Abstract
Early-onset cannabis use is widespread in many countries and might cause later onset of depression. Sound epidemiologic data across countries are missing. The authors estimated the suspected causal association that links early-onset (age <17 years) cannabis use with later-onset (age ≥17 years) risk of a depression spell, using data on 85,088 subjects from 17 countries participating in the population-based World Health Organization World Mental Health Survey Initiative (2001–2005). In all surveys, multistage household probability samples were evaluated with a fully structured diagnostic interview for assessment of psychiatric conditions. The association between early-onset cannabis use and later risk of a depression spell was studied using conditional logistic regression with local area matching of cases and controls, controlling for sex, age, tobacco use, and other mental health problems. The overall association was modest (controlled for sex and age, risk ratio = 1.5, 95% confidence interval: 1.4, 1.7), was statistically robust in 5 countries, and showed no sex difference. The association did not change appreciably with statistical adjustment for mental health problems, except for childhood conduct problems, which reduced the association to nonsignificance. This study did not allow differentiation of levels of cannabis use; this issue deserves consideration in future research.
Keywords: cannabis, depression, mental health, world health
Recreational cannabis use occurs across the globe (1). In recent decades, the age of initiation has declined, and concern about the public health impact has mounted (1, 2). Largely based on evidence from Western countries, various reviews have concentrated on a suspected association between cannabis use and psychosis (3–5). Statistically significant associations between cannabis use and anxiety and major depressive symptoms or disorders have been found in some high-income countries (6–14), although several studies have failed to identify such a relation (15–22). Brook et al. (23) proposed that the risk of depression increases when cannabis use starts early. Schneider (24) suggested a mechanism for this potential age-specific association, namely that cannabis onset during puberty exacerbates morphologic and behavioral disturbances not seen with adult-onset cannabis use.
Here, with community samples from diverse countries, we estimated whether cannabis onset by the middle of adolescence (before age 17 years) is followed by greater risk of first onset of a depression spell, with due attention to sex and age variations. We used a local area matching approach (25) to constrain the effects of geographic variation in enforcement of cannabis laws, local jurisdictional variation in subnational law, and other socially shared local area variations that might confound cannabis-depression estimates and which also account for potential geographic variation in odds of exposure to cannabis use (26). Furthermore, we considered an array of possibly confounding mental health problems, including childhood conduct problems. Childhood conduct problems and early rule violations have been described to precede early peer rejection, affiliation with drug-using peers, early-onset cannabis use, and subsequent low self-esteem and mood disturbances (27, 28) and thus are important confounding variables.
To our knowledge, this study is the largest study of this issue to date and the first to examine the association between early-onset cannabis use and risk of later depressive spells across 17 countries with varied economic, social, and cultural norms.
MATERIALS AND METHODS
Participants
Data were obtained from cross-sectional surveys carried out at 18 sites within the World Mental Health Survey (WMHS) Consortium during 2001–2005: the Americas (Colombia, Mexico, the United States), Europe (Belgium, France, Germany, Italy, the Netherlands, Spain, Ukraine), the Middle East and Africa (Israel, Lebanon, Nigeria, South Africa), Asia (Japan and 2 separate surveys carried out in Beijing and Shanghai, China), and Oceania (New Zealand). The minimum age of subjects was generally 18 years. Table 1 provides an overview of each site's sample, methods, and recruitment results. Details about consistent use of standardized interview translation protocols, training procedures, and field quality control monitoring for reduction of between-site variation in data quality and institutional review board approval of the participation of human subjects can be found elsewhere (29; http://www.hcp.med.harvard.edu/wmh). Sample sizes ranged from 2,372 (the Netherlands) to 12,992 (New Zealand), with a total of 85,088 participants. Response rates averaged 70%, ranging upward to 88% (Colombia).
Table 1.
Cross-sectional Survey and Sample Characteristics for Cases With a Depression Spell, Noncases, and Local Area Risk Sets, World Health Organization World Mental Health Survey Initiative, 2001–2005a
| Sample Characteristic |
Depression Spell With First Onset at Age 17 Years or Older |
||||||
| Region and Country | Survey | Field Dates | Response Rate, % | Survey Sample Size, no. | No. of Cases | No. of Noncases | No. of Risk Sets |
| Americas | |||||||
| Colombia | NSMH | 2003 | 88 | 4,426 | 483 | 3,748 | 32 |
| Mexico | M-NCS | 2001–2002 | 77 | 5,782 | 468 | 5,125 | 58 |
| United States | NCS-R | 2002–2003 | 71 | 9,282 | 1,299 | 3,695 | 42 |
| Europe | |||||||
| Belgium | ESEMeD | 2001–2002 | 51 | 2,419 | 388 | 588 | 23 |
| France | ESEMeD | 2001–2002 | 46 | 2,894 | 613 | 683 | 27 |
| Germany | ESEMeD | 2002–2003 | 58 | 3,555 | 381 | 868 | 33 |
| Italy | ESEMeD | 2001–2002 | 71 | 4,712 | 496 | 1,161 | 42 |
| Netherlands | ESEMeD | 2002–2003 | 56 | 2,372 | 427 | 551 | 23 |
| Spain | ESEMeD | 2001–2002 | 79 | 5,473 | 717 | 1,266 | 48 |
| Ukraine | CMDPSD | 2002 | 78 | 4,725 | 642 | 860 | 38 |
| Middle East and Africa | |||||||
| Israel | INHS | 2002–2004 | 73 | 4,859 | 298 | 4,141 | 61 |
| Lebanon | LEBANON | 2002–2003 | 70 | 2,857 | 317 | 636 | 29 |
| Nigeria | NSMHW | 2002–2004 | 79 | 6,752 | 238 | 1,798 | 53 |
| South Africa | SASH | 2002–2003 | 87 | 4,351 | 429 | 3,453 | 61 |
| Asia | |||||||
| Japan | WMHJ | 2002–2003 | 51 | 2,436 | 222 | 1,029 | 39 |
| China | B-WMH, S-WMH | 2002–2003 | 75 | 5,201 | 191 | 1,390 | 46 |
| Oceania | |||||||
| New Zealand | NZMHS | 2004–2005 | 73 | 12,992 | 2,038 | 10,079 | 166 |
| Total | 70 | 85,088 | 9,647 | 41,071 | 821 | ||
Abbreviations: B-WMH, Beijing World Mental Health Survey; CMDPSD, Ukraine Comorbid Mental Disorders during Periods of Social Disruption; ESEMeD, European Study of the Epidemiology of Mental Disorders; INHS, Israel National Health Survey; LEBANON, Lebanese Evaluation of the Burden of Ailments and Needs of the Nation; M-NCS, Mexico National Comorbidity Survey; NCS-R, US National Comorbidity Survey Replication; NSMH, Colombian National Study of Mental Health; NSMHW, Nigerian Survey of Mental Health and Wellbeing; NZMHS, New Zealand Mental Health Survey; S-WMH, Shanghai World Mental Health Survey; SASH, South Africa Health Survey; WMHJ, World Mental Health Japan Survey.
