Abstract
We present a novel solid-state NMR method for heteronuclear dipolar recoupling without decoupling. The method, which introduces the concept of exponentially modulated rf fields, provides efficient broadband recoupling with large flexibility with respect to hetero- or homonuclear applications, sample spinning frequency, and operation without the need for high-power 1H decoupling. For previous methods, the latter has been a severe source of sample heating which may cause detoriation of costly samples. The so-called EXPonentially mOdulated Recoupling Technique (EXPORT) is described analytically and numerically, and demonstrated experimentally by 1D 13C spectra and 2D 13C-15N correlation spectra of 13C,15N-labeled samples of GB1, ubiquitin, and fibrils of the SNNFGAILSS fragment of amylin. Through its flexible operation, robustness, and strong performance, it is anticipated that EXPORT will find immediate application for both hetero- and homonuclear dipolar recoupling in solid-state NMR of 13C,15N-labeled proteins and compounds of relevance in chemistry.
Keywords: Solid-State NMR, Dipolar recoupling without decoupling, proteins
Over the past few years solid-state NMR has demonstrated capability to undertake a long-standing need in structural biology, namely resolving atomic-resolution structure and dynamics information for so-called insoluble proteins that reside in, e.g., membranes,1–7 filaments,8 and amyloid fibrils.9–14 This has been facilitated by development of advanced instrumentation, isotope-labeling procedures,15–17 and pulse sequences extracting information from isotropic and anisotropic nuclear spin interactions to provide information about molecular structure and dynamics. Particular efforts have been devoted to the design of experiments which combines the high-resolution properties of magic-angle spinning (MAS) and recoupling methods to probe desired nuclear spin interactions while not using excessively long periods of strong rf irradiation.
To reduce sample detoriating effect of high-frequency rf heating focus has recently been devoted to the design of experiments that enable recoupling of dipolar interactions between low-γ nuclei without the need for intense 1H irradiation for decoupling of dipolar interactions to protons. Until now this has been realized for experiments recoupling homonuclear 13C-13C couplings, as introduced through the experiments CMAR,18 RFDR without decoupling,19,20 and variants of these.21 For the important class of heteronuclear recoupling experiments, being fundamental in solid-state NMR relying on 13C,15N-labeled compounds, broadband recoupling under fast MAS and high-field conditions in combination with released demands to decoupling remains a challenge.
In this Letter, we introduce a novel concept for dipolar recoupling taking advantage of multi-axis phase-modulated rf irradiation to enable recoupling of heteronuclear dipolar couplings with (a) extreme broadbandedness even at high field, (b) high efficiency through γ-encoded recoupling,22 (c) compensation for rf inhomogeneity, (d) operation at all relevant spinning frequencies, and (e) realization without high-power 1H decoupling. This is accomplished by introducing exponentially modulated rf fields which, with inspiration from recent multiple-field-oscillating recoupling methods,23,24 exploit the effect of two intertwined modulations to facilitate simultaneous 13C-15N (or 13C-13C) recoupling and 1H decoupling. The method will be referred to as the EXPonentially mOdulated Recoupling Technique (EXPORT).
The recoupling experiment, illustrated schematically in Fig. 1, is governed by the rf Hamiltonian
| (1) |
where Iq and Sq (q=x,y,z) represent Cartesian operators for the I and S spins, respectively, and BI, BS, CI, and CS amplitudes for the I- and S-spin components of the rf field. Although the method works equally well for homonuclear dipolar recoupling, we here restrict ourselves to the heteronuclear case with I=15N and S=13C. In pratice, the rf fields may be expessed in terms of an amplitude and phase . While the use of different CK values on the two rf channels offers interesting possibilities to adapt the rf fields most appropriately to the instrumentation and specific desires in terms of broadbandedness, we will for the sake of simplicity assume C=CI=CS.
