 cm | 86 (24.3%) | 93 (25.9%) |
| Margins, n (%) | ||
| R0 | 330 (93.2%) | 325 (90.5%) |
| R1 | 23 (6.5%) | 34 (9.5%) |
| Unknown | 1 (0.3%) | 0 (0.0%) |
| Tumor origin, n (%) | ||
| Stomach | 235 (66.4%) | 209 (58.2%) |
| Small intestine | 102 (28.8%) | 125 (34.8%) |
| Rectum | 5 (1.4%) | 5 (1.4%) |
| Other | 12 (3.4%) | 18 (5.0%) |
| Unknown | 0 (0.0%) | 2 (0.6%) |
R0 – negative microscopic margins; R1 – positive microscopic margins
Tolerability/Safety
Treatment was stopped prematurely in 184 (25.8%) patients (Fig. 1). Discontinuation was more likely due to adverse events in the imatinib arm (p<0.0001) and tumor recurrence in the placebo arm (p<0.0001). A dose reduction and/or interruption occurred for any reason in 59 (16.4%) patients on the imatinib arm and 17 (4.8%) on the placebo arm and occurred due to adverse events in 52 (14.5%) and 10 (2.8%) patients, respectively. When considering just the 682 patients who received at least one dose of either imatinib or placebo, 647 (94.9%) patients experienced at least one adverse event. Grade 1 and 2 events were common and mostly involved gastrointestinal effects (mild diarrhea, nausea, and flatulence), headache, rash, periorbital or peripheral edema, fatigue, or myalgias/arthralgias (Table 3A). There were 251 (72.8%) patients on the placebo arm and 229 (68.0%) on the imatinib arm who experienced a Grade 1 or Grade 2 event (Table 3B). Grade 3 or 4 events occurred in 63 (18.3%) patients on the placebo arm and 104 (30.9%) patients on the imatinib arm.
Table 3.
| Table 3A. Common adverse events, n (%). | ||||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=345) | Imatinib (n=337) | |||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Neutropenia | 11 (3%) | 8 (2%) | 3 (<1%) | 1 (<1%) | 23 (6%) | 26 (7%) | 7 (2%) | 5 (1%) |
| Fatigue | 134 (39%) | 51 (15%) | 4 (1%) | 0 (0%) | 117 (33%) | 20 (5%) | 5 (1%) | 2 (<1%) |