Estimates were based on data obtained from 17 countries participating in the World Mental Health Surveys Consortium.
Local area matching within each site
The WMHS sampling design makes it possible to form matched local area risk sets based upon how a country's multistage area probability sample was constructed. For example, at the Beijing site, sampling was done from lists of neighborhoods organized around local area committees of the Communist Party. In the United States, some sampling strata were counties or combinations of counties (30). Via conditioning on these strata, this “matching” within a local area risk set holds constant shared local area variables that otherwise might confound cannabis-mood associations and might be difficult to measure directly (e.g., prevailing cannabis smoking norms, levels of cannabis availability, police presence, levels of socially shared anomie, socioeconomic status).
Measures
Composite International Diagnostic Interview.
All assessments were from the WMHS Composite International Diagnostic Interview (CIDI), version 3.0, a fully structured diagnostic interview for evaluation of psychiatric conditions (31, 32). Within this assessment, participants were asked whether they had ever smoked cannabis and whether they had ever experienced mood disturbances, including first onset of a depression spell as described below. The CIDI 3.0 starts with a screening section, with key questions for most disorders. The reason for this is to reduce interview duration, because of the large number of disorders evaluated and the large number of questions per section, and to minimize the possibility that respondents will learn how to shorten the interview by answering key questions negatively if these questions are asked each time at the beginning of a disorder section. Participants responding affirmatively to a key question in this screening section are administered the section of the CIDI pertaining to that disorder.
Except for Israel and South Africa, where all respondents were administered the full interview, investigators in all WMHS countries conducted subsampling for a 2-part CIDI that reduced respondent burden, such that 100% completed part I (core standardized item sets) but a smaller probability subsample completed part II (noncore item sets) (29). For survey analysis purposes and to account for differential selection probabilities, each participant is assigned a weight based on the inverse of the probability of selection for the survey sample and for each part of the CIDI.
Assessment of first depression spell at or after age 17 years.
A “depression spell” refers to a negative and distressing change in mood, manifest as feeling sad, discouraged, or uninterested, that is present throughout most of each day and nearly every day for at least 2 weeks, accompanied by at least 1 other mood problem (problems with sleep, appetite, energy, ability to concentrate and remember, or self-worth). This definition of a depression spell is less strict than the criteria used for major depressive disorder and major depressive episode in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (33). Furthermore, for the depression spell, no diagnostic hierarchies or exclusion rules can thwart attempts to understand cannabis-induced mood disturbances, as occurs when current major depression diagnostic criteria with exclusion rules are fully implemented. Indeed, variations in the handling of diagnostic hierarchies may account for some observed variability in estimates regarding the cannabis-major depression association (34).
Based on the CIDI assessment, participants experiencing such a depression spell were asked about age of onset; 9,647 qualified as having experienced onset at or after age 17 years. These “cases” were matched within 821 local areas to a total of 41,071 noncase “controls” who had never experienced such a spell (Table 1). Respondents whose first depression spell occurred before age 17 years and respondents younger than age 17 years were excluded.
Assessment of early-onset cannabis use.
Valid estimation of the cannabis-depression hypothesis requires earlier onset of cannabis use and later onset of a depression spell. Prior publications helped to set the age of initiation of cannabis use at a threshold of 16 years (34–38). The assessment asked, “Have you ever used either marijuana or hashish, even once?” and “How old were you the first time you used marijuana or hashish?” Cannabis onset age before 17 years was set by responses to these CIDI items.
Assessment of other mental health problems.
Other mental health problems or behaviors, such as persistent adult cannabis use and tobacco smoking, might be related to the first onset of a depression spell, temporally subsequent to early-onset cannabis use. Therefore, in analysis of the association between early-onset cannabis use and first onset of a depression spell, we took these variables into account. The resulting estimates of the association between early-onset cannabis smoking and an adult-onset depression spell might well be downwardly biased in these exploratory analyses, as would be the case if persistent adult cannabis smoking or adult tobacco smoking actually lay on mediational pathways leading from early cannabis use to a later depression spell. Data on all covariates of interest were taken from CIDI standardized items, including tobacco smoking, which almost always preceded first use of cannabis in these data (1).
Persistent adult cannabis use was measured via assessment of cannabis use during the 12 months prior to assessment. Adult cannabis use was not assessed in the European countries, except for Ukraine. Other mental health problems were measured by means of the lifetime symptoms in the CIDI screening section: fear and panic attack; other panic symptoms; manifestations of manic spells; excessive irritability, grumpiness, or bad mood; worry; nervous or anxious feelings; miscellaneous phobia-like fears (e.g., an irrational fear of crowds, animals, insects, blood, closed spaces, high places, or flying); shyness; childhood problems with attention; childhood restlessness/fidgetiness; anger in childhood or adolescence; and separation anxiety. Furthermore, lifetime alcohol use was assessed from the CIDI alcohol section.