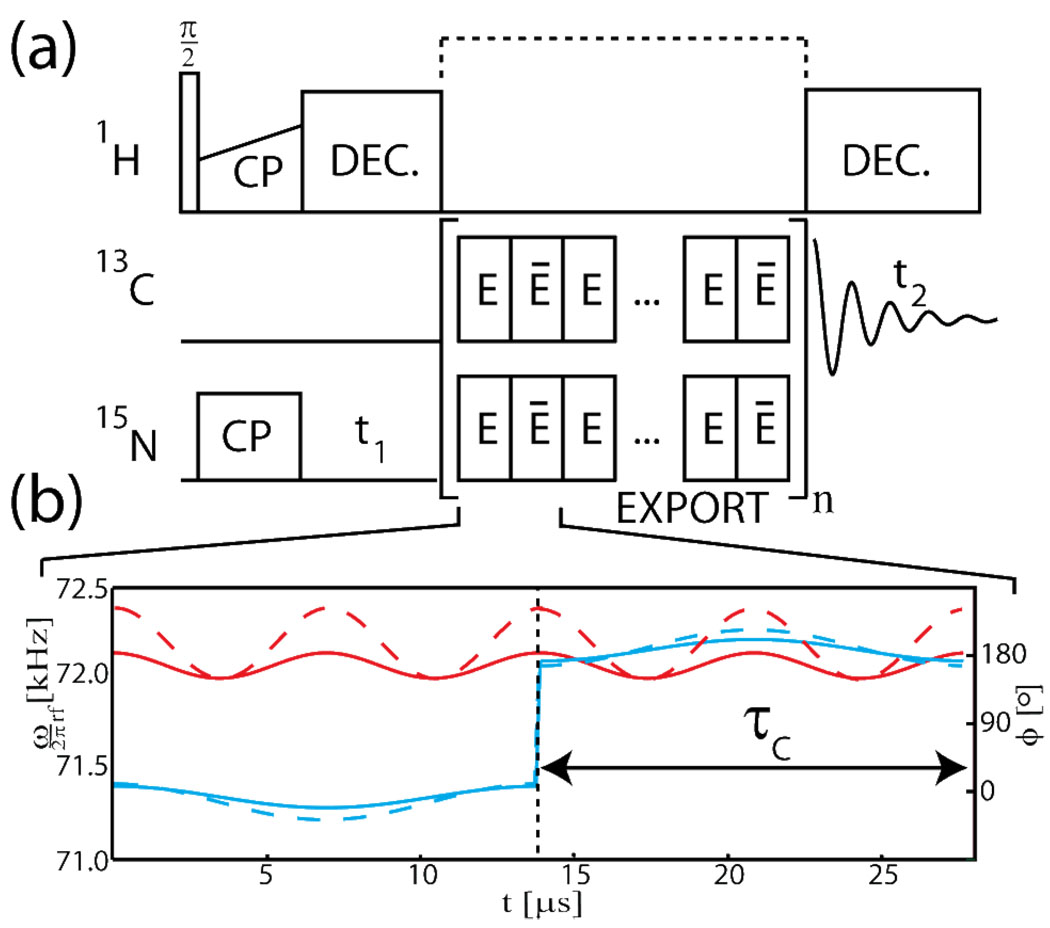
Figure 1.
(a) The EXPORT pulse sequence for heteronuclear dipolar recoupling without decoupling embedded in a typical 2D NCO/NCA chemical shift correlation experiment. (b) The amplitude (red) and phase (blue) modulation schemes of the basic elements with the 13C and 15N fields represented by solid and broken lines, respectively, for EXPORT with C=6ωr, BI=3ωr/8, BS=5ωr/8, and ωr/2π=12 kHz.
The impact of the rf field on chemical shift and dipolar couplings may be described by transforming the Hamiltonians for these interactions, HK (t) = ωK (t)Kz and HIS(t) = ωIS (t)2Iz Sz, respectively, into the interaction frame of the rf irradiation. By first transforming into the frame of the modulating field C(Ix+Sx) using F̃(t) = eiCt(Ix + Sx)Fe−iCt(Ix + Sx) and subsequently into the frame of the weak rf fields BIIy and BSSy using F͌(t) = eiBItIx eiBStSx F̃(t)ei̶BS tSx ei̶BItIx one arrives at
| (2) |
| (3) |
using the shorthand notation cx=cos(x) and sx=sin(x). By assuming C>>BI,BS,ωr, it is evident that all linear terms (eq. (2)) – covering chemical shift on the I and S spins as well as dipolar couplings to protons (the Hamiltonian is modified by multiplying with 2Hz, with Hz being the 1H Zeeman operator, and changing the angular frequency to that of the coupling) vanish to first-order upon averaging over the period 1/C. It can also be shown that the first and third of the three terms within the square bracket in eq. (3) does not lead to recoupling. Using with the Fourier coefficients given elsewhere,25 the second term lead to the first-order average over the period 1/|BI−BS|:
| (5) |
using denoting the dipole-dipole coupling in angular units and βPR and γPR the polar an azimutal angles, respectively, relating the internuclear axis to the rotor axis. It is evident that γPR-encoded22 zero- or double-quantum dipolar recoupling (in a frame tilted by π/2 around Iy and Sy) may be obtained by adjusting the sum and difference of the recoupling BI and BS field amplitudes to match nωr (n=±1 or ±2). Efficient cancellation of chemical shift and couplings to protons and avoiding interference with recoupling require that the decoupling field C is substantially larger than the B fields. Effects from rf inhomogeneity may be reduced by phase-alternating ±C for each τ C=τr/C as illustrated by the E and E̅ elements in Fig. 1.