| Dermatitis | 75 (22%) | 32 (9%) | 0 (0%) | 0 (0%) | 54 (15%) | 15 (4%) | 11 (3%) | 0 (0%) |
| Abdominal pain | 64 (18%) | 10 (2%) | 6 (1%) | 0 (0%) | 61 (17%) | 25 (7%) | 12 (3%) | 0 (0%) |
| Nausea | 144 (42%) | 27 (8%) | 4 (1%) | 0 (0%) | 78 (22%) | 14 (4%) | 8 (2%) | 0 (0%) |
| Vomiting | 60 (17%) | 18 (5%) | 2 (<1%) | 0 (0%) | 37 (10%) | 9 (2%) | 8 (2%) | 0 (0%) |
| Diarrhea | 147 (43%) | 42 (12%) | 5 (1%) | 0 (0%) | 79 (22%) | 17 (4%) | 10 (2%) | 0 (0%) |
| ALT | 42 (12%) | 6 (1%) | 0 (0%) | 0 (0%) | 38 (11%) | 9 (2%) | 7 (2%) | 2 (<1%) |
| AST | 27 (7%) | 3 (<1%) | 0 (0%) | 0 (0%) | 31 (9%) | 4 (1%) | 4 (1%) | 3 (<1%) |
| Edema | 96 (28%) | 5 (1%) | 1 (<1%) | 0 (0%) | 220 (65%) | 32 (9%) | 7 (2%) | 0 (0%) |
| Hyperglycemia | 34 (9%) | 6 (1%) | 7 (2%) | 0 (0%) | 27 (8%) | 9 (2%) | 2 (<1%) | 0 (0%) |
| Hypokalemia | 9 (2%) | 1 (<1%) | 3 (<1%) | 0 (0%) | 28 (8%) | 0 (0%) | 4 (1%) | 0 (0%) |
| Syncope | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (<1%) | 0 (0%) | 4 (1%) | 0 (0%) |
| Dyspnea | 16 (4%) | 5 (1%) | 2 (<1%) | 0 (0%) | 13 (3%) | 1 (1%) | 4 (1%) | 0 (0%) |
| Table 3B. Maximum grade of adverse events per patient, n (%). | ||
|---|---|---|
| Grade | Placebo (n=345) | Imatinib (n=337) |
| 1 | 101 (29%) | 81 (24%) |
| 2 | 150 (43%) | 148 (44%) |
| 3 | 56 (16%) | 86 (26%) |
| 4 | 7 (2%) | 15 (4%) |
| 5 | 0 (0%) | 3 (1%) |
ALT – alanine aminotransferase; AST – aspartate aminotransferase
Efficacy
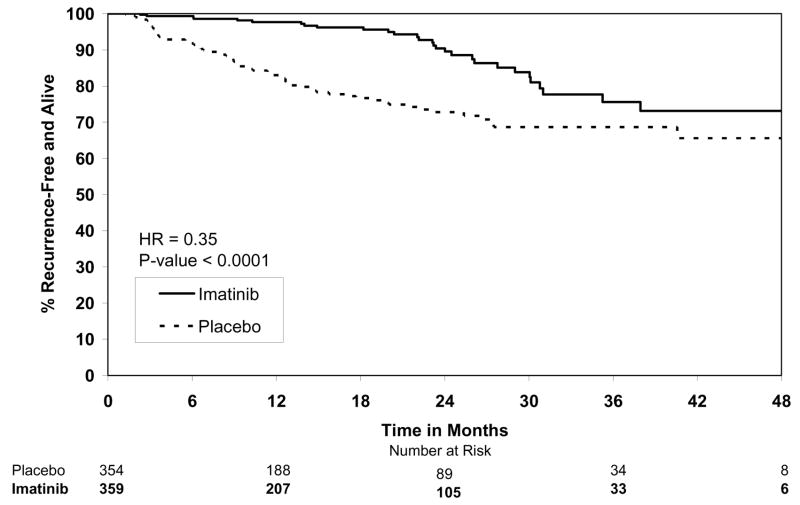
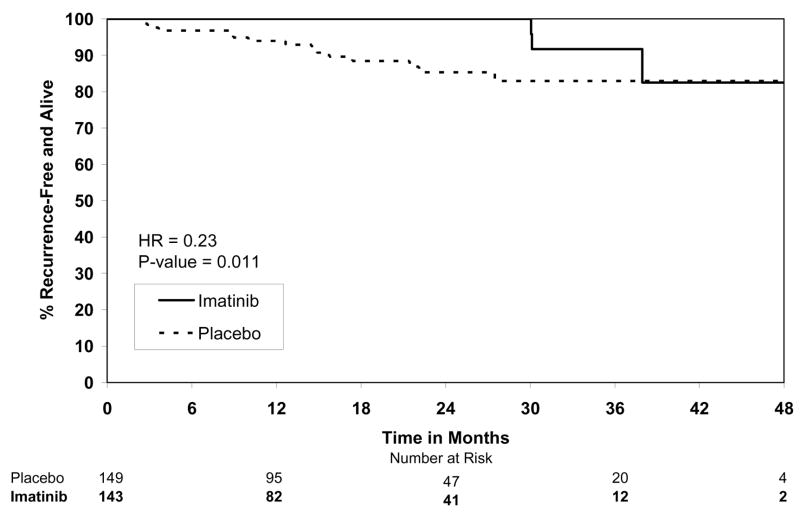
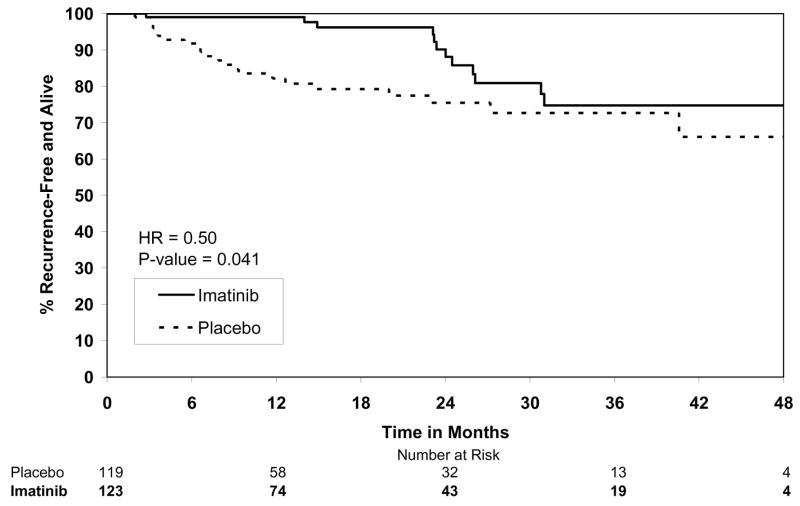
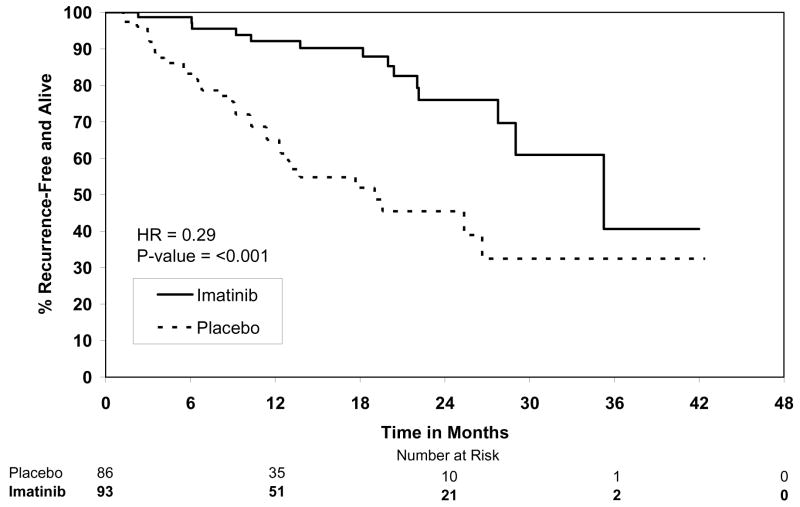
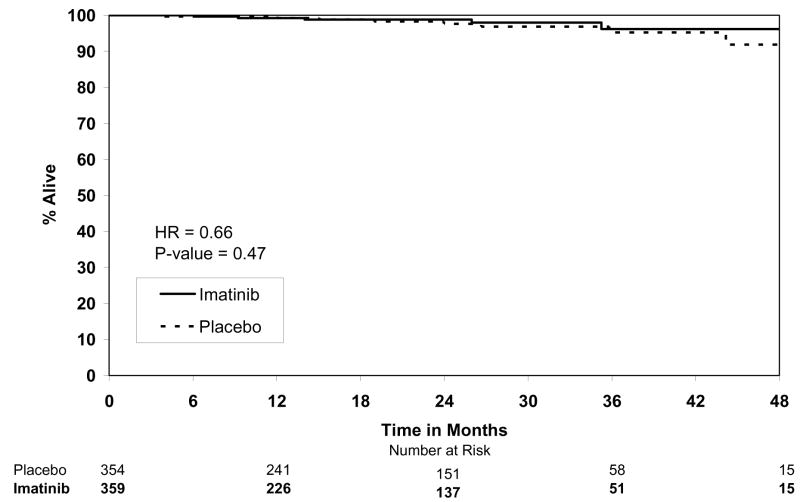
By the final analysis of RFS, 30 (8.4%) patients on the imatinib arm and 70 (19.8%) patients on the placebo arm had experienced events. With a median follow-up for surviving patients of 19.7 months (min-max: 0–56.4 months), the estimated one-year RFS was 98% (95% CI 0.96–1.00) on the imatinib