Precannabis violations of social norms and conduct problems might confound estimation of any cannabis-depression association. Therefore, we created an “early conduct problems” index (sum score) from the multiple-item assessment of childhood/early adolescence conduct problems from CIDI part II (see Appendix Table 1). These problems were assessed in all countries except Israel, South Africa, Japan, and New Zealand.
Data analysis
Associations linking early-onset cannabis use and a later-onset depression spell were estimated via odds ratios from conditional logistic regression analyses with local area-matched cases and controls, with and without statistical adjustment for covariates, using STATA, version 10, “clogit” commands (Stata Corporation, College Station, Texas) that yield regression slope coefficients. After exponentiation, odds ratios estimate risk ratios. The complex survey sample structure is accommodated by local area matching within risk sets (25).
RESULTS
Association between early-onset cannabis use and later depression spells
Early-onset cannabis use was moderately associated with later onset of a depression spell at a bivariate level (risk ratio (RR) = 1.2, 95% confidence interval (CI): 1.1, 1.3; P < 0.001; Table 2). Females were more likely than males to have experienced a depression spell after age 16 years (RR = 1.9, 95% CI: 1.8, 2.0; P < 0.001). Age was also associated with a later-onset depression spell, with an age-squared term being required to address residual nonlinearity (both P’s < 0.05; higher-order polynomials of age were not required). Tobacco smoking and a depression spell after age 16 years were associated too modestly for tobacco to function as an important confounder here (RR = 1.2, 95% CI: 1.1, 1.2; P < 0.001).
Table 2.
Estimated Association Between Early-Onset (Age <17 Years) Cannabis Use and Later Onset (Age ≥17 Years) of a Depression Spell Before Adjustment for Covariates, World Health Organization World Mental Health Survey Initiative, 2001–2005a
| Depression Spell Status | Casesb (n = 9,647) |
Noncase Controlsb (n = 41,071) |
Estimated Risk Ratio | 95% Confidence Interval | P Value | ||
| No. | % | No. | % | ||||
| Main covariates under study | |||||||
| Early-onset cannabis use | |||||||
| Yes | 904 | 9 | 2,955 | 7 | 1.2 | 1.1, 1.3 | <0.001 |
| No (referent) | 8,743 | 91 | 38,116 | 93 | 1.0 | ||
| Sex | |||||||
| Female | 6,551 | 68 | 22,295 | 54 | 1.9 | 1.8, 2.0 | <0.001 |
| Male (referent) | 3,096 | 32 | 18,776 | 46 | 1.0 | ||
| Age and age squaredc | 9,647 | 100 | 41,071 | 100 | 1.0 | 1.0, 1.0 | <0.001 |
| Primary additional covariate under study | |||||||
| History of tobacco smoking | |||||||
| Yes | 5,040 | 52 | 18,968 | 46 | 1.2 | 1.1, 1.2 | <0.001 |
| No (referent) | 4,605 | 48 | 22,083 | 54 | 1.0 | ||
Estimates were based on data obtained from 17 countries participating in the World Mental Health Surveys Consortium.
The mean age of cases was 46.2 years (standard deviation, 15.3); the mean age of controls was 43.0 years (standard deviation, 17.0).
After age was centered on its mean value for the sample, the estimated age-related log odds difference changes in relation to the following equation with each 1-year increase in age: 0.0121918 × (age) + (−0.0009107) × (age2).
Crude and sex- and age-adjusted risk ratios for the association between early-onset cannabis use and a later spell of depression are shown in Table 3, according to country. The overall sex- and age-adjusted risk ratio estimate (hereafter called the adjusted RR (aRR)) was 1.5 (95% CI: 1.4, 1.7; P < 0.001). Germany, Ukraine, Nigeria, South Africa, and New Zealand had statistically robust aRR estimates, with the strongest associations being seen in sub-Saharan Africa (in South Africa, aRR = 3.9, 95% CI: 2.0, 7.5 (P < 0.001); in Nigeria, aRR = 3.2, 95% CI: 1.2, 8.5 (P = 0.02)). Estimations for Lebanon, Japan, and China failed because early-onset cannabis use was rare there.
Table 3.
Estimated Association Between Early-Onset (Age <17 Years) Cannabis Use and Later Onset (Age ≥17 Years) of a Depression Spell, World Health Organization World Mental Health Survey Initiative, 2001–2005a
| Region and Country | No Covariatesb |
Sex, Age, and Age Squaredc |
“Leave 1 Out”d |
||||||
| Estimated RR | 95% CI | P Value | Estimated RR | 95% CI | P Value | Estimated RR | 95% CI | P Value | |
| Americas | |||||||||
| Colombia | 1.1 | 0.7, 1.9 | 0.635 | 1.6 | 1.0, 2.8 | 0.069 | 1.5 | 1.4, 1.7 | <0.001 |
| Mexico | 0.4 | 0.2, 1.1 | 0.082 | 0.8 | 0.3, 1.9 | 0.545 | 1.5 | 1.4, 1.7 | <0.001 |
| United States | 1.0 | 0.9, 1.2 | 0.932 | 1.1 | 1.0, 1.4 | 0.113 | 1.7 | 1.5, 1.9 | <0.001 |
| Europe | |||||||||
| Belgium | 0.6 | 0.3, 1.4 | 0.262 | 0.8 | 0.3, 1.8 | 0.548 | 1.5 | 1.4, 1.7 | <0.001 |
| France | 0.7 | 0.4, 1.2 | 0.161 | 1.0 | 0.6, 1.7 | 0.962 | 1.5 | 1.4, 1.7 | <0.001 |
| Germany | 1.8 | 1.1, 3.2 | 0.031 | 2.0 | 1.1, 3.6 | 0.021 | 1.5 | 1.4, 1.6 | <0.001 |
| Italy | 1.2 | 0.6, 2.6 | 0.659 | 2.1 | 1.0, 4.8 | 0.062 | 1.5 | 1.4, 1.6 | <0.001 |
| Netherlands | 1.4 | 0.8, 2.5 | 0.227 | 1.4 | 0.8, 2.5 | 0.246 | 1.5 | 1.4, 1.7 | <0.001 |