Independent adjustment of the recoupling and decoupling fields opens up the possibility to perform experiments with (small values of C) or without (large values of C) 1H decoupling with great flexibility on the spinning frequency. This solves a major problem for heteronuclear dipolar recoupling, where typical combinations of recoupling fields at the low-γ spins species and simultaneous need for high-power 1H decoupling (typical recommendation: ωrf(1H) > 2.5ωrf(I,S)) may lead to excessively strong 1H rf fields in the regime of medium to fast sample spinning with the inherent risk of sample damage. We note that at very spinning frequencies (>35 kHz), 1H decoupling with low rf field strengths may be feasible.26,27
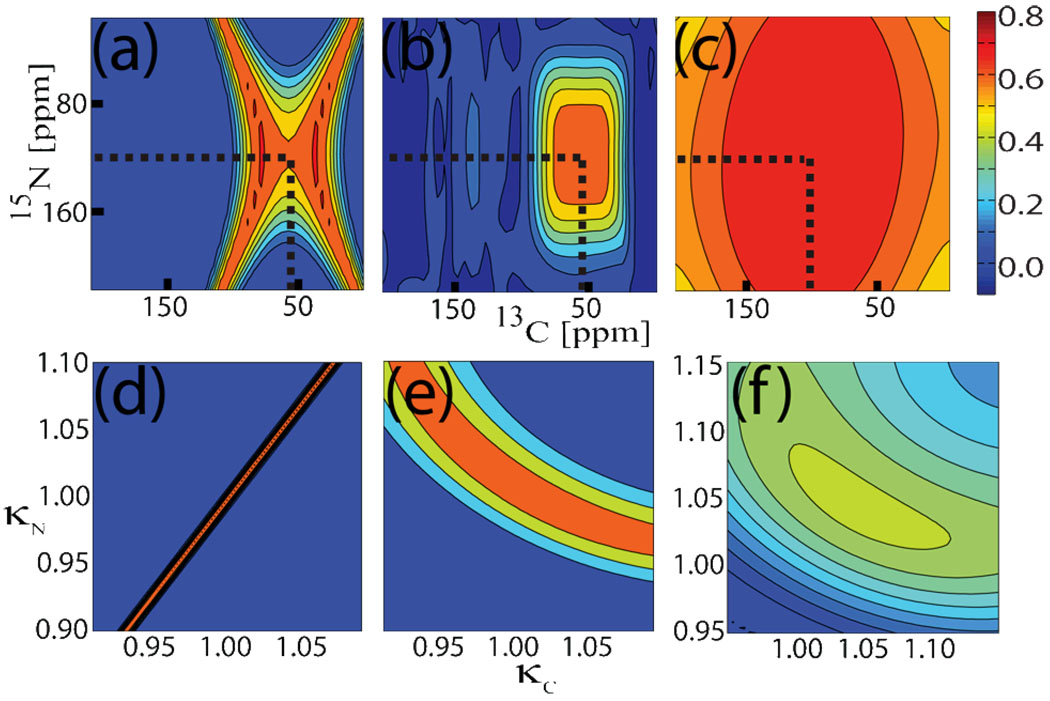
Through numerical simulations of 15N to 13Cα coherence transfer in a typical 15N-13Cα spin-pair system at 16.4 T using MAS with ωr/2π=12 kHz, Fig. 2c demonstrates that EXPORT (C=6ωr, BI=3ωr/8, and BS=5ωr/8) is substantially more robust with respect to resonance offsets than standard double-cross-polarization29 (DCP, ωrf,N=3ωr, ωrf,C=4ωr) (Fig. 2a) and the deliberately bandselective OCNCA experiment (Fig. 2b).28 EXPORT is also superior to previous experiments with respect to rf mismatch (and thereby rf inhomogenity) as illustrated in Figs. 2d–2f by numerical simulations for DCP (Fig. 2d), EXPORT with the rf wave digitized in 100 (Fig. 2e) and 20 (Fig. 2f) steps over each rotor period. Figures 2e and 2f also illustrate an important issue of EXPORT, namely the need for good digitization of the C-field phase modulation. Too low digitization may degrade the sensitivity of the experiment, but conversely improve the robustness towards rf inhomogeneity.