arm versus 83% (95% CI 0.78–0.88) on the placebo arm (Fig. 2). The overall hazard ratio was 0.35 (95% CI 0.22–0.53) with a p-value <0.0001 (Table 4). Although the trial was not designed to assess patient subsets, we analyzed the effect of tumor size (the stratification factor) and found that RFS was prolonged on the imatinib arm in each size category (Figs. 3). Five (1.4%) patients died on the imatinib arm, all from causes unrelated to GIST. There were 8 (2.3%) deaths on the placebo arm, 5 of which were related to GIST. At this time, there is no difference in OS (Fig. 4, Table 4). The observed hazard ratio is 0.66 (95% CI 0.22–2.03).
Figure 2.
Recurrence-free survival.
Table 4.
Summary of RFS and OS results.
| Outcome | No. of pts | No. of events | HR (95% CI) | p-value |
|---|---|---|---|---|
| RFS (primary) | 713 | 100 (14.0%) | 0.35 (0.22 to 0.53) | < 0.0001 |
| OS (secondary) | 713 | 13 (1.8%) | 0.66 (0.22 to 2.03) | 0.4714 |
RFS – recurrence-free survival; OS – overall survival; HR – hazard ratio; CI – confidence interval
Figure 3.
Figure 3A. Recurrence-free survival for tumor size ≥3 and <6 cm.
Figure 3B. Recurrence-free survival for tumor size ≥6cm and <10cm.
Figure 3C. Recurrence-free survival for tumor size ≥10cm.
Figure 4.
Overall survival.
DISCUSSION
We found that assignment to 1 year of adjuvant imatinib compared to placebo prolonged RFS following the complete resection of primary GIST. In addition, adjuvant imatinib was safe and well-tolerated. The adverse event rate was low and consistent with imatinib use in chronic myelogenous leukemia and metastatic GIST.11, 13 We did not observe significant cardiac toxicity that was recently suggested by one group,19 but refuted by others.20