| Spain | 0.9 | 0.6, 1.4 | 0.563 | 1.3 | 0.8, 2.1 | 0.239 | 1.5 | 1.4, 1.7 | <0.001 |
| Ukraine | 0.5 | 0.2, 1.3 | 0.176 | 3.0 | 1.1, 8.4 | 0.031 | 1.5 | 1.3, 1.6 | <0.001 |
| Middle East and Africa | |||||||||
| Israel | 2.0 | 0.8, 4.8 | 0.126 | 2.0 | 0.8, 4.9 | 0.114 | 1.5 | 1.4, 1.6 | <0.001 |
| Lebanon | —e | — | — | — | 1.5 | 1.4, 1.7 | <0.001 | ||
| Nigeria | 2.4 | 0.9, 6.4 | 0.070 | 3.2 | 1.2, 8.5 | 0.023 | 1.5 | 1.4, 1.6 | <0.001 |
| South Africa | 2.8 | 1.5, 5.4 | 0.002 | 3.9 | 2.0, 7.5 | <0.001 | 1.5 | 1.4, 1.6 | <0.001 |
| Asia | |||||||||
| Japan | — | — | — | — | 1.5 | 1.4, 1.7 | <0.001 | ||
| China | — | — | — | — | 1.5 | 1.4, 1.7 | <0.001 | ||
| Oceania | |||||||||
| New Zealand | 1.4 | 1.2, 1.6 | <0.001 | 1.8 | 1.5, 2.0 | <0.001 | 1.3 | 1.2, 1.5 | <0.001 |
| All countries | 1.2 | 1.1, 1.3 | <0.001 | 1.5 | 1.4, 1.7 | <0.001 | 1.5 | 1.4, 1.7 | <0.001 |
Abbreviations: CI, confidence interval; RR, relative risk.
Unadjusted and covariate-adjusted models along with “leave-1-out” influence analysis. Estimates were based on data obtained from 17 countries participating in the World Mental Health Surveys Consortium.
RR was estimated via the odds ratio under a conditional form of the logistic regression model, with “risk set” matching on local area; no covariate adjustments.
Same as described in footnote b, but here sex, age, and age squared were included as covariates.
Same as described in footnote c, but here the RR estimate was a multisite estimate, with this site left out.
The RR and 95% CI could not be estimated (there were too few informative risk sets at these sites, generally because of the very low occurrence of early-onset cannabis smoking; see Degenhardt et al. (1)).
Figure 1 depicts the site-specific estimates in a horizontal summary plot inspired by the meta-analytic vertical “funnel plot.” The horizontal line shows the overall meta-analytic risk ratio summary estimate, derived as if each site had published its own sex- and age-adjusted risk ratio estimate and 95% confidence interval, with a rather narrow 95% confidence interval for this covariate-adjusted risk ratio summary estimate, as shown on the right-hand side of the figure. Working leftward toward the y-axis, one can see how the width of the 95% confidence interval might grow if we were to eliminate each site from consideration one-by-one, in order of precision of the site-specific summary estimate. The 95% confidence interval for each site-specific covariate-adjusted estimate is also shown, to provide the reader with a sense of the extent to which the site-specific estimates depart from the overall summary estimate for the risk ratio.
Figure 1.
Estimated odds ratios for the association between early-onset (age <17 years) cannabis use and later onset (age ≥17 years) of a depression spell in different countries, World Health Organization World Mental Health Survey Initiative, 2001–2005. The solid horizontal line is the meta-analytic pooled odds ratio estimate. This summary estimate is slightly larger than the summary odds ratio estimate obtained from multisite conditional logistic regression, because the meta-analysis approach is based upon different site-specific information weighting (i.e., as a function of the site-specific precision of each odds ratio estimate), as compared with the information weighting used to produce an overall odds ratio summary estimate in the conditional logistic regression analysis. Bars, 95% confidence interval.
The possibility of different associations between the sexes and between different age categories deserves attention. In an age-adjusted model with a product term for early cannabis use and sex (male vs. female), the product term P value was 0.084, and we estimated the aRR for the cannabis-depression association as 1.6 for males (95% CI: 1.4, 1.8; P < 0.001) and 1.4 for females (95% CI: 1.2, 1.6; P < 0.001), indicating no difference in the association according to sex. There was a tendency for the cannabis-depression association to be stronger in older participants (age 17–24 years: aRR = 1.6, 95% CI: 1.2, 2.1 (P < 0.001); age 25–34 years: aRR = 1.3, 95% CI: 1.1, 1.6 (P = 0.001); age 35–44 years: aRR = 1.5, 95% CI: 1.3, 1.7 (P < 0.001); and age ≥45 years: aRR = 1.8, 95% CI: 1.4, 2.2 (P < 0.001); the aRR was controlled for sex).
The aRR estimates did not vary appreciably across strata defined by tobacco smoking history (data not shown).
Controlling for other mental health problems
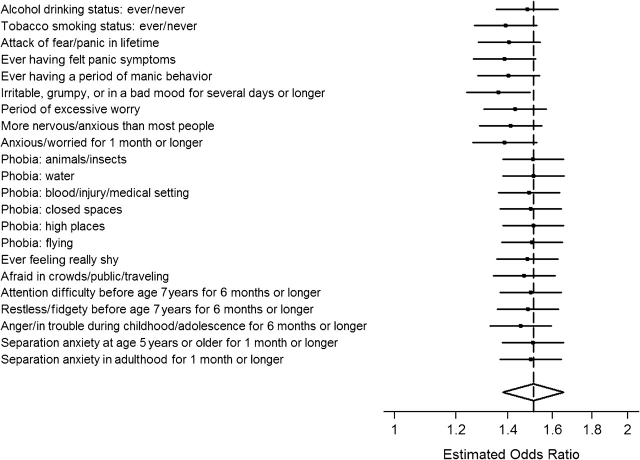
The cannabis-depression association was reestimated with statistical adjustment for persistency of cannabis use and other mental health problems that might be functioning as confounding variables. Persistency of cannabis use into the 12 months prior to assessment did not appear to alter the size of the cannabis-depression association. Moreover, statistical adjustment for a broad array of precannabis, concurrent, and postcannabis mental health problems in a conditional logistic regression model did little to attenuate the originally observed association (Figure 2).