Figure 2.
15N to 13Cα coherence transfer efficiencies calculated for EXPORT using the parameters in Fig. 1b. (a–c) 2D 15N vs 13C offset plots for EXPORT (c), an OCNCA optimal control sequence28 (b), and DCP (a)29. (d–f) 2D 15N vs 13C rf field strength plots (scaling factors relative to the nominal values) for DCP (d), EXPORT with high digitization of the rf field (100 points over 1 rotor period) (e), and EXPORT with lower digitization (20 points over 1 rotor period) (f). Simulations were made using SIMPSON25 with parameters30 for a directly bonded 15N-13Cα spin system, powder averaging with 5 γCR and 144 REPULSION angles,31 and a spinning frequency of 12 kHz at 16.4 T. Broken lines mark carrier frequencies.
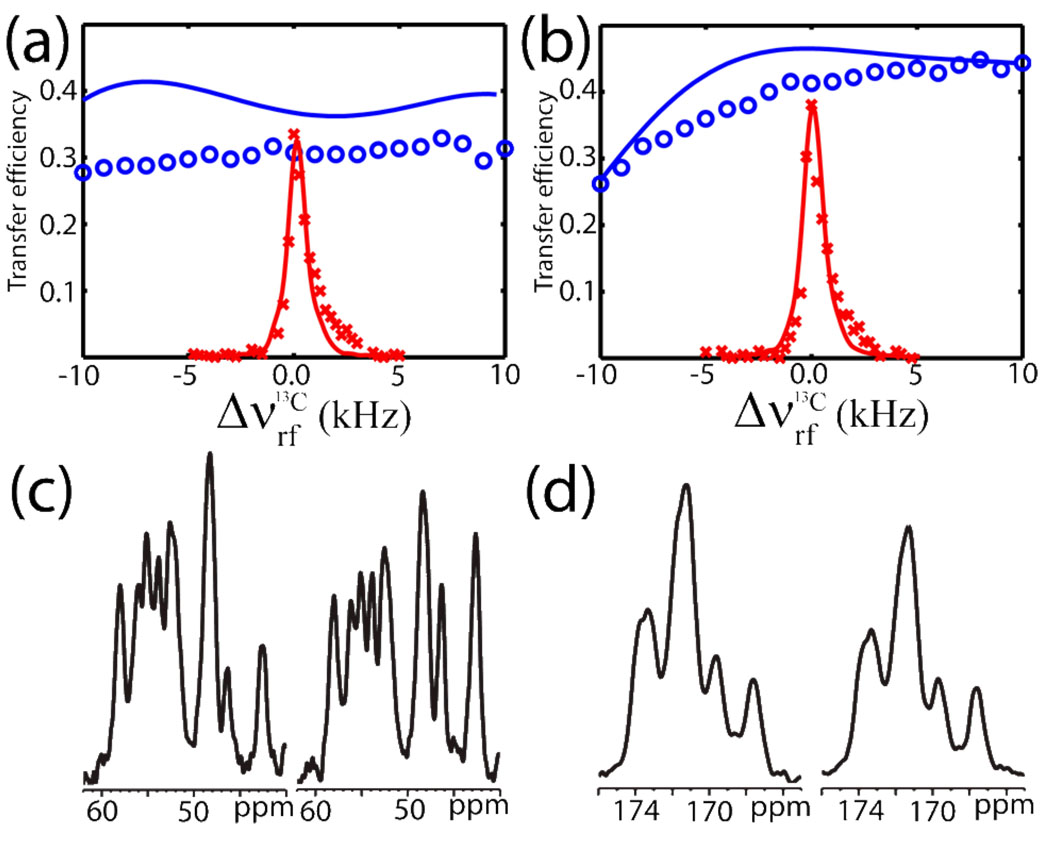
Experimental data in Fig. 3, obtained at 16.4 T for a fibrillar sample of the hIAPP20-29 decapeptide (SNNFGAILSS) of amylin with uniform 13C,15N labeling of the FGAIL stretch using a 1D version of the pulse sequence in Fig. 1 with 11.9 kHz spinning, clearly reinforces that EXPORT in addition to the clear advantage of recoupling without decoupling facilitates experimental realization through robustness towards rf mismatch and inhomogeneity. We note that the doubled number of peaks is a result of antiparallel fibril formation.13 Figure 3 illustrates experimentally and numerically the consequences of rf mismatch on the 13C rf channel for 15N to 13Cα (NCA, Fig. 3a) and 15N to 13C’ (NCO, Fig. 3b) coherence transfers for EXPORT (open circles; C=7ωr, BI=3ωr/8, BS=5ωr/8, no decoupling) and a carefully optimized DCP sequence (crosses; ωrf,C/(2π) = 50.2 kHz, ωrf,N/(2π) = 39.3 kHz with 120 kHz CW 1H decoupling). Both experiments and simulations took into account 5% Lorentzian rf inhomogeneity, which along with a relatively course digitization (time steps of 1.2 µs) reduces the