We chose to stratify patients based only on tumor size. Mitotic rate and tumor site have also been reported to have prognostic importance in retrospective studies of primary GIST. Notably, none of these tumor features has been validated prospectively. Furthermore, the method of determining mitotic rate has never been standardized and the reproducibility of measurements among different pathologists (especially in a large, multicenter trial such as this) has not been proven. Patients on the placebo arm in this study provide the first large prospective cohort of patients with primary GIST in which to identify risk factors for recurrence. This is also the first large prospective evaluation of recurrence using serial radiologic imaging. Additional ad hoc analyses related to risk factors for tumor recurrence will be forthcoming as central pathologic and molecular analyses are underway.
During the year of assigned study therapy, there were 41 recurrences on the placebo arm yet only 1 on the imatinib arm. RFS was increased in each of the 3 size categories on retrospective analysis. Adjuvant therapy is especially relevant for high risk patients (e.g., tumor size ≥10 cm or high mitotic rate), who can have a greater than 50% chance of recurrence at 2 years in the absence of adjuvant therapy (Fig. 3C). Notably, the rate of recurrence on the imatinib arm (Fig. 2) appears to increase after approximately 18 months from surgery (i.e., 6 months following the completion of study therapy). This is consistent with a trial in metastatic GIST in which patients with responding or stable disease on imatinib developed tumor progression at a median of 6 months after randomization to discontinue therapy.21 It is likely that longer use of adjuvant imatinib may further prolong RFS. Ongoing trials based in Europe are testing 0 versus 2 years [European Organization for Research and Treatment of Cancer (EORTC) trial 62024], as well as 1 versus 3 years of adjuvant imatinib therapy [Scandanavian Sarcoma Group (SSG) trial XVIII)], to assess OS and RFS, respectively. The results are not expected for several years.