Figure 2.
Covariate-adjusted estimated odds ratios for the association between early-onset (age <17 years) cannabis use and later onset (age ≥17 years) of a depression spell, according to mental health screening items included in the World Mental Health Survey Composite International Diagnostic Interview, World Health Organization World Mental Health Survey Initiative, 2001–2005. Bars, 95% confidence interval.
To study the possibility of confounding by a propensity to commit early violations of social norms, we created an index of childhood conduct problems as described in Appendix Table 1 and reestimated the cannabis-depression association with this index in the model, along with terms for age, age squared, and sex. This was possible for all but 4 countries, which did not administer this assessment. Based on data from the 13 countries that administered the childhood conduct problems assessment, and prior to statistical adjustment for this index of early norm violations, the aRR for the estimated cannabis-depression association was modest at 1.18 (95% CI: 1.02, 1.37; P = 0.02). With statistical adjustment for the index of early norm violations, the estimated cannabis-depression association dropped towards the null value and lacked statistical significance (aRR = 1.08, 95% CI: 0.93, 1.25; P = 0.32).
DISCUSSION
Key findings
The general summary evidence from this cross-site research with representation of all world regions suggests that there might be a modest but statistically robust sex- and age-adjusted association linking early-onset cannabis use with later occurrence of a depression spell. In most prior research on the hazards of cannabis use, the “controls” have been drawn from the total population, without taking into account the fact that in many places cannabis smoking is a violation of social norms. Unless early propensity for norm violations of this type can be taken into account, or unless norm violation has no association with the hazards of interest, other research teams may find what has been observed here—namely, an initial statistically robust signal of cannabis-related harm that would disappear when cases and controls were selected with attention to the norm-violation distribution or when the propensity toward norm violation was held constant.
Other studies on the relation between cannabis use and major depression symptoms or disorders have also found moderate associations, but no significant association was reported in some studies (21, 39). According to the review by Degenhardt et al. (15) on the association between cannabis use and depression, a number of studies found a modest association between early regular cannabis use and later depression, which persisted after controlling for potentially confounding variables.
Of course, the cannabis-depression association touches upon only 1 possible cannabis-associated psychiatric hazard. The world literature now references an array of possibilities, as recently reviewed by Hall and Degenhardt (40). As they noted in that review, the range of suspected hazards of this type is broad and encompasses anxiety disturbances as well as schizophrenia or schizophrenia-like psychosis reactions. Readers can judge for themselves whether the published evidence justifies a claim that early-onset or later-onset cannabis smoking might represent a generalized vulnerability to psychiatric disturbances (e.g., with the form of the disturbance being shaped by individual-level propensities in addition to the cannabis smoking), whether it might be a causal influence with adverse mental health consequences, or whether the observed associations might be a manifestation of something else (e.g., substantive or methodological possibilities mentioned elsewhere in this discussion).
We found evidence for an association between early cannabis use and later depression spells for 5 individual countries (Germany, Ukraine, Nigeria, South Africa, and New Zealand), representing both developed and developing countries. It is difficult to explain why in some countries the association was significant, while in others it was not. A high prevalence of early cannabis use in a particular country was not a clear explanation for a significant relation. For example, the United States and New Zealand had the highest prevalences of early cannabis use (1), but only in New Zealand was the association significant. On the contrary, in South Africa and Nigeria, the prevalence of early cannabis use was low (1), while the aRR was large and significant. It might be that in these countries the deviancy of cannabis use influences the incidence of a later depression spell.
We found no difference between females and males in the association linking early-onset cannabis use with later occurrence of a depression spell. In addition, the study by Poulin et al. (13) did not find clear sex differences, while Patton et al. (14) reported females to be more at risk. The cannabis-depression association was highest in the oldest age group. This might be explained by the fact that the oldest age group had more time to develop depressive symptoms. Another explanation might be the higher prevalence of cannabis use in younger age cohorts (1), which makes early cannabis use more normative in younger cohorts and therefore less deviant, with fewer negative consequences (e.g., discrimination, criminalization, use in segregated subcultures), thereby lowering the likelihood of deleterious mental effects such as depressive symptoms. In addition, a shorter duration of cannabis exposure among the younger cohorts could explain this finding, although controlling for persistence of cannabis use in this study did not alter the association.
Strength and limitations
A major strength of this study was its coverage of multiple global regions, with multisite standardized sampling, assessment, and risk-set matching approaches that constrained methodological and local area variations to a large extent.
Limitations included the limited capacity of cross-sectional surveys to shed light on suspected causal associations, although some of these limitations were constrained via the use of specific ages of onset of cannabis use and depression spells to assure temporal sequencing. Retrospective reports on age at first cannabis use can be questioned, although these have been found to be more reliable than reports on tobacco and alcohol use (41). Repeated measurements over time show that reported age of first substance use tends to increase as people age (42–44). Thus, it might be possible that in our study, some older subjects inaccurately estimated their age at first cannabis use as older and were misclassified as later-onset cannabis users. Therefore, the greater cannabis-depression association found in older cohorts might be even larger than was estimated here. Symptoms of depression and their age of onset were also assessed by means of retrospective reports, which might be biased by the reconstruction of memories based on current affective states (10). Subjects who incorrectly reported onset of their depression symptoms as occurring at a later age (i.e., after their 17th birthday) would have been inadvertently included in the analyses as cases.