overall transfer to 0.4–0.5 in this specific case. We note that the corresponding homonuclear experiments are considerably less influenced by digitization and rf inhomogeneity effects. Nonetheless, the experiments (including the representative spectra in Figs. 3c and 3d) and the good numerical reproductions clearly highlight the robustness of EXPORT relative to DCP with respect to rf field variations, which removes one of the major drawbacks of DCP, namely the need for very precise calibrations and long-term stability of the involved rf field strengths. It should be mentioned that under the present modest sample-spinning conditions some intensity loss is observed for the methylene groups using EXPORT without decoupling, which is understandable considering the strong couplings present in this system and the associated general difficulties in decoupling of methylene carbons.
Figure 3.
Experimental (signal integrals for the target spin spectral region) and simulated 15N to 13C’ (a) and 15N to 13Cα (b) coherence transfer efficiencies for DCP (experiment: red crosses, simulation: red line) and EXPORT (experiment: blue circles, simulation: blue line) as function of the 13C rf field strength mismatch. Experimental spectra were obtained for U-13C,15N-labeled FGAIL in a SNNFGAILSS fibril sample. Representative 13C spectra following (c) 15N to 13Cα and (d) 15N to 13C’ transfer for EXPORT (left) and DCP (right). In accord with Fig. 1a, the spectra were recorded using CP for the initial 1H-15N transfer and SPINAL-64 decoupling32 (80 kHz) was used during acqusition. All spectra were recorded at 11.9 kHz spinning with carrier frequencies at 120 ppm for 15N and 50/172 ppm for NCA/NCO transfer. DCP used ωrf,C/2π = 50.2 kHz, ωrf,N/2π = 39.3 kHz, and 120 kHz 1H decoupling. EXPORT used C=7ωr, BI=3ωr/8, BS=5ωr/8, and no 1H decoupling.