In metastatic GIST, imatinib achieves a partial response or stable disease in approximately 80% of patients and a median survival of nearly 60 months.13–16 Tumor mutation status predicts response to imatinib and survival. In a combined analysis of 1,640 patients with metastatic GIST treated on two phase 3 trials, patients with exon 11 mutations had the longest progression-free survival (PFS), those with an exon 9 mutation had the worst outcome, and patients without a KIT or PDGFRα mutation had an intermediate course.22 Mutation studies are ongoing in tumor specimens from the current study.
Acquired resistance is a frequent event in patients with metastatic GIST who initially respond to imatinib. Tumor progression occurs at a median of 18–24 months,15, 16 commonly from the development of a secondary mutation in KIT.23–25 Once clinical progression develops, higher dose imatinib or sunitinib (Sutent®, Pfizer Inc., New York) can restore tumor control in some patients, at least temporarily.26, 27 Currently, there are no other FDA approved agents for metastatic GIST. Thus, the possibility to delay or prevent recurrence with adjuvant treatment is critical given that acquired resistance to tyrosine kinase inhibitors eventually occurs in most patients with measurable metastatic GIST. How cumulative exposure to imatinib (i.e., in the adjuvant and metastatic settings combined) affects the development of imatinib resistance is unknown.
It is not surprising that OS between the study arms is comparable given the relatively short follow-up time and the crossover design of the study, which allowed patients assigned to the placebo arm to receive imatinib upon tumor recurrence. While imatinib is rarely curative in metastatic GIST,15, 16 it may eradicate residual microscopic disease in some patients following removal of the primary tumor. Longer patient follow-up is necessary to determine whether adjuvant imatinib increases the cure rate of surgery alone for localized, primary GIST. Quality of life instruments were not used in this study. The advantage of improved RFS by taking adjuvant imatinib must be weighed against the potential toxicity of the drug, even though it was generally well-tolerated.
Pediatric patients and patients with GISTs lacking KIT staining by immunohistochemistry were excluded from the present trial. Our findings are probably not applicable to GISTs in pediatric patients which typically lack KIT or PDGFRα mutations and seem to be more responsive to sunitinib than imatinib.28, 29 Our results may be relevant to the 4% of GISTs that lack KIT expression, which often contain a KIT or PDGFRα mutation and can respond to imatinib.30 It is likely that patients with certain mutations (i.e., PDGFRα exon 18 D842V) that are known to be insensitive to imatinib in vitro and in metastatic GIST may not benefit from adjuvant imatinib.
Only the starting dose of 400 mg/day of imatinib was tested in the current study. The recent meta-analysis of the two phase 3 studies in metastatic GIST showed that 800 mg compared to 400 mg per day did not alter OS but slightly improved PFS (34% vs. 30% at 3 years).22 In particular, patients with KIT exon 9 mutations treated with the higher dose had greater PFS. Further studies will be needed to determine whether doses greater than 400 mg/day should be used in the adjuvant setting.
With the advent of tyrosine kinase inhibitors, there are now effective agents against GIST. In the first phase 3 adjuvant trial of targeted therapy following the resection of localized, primary GIST, we have found that imatinib prolongs RFS. Our findings will impact the management of patients with primary GIST and may have relevance to the adjuvant use of other molecular agents for cancer.