A limitation that might have affected this study's estimates is the level of survey nonresponse. This is a likely source of underestimation of illegal drug involvement but not necessarily a source of bias with respect to estimated associations with other variables (45). Furthermore, underreporting of cannabis use might have occurred, because some respondents may be unwilling to disclose an illegal behavior, and this might vary across countries. This bias might have resulted in underestimation of the association between early cannabis use and depression spells. However, we assume that the assurance of anonymity, as was done clearly before and at the start of the interview, will have helped to limit this.
The data did not allow differentiation between levels of cannabis use as reported elsewhere (9, 14). Degenhardt et al. (15) concluded that infrequent cannabis use has no association with depression. Perhaps our study's estimates can be attributed to what generally is a low level of cannabis involvement among persons who start to use this drug. Possibly, if the study focus had been on early onset of regular cannabis use, the observed cannabis-depression association might have been stronger. On the other hand, the youngest cannabis users may be most at risk, simply because their cannabis use is more likely to become long-standing (21).
Multiple covariates were considered, but there remain possible model misspecifications, with some suspected confounding variables not being measured at all or not being measured with sufficient accuracy and precision. Because ages of onset of the reported symptoms were not assessed, in some cases onset might have happened after the occurrence of the depression spell. The influences of family and parenting factors and childhood trauma were not studied; their confounding effects could be studied in future research. This study's control of precannabis norm violations is a case in point. The assessment of alleged cannabis toxicity will depend upon more complete attention to this source of confounding, when the toxic outcome is in the domain of mood disturbances or other mental health problems known to vary with premorbid norm violations and conduct problems.
Finally, in a prospective study of the cannabis-depression association, one might be able to match cannabis users and nonusers at the time of a baseline assessment, with subsequent follow-up for ascertainment of later depression and with formation of area-matched risk sets based on neighborhood of residence at the time of initiation of cannabis use. This approach was not possible in the present context but could be used in future prospective studies.
Acknowledgments
Author affiliations: Department of Epidemiology, Netherlands Institute of Mental Health and Addiction, Utrecht, the Netherlands (Ron de Graaf, Margriet van Laar); Department of Epidemiology, College of Human Medicine, Michigan State University, East Lansing, Michigan (Mirjana Radovanovic, Brian Fairman, James C. Anthony); University Psychiatric Hospital, Ljubljana, Slovenia (Mirjana Radovanovic); National Drug and Alcohol Research Centre, University of New South Wales, Sydney, Australia (Louisa Degenhardt); Center for Reducing Health Disparities, School of Medicine, University of California, Davis, Davis, California (Sergio Aguilar-Gaxiola); University Hospital Gasthuisberg, Leuven, Belgium (Ronny Bruffaerts); IRCCS Centro S. Giovanni di Dio Fatebenefratelli, Brescia, Italy (Giovanni de Girolamo); Institute of Development, Research, Advocacy and Applied Care, Department of Psychiatry and Clinical Psychology, St. George Hospital University Medical Centre, Balamand University, Beirut, Lebanon (John Fayyad); University College Hospital, Ibadan, Nigeria (Oye Gureje); Parc Sanitari Sant Joan de Déu, Fundació Sant Joan de Déu, CIBER en Salud Mental, Sant Boi de Llobregat, Barcelona, Spain (Josep Maria Haro); Institute of Mental Health, Peking University, Beijing, China (Yueqin Huang); Ukrainian Psychiatric Association, Kiev, Ukraine (Stanislav Kostychenko); Institut National de la Santé et de la Recherche Médicale, U705, CNRS UMR 7157, University Paris Diderot, Hôpital Lariboisière Fernand Widal, Assistance Publique Hôpitaux de Paris, Paris, France (Jean-Pierre Lépine); Department of Psychiatry, University of Leipzig, Leipzig, Germany (Herbert Matschinger); National Institute of Psychiatry, Mexico City, Mexico (Maria Elena Medina Mora); Braun School of Public Health and Community Medicine, Hebrew University, Hadassah, Israel (Yehuda Neumark); Department of Psychiatry, University Medical Centre, Groningen, the Netherlands (Johan Ormel); Department of Epidemiology and Bioinformatics, University Medical Centre, Groningen, the Netherlands (Johan Ormel); Pontificia Universidad Javeriana, Centro Medico de la Sabana, Bogota, Colombia (Jose Posada-Villa); Department of Psychiatry and Mental Health, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa (Dan J. Stein); National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan (Hisateru Tachimori); and Department of Public Health and General Practice, School of Medicine and Health Sciences, University of Otago, Christchurch, New Zealand (J. Elisabeth Wells).
This study was carried out in conjunction with the World Health Organization World Mental Health Survey (WMHS) Initiative. This work was supported by the US National Institute of Mental Health (grant R01MH070884), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service (grants R13-MH066849, R01-MH069864, and R01 DA016558), the Fogarty International Center (grant FIRCA R03-TW006481), the Pan American Health Organization, the Eli Lilly & Company Foundation, Ortho-McNeil Pharmaceutical, Inc., GlaxoSmithKline, Bristol-Myers Squibb, and Shire. A complete list of WMHS publications can be found at http://www.hcp.med.harvard.edu/wmh/.