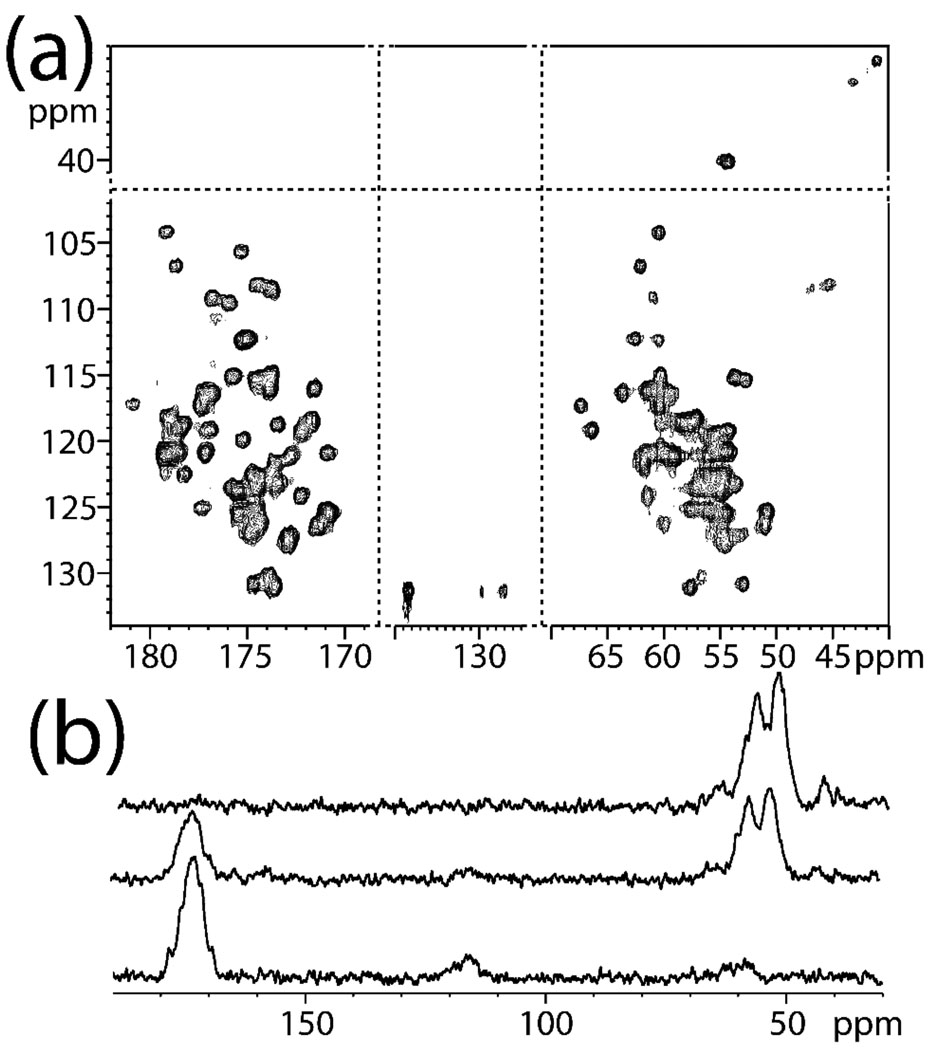
Figure 4a illustrates the use of EXPORT to obtain 15N-13C 2D chemical shift correlation for a uniformly-13C,15N-labelled sample of the β1-domain of the immunoglobulin binding protein G (GB1)33 at 23.81 kHz spinning without 1H decoupling during the 13C-15N mixing. The static fields were adjusted to C=3ωr (i.e., 71.5 kHz) with the 13C rf carrier between the 13Cα and 13C’ regions leading to efficient transfer to both types of spins. We note that recoupling without decoupling may alternatively be obtained using DCP or adiabatic variants with comparatively strong rf fields on the 13C and 15N channels, although with higher sensitivity towards rf inhomogeneity and mismatch. In Fig. 4b, we experimentally demonstrate the broadband recoupling of 13C,15N dipolar interactions for EXPORT on U-13C,15N-ubiquitin at 10.02 kHz spinning, using C=3ωr, BI=3ωr/8, and BS=5ωr/8. By varying the 13C carrier frequency, polarization can be directed to both 13Cα’s and 13C`’s (top) or in a band-selective manner to either 13Cα’s (middle) or 13C`’s (bottom). The spectra were obtained using relatively weak 13C,15N rf fields (~31 kHz) and standard 1H CW decoupling (90 kHz) during the heteronuclear transfer.
Figure 4.
Experimental spectra for U-13C,15N-labeled samples of (a) GB1 and (b) ubiquitin recorded at 16.4 T using EXPORT for 15N-13C transfer with C =3ωr, BI=3ωr/8, BS=5ωr/8, and τmix=2.35 ms. In accord with Fig. 1a, the spectra were recorded using CP for the initial 1H-15N transfer. SPINAL-64 decoupling (70 kHz) was used in the detection periods. The 2D spectrum (a) used 23.81 kHz sample spinning without 1H decoupling during EXPORT, 560 points in the indirect and 4096 point in the direct dimensions. The 1D spectra (b) used 10.02 kHz spinning, carrier frequencies at 40 (top), 120 (middle), and 180 (bottom) ppm, and 90 kHz 1H decoupling during EXPORT.