Acknowledgments
This work was supported by Public Health Service Grants U10 CA076001 (ACOSOG) and CA94503 and CA102613 (RPD) from the National Cancer Institute, National Institutes of Health, and by Novartis. ACOSOG Z9001 was conducted through a contract between Novartis and NCI under CRADA 1111.1.
We are indebted to members of CTEP who made this trial possible. We thank Samuel A. Wells, Jr. M.D. and Vijaya Chadaram who were instrumental in the development and early conduct of this trial and Sue Budinger. Chris Corless M.D. Ph.D. provided assistance with pathologic review. Finally, we would like to recognize Linda McCall M.S. for performing the analyses and creating the tables and graphs.
Funding: NIH, Novartis Pharmaceuticals
Footnotes
The trial was endorsed by the Southwest Oncology Group (SWOG), Cancer and Leukemia Group B (CALGB), Eastern Cooperative Oncology Group (ECOG), and the National Cancer Institute of Canada (NCI-C), all of which participated through the Cancer Trials Support Unit (CTSU).
Drs. DeMatteo, Maki, Pisters, Blackstein, Blanke, von Mehren, Demetri, and Patel report receiving honoraria from Novartis and have served on Novartis advisory boards. No other conflicts of interest were reported.
The final manuscript was written by Dr. DeMatteo with substantial input from the authors. All authors approved the final manuscript. The views expressed are those of the authors and do not necessarily represent the official views of the National Cancer Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Tryggvason G, Gislason HG, Magnusson MK, Jonasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: The Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005 doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156(3):791–795. doi: 10.1016/S0002-9440(10)64946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 6.Debiec-Rychter M, Sciot R, Le CA, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 8.Dematteo RP, Heinrich MC, El Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33(5):466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 9.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215(1):68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dematteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 12.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 13.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 14.Blanke CD, Demetri GD, von MM, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 15.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 16.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Therapy Evaluation Program. Common terminology for adverse events version 3.0 (CTCAE) National Cancer Institute; 2003 . [(accessed April 10, 2008) 2008]. http://ctepcancergov/forms/CTCAEv3.pdf. [Google Scholar]
- 18.Friedlin B, Korn EL, George SL. Data monitoring and interim monitoring guidelines. Controlled Clinical Trials. 1999;20:395–407. doi: 10.1016/s0197-2456(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 19.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 20.Verweij J, Casali PG, Kotasek D, et al. Imatinib does not induce cardiac left ventricular failure in gastrointestinal stromal tumours patients: analysis of EORTC-ISG-AGITG study 62005. Eur J Cancer. 2007;43(6):974–978. doi: 10.1016/j.ejca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Blay JY, Le CA, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25(9):1107–1113. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 22.Van Glabbeke MM, Owzar K, Rankin C, Simes J, Crowley J GIST meta-analysis group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors (GIST): A meta-analysis based on 1,640 patients. J Clin Oncol. 2007;25:10004. [Google Scholar]
- 23.Chen LL, Trent JC, Wu EF, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004;64(17):5913–5919. doi: 10.1158/0008-5472.CAN-04-0085. [DOI] [PubMed] [Google Scholar]
- 24.Debiec-Rychter M, Cools J, Dumez H, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128(2):270–279. doi: 10.1053/j.gastro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11(11):4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 26.Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastrointestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41(12):1751–1757. doi: 10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 28.Prakash S, Sarran L, Socci N, et al. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005;27(4):179–187. doi: 10.1097/01.mph.0000157790.81329.47. [DOI] [PubMed] [Google Scholar]
- 29.Janeway KA, Matthews DC, Butrynski JE, et al. Sunitinib treatment of pediatric metastatic GIST after failure of imatinib. J Clin Oncol. 2006;24:9519. [Google Scholar]
- 30.Blackstein ME, Rankin R, Fletcher C, et al. Clinical benefit of imatinib in patients with metastatic gastrointestinal stromal tumors negative for the expression of CD117 in the S0033 trial. J Clin Oncol. 2005;23(9010) [Google Scholar]