The Chinese World Mental Health Survey Initiative is supported by the Pfizer Foundation. The Colombian National Study of Mental Health is supported by the Ministry of Social Protection of Colombia. The European Study of the Epidemiology of Mental Disorders is funded by the European Commission (contracts QLG5-1999-01042 and SANCO 2004123), the Piedmont Region (Italy), Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (Spain) (grant FIS 00/0028), Ministerio de Ciencia y Tecnología (Spain) (grant SAF 2000-158-CE), Departament de Salut, Generalitat de Catalunya, Instituto de Salud Carlos III (Spain) (grants CIBER CB06/02/0046 and RETICS RD06/0011 REM-TAP), other local agencies, and an unrestricted educational grant from GlaxoSmithKline. The Israel National Health Survey is funded by the Israeli Ministry of Health, with support from the Israeli National Institute for Health Policy and Health Services Research, and by the National Insurance Institute of Israel. The World Mental Health Japan Survey is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (grants H13-SHOGAI-023, H14-TOKUBETSU-026, and H16-KOKORO-013) from the Japanese Ministry of Health, Labour and Welfare. The Lebanese National Mental Health Survey is supported by the Lebanese Ministry of Public Health, the World Health Organization (Lebanon), Fogarty International, anonymous private donations to the Institute for Development, Research, Advocacy and Applied Care (Lebanon), and unrestricted grants from Janssen Cilag, Eli Lilly, GlaxoSmithKline, Roche, and Novartis. The Mexican National Comorbidity Survey is supported by the National Institute of Psychiatry Ramon de la Fuente (grant INPRFMDIES 4280) and the National Council on Science and Technology (grant G30544-H), with supplemental support from the Pan American Health Organization. Te Rau Hinengaro: The New Zealand Mental Health Survey is supported by the New Zealand Ministry of Health, the Alcohol Advisory Council, and the Health Research Council. The Nigerian Survey of Mental Health and Wellbeing is supported by the World Health Organization (Geneva and Nigeria) and the Federal Ministry of Health, Abuja, Nigeria. The South Africa Stress and Health Study is supported by the US National Institute of Mental Health (NIMH) (R01-MH059575) and the US National Institute on Drug Abuse (NIDA), with supplemental funding from the South African Department of Health and the University of Michigan. The Ukraine Comorbid Mental Disorders during Periods of Social Disruption Study is funded by the NIMH (grant RO1-MH61905). The US National Comorbidity Survey Replication is supported by the NIMH (grant U01-MH60220), with supplemental support from the NIDA, the Substance Abuse and Mental Health Services Administration (US Department of Health and Human Services), the Robert Wood Johnson Foundation (grant 044708), and the John W. Alden Trust.
Preparation of the manuscript was supported by a National Institutes of Health (NIH)/National Institute on Drug Abuse (NIDA) R01 research grant award (grant DA016558), as well as J. C. A.’s NIH/NIDA K05 Senior Scientist Award (grant DA015799) and B. F.’s T32 fellowship award (grant DA021129); Michigan State University provided postdoctoral fellowship support for M. R.
The authors thank the WMHS staff for assistance with instrumentation, fieldwork, and data analysis.
The study sponsors played no role in the study design or the collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit this paper for publication.
Dr. Dan J. Stein has received research grants and/or consultancy honoraria from AstraZeneca, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Lundbeck, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, Tikvah, and Wyeth. The other authors report no potential conflicts of interest.
Glossary
Abbreviations
- aRR
adjusted risk ratio
- CI
confidence interval
- CIDI
Composite International Diagnostic Interview
- RR
risk ratio
- WMHS
World Mental Health Survey
Appendix Table 1.
Items Included in the Early Conduct Problems Index and the Screening Section of the World Mental Health Survey Composite International Diagnostic Interview, World Health Organization World Mental Health Survey Initiative, 2001–2005
| Conduct problems in childhood or early adolescence |
| Telling lies to trick people |
| Getting out of doing things by fooling people or lying |
| Staying out later than your parents wanted |
| Skipping school |
| Shoplifting |
| Stealing from people you lived with |
| Breaking into a car, home, or building |
| Setting a fire to try to cause damage |
| Damaging property |
| Running away from home |
| Bullying, threatening, or frightening others |
| Often getting involved in physical fights |
| Using a weapon on another person |
| Being physically cruel to an animal and hurting it on purpose |
| Being physically cruel to a person and hurting them on purpose |
| Forcing someone to give you something like money, jewelry, or clothing by threatening them or causing them injury |
| Stealing someone's purse, wallet, luggage, package, or bag by grabbing it from them |
| Making anyone do something sexual by forcing, intimidating, or threatening them |
| Ever being suspended or expelled from school as a result of your behavior or aggression |
| Being fired from a job because of your behavior or aggression |
| Getting in trouble with the police as a result of your behavior or aggression |
| Being arrested because of your behavior or aggression |
| Being sent to jail, prison, or a juvenile correction facility because of your behavior or aggression |
| Screener items on lifetime mental problems and alcohol status |
| Alcohol drinking status: ever/never |
| Tobacco smoking status: ever/never |
| Attack of fear/panic during one's lifetime |
| Ever feeling panic symptoms |
| Ever having a period of manic behavior |
| Irritability, grumpiness, or bad mood for several days or longer |
| Period of excessive worry |
| Being more nervous/anxious than most people |
| Being anxious/worried for 1 month or longer |
| Phobia |
| Animals/insects |
| Water |
| Blood/injury/medical setting |
| Closed spaces |
| High places |
| Flying |
| Ever feeling really shy |
| Feeling afraid in crowds, in public, or while traveling |
| Attention difficulty before age 7 years for 6 months or longer |
| Restlessness/fidgetiness before age 7 years for 6 months or longer |
| Being angry or in trouble during childhood/adolescence for 6 months or longer |
| Separation anxiety at age 5 years or older for 1 month or longer |
| Separation anxiety in adulthood for 1 month or longer |
References
- 1.Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7) doi: 10.1371/journal.pmed.0050141. e141. (doi: 10.1371/journal.pmed.0050141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monshouwer K, Smit F, de Graaf R, et al. First cannabis use: does onset shift to younger ages? Findings from 1988 to 2003 from the Dutch National School Survey on Substance Use. Addiction. 2005;100(7):963–970. doi: 10.1111/j.1360-0443.2005.01088.x. [DOI] [PubMed] [Google Scholar]