In conclusion, we have introduced the concept of exponentially modulated dipolar recoupling and demonstrated its strong capabilities to accomplish 13C-15N dipolar recoupling at varying MAS frequencies with or without 1H decoupling. Through its superior performance relative to previous methods, and the straightforward use of the EXPORT principle in numerous other applications, we anticipate that the presented methods will find widespread applications for solid-state NMR spectroscopy of biological molecules, as well as problems in materials chemistry where heteronuclear correlation experiments have broad applicability.
ACKNOWLEDGMENT
We acknowledge support from the Danish National Research Foundation, the Danish Natural Science Research Council, and the Danish Center for Scientific Computing. CMR acknowledges support from the National Institutes of Healt (grant no. NIH R01-GM073770).
REFERENCES
- 1.Etzkorn M, Martell S, Andronesi OC, Seidel K, Engelhard M, Baldus M. Secondary Structure, Dynamics, and Topology of a Seven-Helix Receptor in Native Membranes, Studied by Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2007;46:459–462. doi: 10.1002/anie.200602139. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Berthold DA, Gennis RB, Rienstra CM. Chemical shift assignment of the transmembrane helices of DsbB, a 20-kDa integral membrane enzyme, by 3D magic-angle spinning NMR spectroscopy. Protein Science. 2008;17:199–204. doi: 10.1110/ps.073225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiller M, Higman VA, Jehle S, van Rossum BJ, Kuhlbrandt W, Oschkinat H. [2,3-C-13]-labeling of aromatic residues-getting a head start in the magic-angle-spinning NMR assignment of membrane proteins. J. Am. Chem. Soc. 2008;130:408–409. doi: 10.1021/ja077589n. [DOI] [PubMed] [Google Scholar]
- 4.Vosegaard T, Kamihira-Ishijima M, Watts A, Nielsen NC. Helix conformations in 7TM membrane proteins determined using oriented-sample solid-state NMR with multiple residue-specific N-15 labeling. Biophys. J. 2008;94:241–250. doi: 10.1529/biophysj.107.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi MG, Cross TA, Zhou HX. Conformational heterogeneity of the M2 proton channel and a structural model for channel activation. Proc. Natl. Acad. Sci. 2009;106:13311–13316. doi: 10.1073/pnas.0906553106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traaseth NJ, Shi L, Verardi R, Mullen DG, Barany G, Veglia G. Structure and topology of monomeric phospholamban in lipid membranes determined by a hybrid solution and solid-state NMR approach. Proc. Natl. Acad. Sci. 2009;106:10165–10170. doi: 10.1073/pnas.0904290106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorieau JL, Day LA, McDermott AE. Conformational dynamics of an intact virus: Order parameters for the coat protein of Pf1 bacteriophage. Proc. Natl. Acad. Sci. 2008;105:10366–10371. doi: 10.1073/pnas.0800405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. A structural model for Alzheimer's beta -amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Wel PCA, Lewandowski JR, Griffin RG. Solid-state NMR study of amyloid nanocrystals and fibrils formed by the peptide GNNQQNY from yeast prion protein Sup35p. J. Am. Chem. Soc. 2007;129:5117–5130. doi: 10.1021/ja068633m. [DOI] [PubMed] [Google Scholar]
- 11.Iwata K, Fujiwara T, Matsuki Y, Akutsu H, Takahashi S, Naiki H, Goto Y. 3D structure of amyloid protofilaments of beta(2)-microglobulin fragment probed by solid-state NMR. Proc. Natl. Acad. Sci. 2006;103:18119–18124. doi: 10.1073/pnas.0607180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen JT, Bjerring M, Jeppesen MD, Pedersen RO, Pedersen JM, Hein KL, Vosegaard T, Skrydstrup T, Otzen DE, Nielsen NC. Unique Identification of Supramolecular Structures in Amyloid Fibrils by Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2009;48:2118–2121. doi: 10.1002/anie.200804198. [DOI] [PubMed] [Google Scholar]
- 14.Walsh P, Simonetti K, Sharpe S. Core Structure of Amyloid Fibrils Formed by Residues 106–126 of the Human Prion Protein. Structure. 2009;17:417–426. doi: 10.1016/j.str.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 15.LeMaster DM, Kushlan DM. Dynamical mapping of E-coli thioredoxin via C-13 NMR relaxation analysis. J. Am. Chem. Soc. 1996;118:9255–9264. [Google Scholar]
- 16.Hong M, Jakes K. Selective and extensive C-13 labeling of a membrane protein for solid-state NMR investigations. J. Biomol. NMR. 1999;14:71–74. doi: 10.1023/a:1008334930603. [DOI] [PubMed] [Google Scholar]
- 17.Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature. 2002;420:98–102. doi: 10.1038/nature01070. [DOI] [PubMed] [Google Scholar]
- 18.De Paepe G, Bayro MJ, Lewandowski J, Griffin RG. Broadband homonuclear correlation spectroscopy at high magnetic fields and MAS frequencies. J. Am. Chem. Soc. 2006;128:1776–1777. doi: 10.1021/ja0550430. [DOI] [PubMed] [Google Scholar]
- 19.Ishii Y. C-13-C-13 dipolar recoupling under very fast magic angle spinning in solid-state nuclear magnetic resonance: Applications to distance measurements, spectral assignments, and high-throughput secondary-structure determination. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- 20.Bayro MJ, Ramachandran R, Caporini MA, Eddy MT, Griffin RG. Radio frequency-driven recoupling at high magic-angle spinning frequencies: Homonuclear recoupling sans heteronuclear decoupling. J. Chem. Phys. 2008;128:052321. doi: 10.1063/1.2834736. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Bayro MJ, Griffin RG, Khaneja N. Dipolar recoupling in solid state NMR by phase alternating pulse sequences. J. Magn. Reson. 2009;197:145–152. doi: 10.1016/j.jmr.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen NC, Bildsoe H, Jakobsen HJ, Levitt MH. Double-Quantum Homonuclear Rotary Resonance - Efficient Dipolar Recovery in Magic-Angle-Spinning Nuclear-Magnetic-Resonance. J. Chem. Phys. 1994;101:1805–1812. [Google Scholar]
- 23.Khaneja N, Nielsen NC. Triple oscillating field technique for accurate distance measurements by solid-state NMR. J. Chem. Phys. 2008;128:015103. doi: 10.1063/1.2816140. [DOI] [PubMed] [Google Scholar]
- 24.Straaso LA, Bjerring M, Khaneja N, Nielsen NC. Multiple-oscillating-field techniques for accurate distance measurements by solid-state NMR. J. Chem. Phys. 2009;130:225103. doi: 10.1063/1.3147010. [DOI] [PubMed] [Google Scholar]
- 25.Bak M, Rasmussen JT, Nielsen NC. SIMPSON: A general simulation program for solid-state NMR spectroscopy. J. Magn. Reson. 2000;147:296–330. doi: 10.1006/jmre.2000.2179. [DOI] [PubMed] [Google Scholar]
- 26.Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LWM, Past J, Samoson A, Ishii Y. Nanomole-scale protein solid-state NMR by breaking intrinsic H-1 T-1 boundaries. Nature Methods. 2009;6:215–218. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholz I, Hodgkinson P, Meier BH, Ernst M. Understanding two-pulse phase-modulated decoupling in solid-state NMR. J. Chem. Phys. 2009;130:114510. doi: 10.1063/1.3086936. [DOI] [PubMed] [Google Scholar]
- 28.Kehlet C, Bjerring M, Sivertsen AC, Kristensen T, Enghild JJ, Glaser SJ, Khaneja N, Nielsen NC. Optimal control based NCO and NCA experiments for spectral assignment in biological solid-state NMR spectroscopy. J. Magn. Reson. 2007;188:216–230. doi: 10.1016/j.jmr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer J, Mckay RA, Stejskal EO. Double-Cross-Polarization Nmr of Solids. J. Magn. Reson. 1979;34:443–447. [Google Scholar]
- 30.Bak M, Schultz R, Vosegaard T, Nielsen NC. Specification and visualization of anisotropic interaction tensors in polypeptides and numerical simulations in biological solid-state NMR. J. Magn. Reson. 2002;154:28–45. doi: 10.1006/jmre.2001.2454. [DOI] [PubMed] [Google Scholar]
- 31.Bak M, Nielsen NC. REPULSION, a novel approach to efficient powder averaging in solid-state NMR. J. Magn. Reson. 1997;125:132–139. doi: 10.1006/jmre.1996.1087. [DOI] [PubMed] [Google Scholar]
- 32.Fung BM, Khitrin AK, Ermolaev K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 33.Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM. Magic-angle spinning solid-state NMR spectroscopy of the beta1 immunoglobulin binding domain of protein G (GB1): 15N and 13C chemical shift assignments and conformational analysis. J. Am. Chem. Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]