- 3.Arseneault L, Cannon M, Witton J, et al. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184(2):110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 4.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 5.Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction. 2004;99(4):425–430. doi: 10.1111/j.1360-0443.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 6.Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28(4):643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- 7.Rey JM, Tennant CC. Cannabis and mental health. BMJ. 2002;325(7374):1183–1184. doi: 10.1136/bmj.325.7374.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant BF, Hasin DS, Stinson FS, et al. Co-occurrence of 12-month mood and anxiety disorders and personality disorders in the US: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Psychiatr Res. 2005;39(1):1–9. doi: 10.1016/j.jpsychires.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen CY, Wagner FA, Anthony JC. Marijuana use and the risk of major depressive episode. Epidemiological evidence from the United States National Comorbidity Survey. Soc Psychiatry Psychiatr Epidemiol. 2002;37(5):199–206. doi: 10.1007/s00127-002-0541-z. [DOI] [PubMed] [Google Scholar]
- 10.Bovasso GB. Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry. 2001;158(12):2033–2037. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97(9):1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 12.van Laar M, van Dorsselaer S, Monshouwer K, et al. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102(8):1251–1260. doi: 10.1111/j.1360-0443.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 13.Poulin C, Hand D, Boudreau B, et al. Gender differences in the association between substance use and elevated depressive symptoms in a general adolescent population. Addiction. 2005;100(4):525–535. doi: 10.1111/j.1360-0443.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 14.Patton GC, Coffey C, Carlin JB, et al. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325(7374):1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 16.Monshouwer K, Van Dorsselaer S, Verdurmen J, et al. Cannabis use and mental health in secondary school children. Findings from a Dutch survey. Br J Psychiatry. 2006;188(2):148–153. doi: 10.1192/bjp.188.2.148. [DOI] [PubMed] [Google Scholar]
- 17.Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use, depression and anxiety among Australian adults: findings from the National Survey of Mental Health and Well-Being. Soc Psychiatry Psychiatr Epidemiol. 2001;36(5):219–227. doi: 10.1007/s001270170052. [DOI] [PubMed] [Google Scholar]
- 18.Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- 19.Harder VS, Morral AR, Arkes J. Marijuana use and depression among adults: testing for causal associations. Addiction. 2006;101(10):1463–1472. doi: 10.1111/j.1360-0443.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 20.Brook JS, Cohen P, Brook DW. Longitudinal study of co-occurring psychiatric disorders and substance use. J Am Acad Child Adolesc Psychiatry. 1998;37(3):322–330. doi: 10.1097/00004583-199803000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction. 1997;92(3):279–296. [PubMed] [Google Scholar]
- 22.Harder VS, Stuart EA, Anthony JC. Adolescent cannabis problems and young adult depression: male-female stratified propensity score analyses. Am J Epidemiol. 2008;168(6):592–601. doi: 10.1093/aje/kwn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brook DW, Brook JS, Zhang C. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry. 2002;59(11):1039–1044. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- 24.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13(2):253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 25.Schlesselman JJ, Stolley PD. Case-Control Studies: Design, Conduct, Analysis. New York, NY: Oxford University Press; 1982. [Google Scholar]
- 26.Wells JE, Degenhardt L, Bohnert KM, et al. Geographical clustering of cannabis use: results from the New Zealand Mental Health Survey 2003–2004. Drug Alcohol Depend. 2009;99(1–3):309–316. doi: 10.1016/j.drugalcdep.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson GR, Reid J, Dishion T. Antisocial Boys. Eugene, OR: Castalia Press; 1992. [Google Scholar]
- 28.Ialongo NS, Vaden-Kieman N, Kellam S. Early peer rejection and aggression: longitudinal relations with adolescent behavior. J Dev Phys Disabil. 1998;10(2):199–213. [Google Scholar]
- 29.Kessler RC, Üstün B, editors. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York, NY: Cambridge University Press; 2008. [Google Scholar]
- 30.Kessler RC, Berglund P, Chiu WT, et al. The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int J Methods Psychiatr Res. 2004;13(2):69–92. doi: 10.1002/mpr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler RC, Abelson J, Demler O, et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI) Int J Methods Psychiatr Res. 2004;13(2):122–139. doi: 10.1002/mpr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anthony JC, Petronis KR. Suspected risk factors for depression among adults 18–44 years old. Epidemiology. 1991;2(2):123–132. doi: 10.1097/00001648-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Lynskey MT, Glowinski AL, Todorov AA, et al. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Arch Gen Psychiatry. 2004;61(10):1026–1032. doi: 10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- 35.Pope HG, Jr, Gruber AJ, Hudson JI, et al. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 36.Baumeister SE, Tossmann P. Association between early onset of cigarette, alcohol and cannabis use and later drug use patterns: an analysis of a survey in European metropolises. Eur Addict Res. 2005;11(2):92–98. doi: 10.1159/000083038. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal A, Lynskey MT, Pergadia ML, et al. Early cannabis use and DSM-IV nicotine dependence: a twin study. Addiction. 2008;103(11):1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swift W, Coffey C, Carlin JB, et al. Adolescent cannabis users at 24 years: trajectories to regular weekly use and dependence in young adulthood. Addiction. 2008;103(8):1361–1370. doi: 10.1111/j.1360-0443.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- 39.McGee R, Williams S, Poulton R, et al. A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction. 2000;95(4):491–503. doi: 10.1046/j.1360-0443.2000.9544912.x. [DOI] [PubMed] [Google Scholar]
- 40.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 41.Johnson TP, Mott JA. The reliability of self-reported age of onset of tobacco, alcohol and illicit drug use. Addiction. 2001;96(8):1187–1198. doi: 10.1046/j.1360-0443.2001.968118711.x. [DOI] [PubMed] [Google Scholar]
- 42.Henry B, Moffitt T, Caspi A, et al. On the “remembrance of things past”: a longitudinal evaluation of the retrospective method. Psychol Assess. 1994;6(2):92–101. [Google Scholar]
- 43.Engels RC, Knibbe RA, Drop MJ. Inconsistencies in adolescents’ self-reports of initiation of alcohol and tobacco use. Addict Behav. 1997;22(5):613–623. doi: 10.1016/s0306-4603(96)00067-6. [DOI] [PubMed] [Google Scholar]
- 44.Labouvie E, Bates ME, Pandina RJ. Age of first use: its reliability and predictive utility. J Stud Alcohol. 1997;58(6):638–643. doi: 10.15288/jsa.1997.58.638. [DOI] [PubMed] [Google Scholar]
- 45.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev. 1995;17(1):192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]