Abstract
This study examined how adiposity influences racial/ethnic differences in diabetes incidence by exploring whether relations between anthropometric measures and incident diabetes vary by race/ethnicity. Data from the Multi-Ethnic Study of Atherosclerosis initiated in 2000 (n = 5,446 US men and women aged 45–84 years) were analyzed by using proportional hazards and Poisson regression. The diabetes incidence rate was 2/100 person-years (n = 479 cases). Interactions were present between race and anthropometry (P-interaction(race × body mass index) = 0.002). The slope of incident diabetes per anthropometric unit was greatest for Chinese, less for whites and Hispanics, and still less for blacks. For small waist, risk of incident diabetes was <1/100 person-years for all racial/ethnic groups. At intermediate waist levels, Chinese had the highest and whites the lowest rates of incident diabetes. At the respective 95th percentiles of waist circumference, risk of incident diabetes per 100 person-years was 3.9 for Chinese (104 cm), 3.5 for whites (121 cm), 5.0 for blacks (125 cm), and 5.3 for Hispanics (121 cm). Adiposity influenced relative diabetes occurrence across racial/ethnic groups, in that Chinese had a steeper diabetes risk per unit of adiposity. However, the generally low level of adiposity in Chinese led to a relatively low diabetes occurrence.
Keywords: Asian continental ancestry group, body mass index, diabetes mellitus, waist circumference
In the United States, the burden of diabetes varies greatly by race/ethnicity, with blacks having the highest prevalence, followed by Hispanics and then whites (1). Less is known about the prevalence of diabetes in US Asians; however, it is thought to be similar to, or higher than, that observed in whites (1, 2).
Anthropometric measures, such as body mass index (BMI), waist circumference, hip circumference, waist-to-hip ratio, waist-to-height ratio, and waist-to-weight ratio, frequently act as surrogates for body fat distribution (3). Racial and ethnic differences in these markers of obesity are well established (3–6). Furthermore, numerous pathophysiologic mechanisms suggest a relation between adiposity and diabetes risk (3, 7–10).
Relatively little research has prospectively examined interrelationships between race/ethnicity, anthropometry, and diabetes risk. Research in this domain is highly relevant given the changing demography of the United States (11), the present obesity epidemic (12), and the anticipated diabetes epidemic both within the United States (13) and globally (14). Understanding relations of anthropometry to diabetes risk in Asian Americans is of particular interest, given the “Asian paradox” of low general obesity yet high diabetes risk (15).
The purpose of this study was to explore whether the association between anthropometrics and diabetes risk varies by race/ethnicity among participants in the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that whites, blacks, and Hispanics would be similar but that there would be an interaction, such that, compared with other groups, Chinese participants would be at greater risk of diabetes per anthropometric increment.
MATERIALS AND METHODS
Study population and data collection
MESA is a prospective epidemiologic cohort initiated in July 2000 (16). A specific objective of MESA is to assess racial/ethnic, age, and sex differences in subclinical and clinical cardiovascular disease. Local institutional review committees approved the MESA protocol, and all participants gave informed consent.
A total of 6,814 men and women between the ages of 45 and 84 years, all of whom were free of clinical cardiovascular disease at baseline, were recruited in 6 US field centers. Included in this analysis were data collected at examination 1 (July 2000–August 2002), examination 2 (September 2002–February 2004), examination 3 (March 2004–September 2005), and examination 4 (September 2005–May 2007). Participants were excluded from the analysis if they had diabetes at baseline (n (% prevalent in sample) = 859 (12.7%) total: 158 (6.0%) whites, 332 (17.7%) blacks, 105 (13.1%) Chinese, and 264 (17.7%) Hispanics). In their respective racial/ethnic groups, participants with diabetes at baseline had substantially greater waist circumferences than those without diabetes; however, the difference comparing by diabetes status was the smallest among Chinese (data not shown).
Participants self-reported their race/ethnicity and were classified as Hispanic, non-Hispanic black, non-Hispanic white, or non-Hispanic Chinese on the basis of their answers to questions about race, ethnicity, and nationality modeled on questions from the 2000 US Census. In this paper, racial/ethnic groups are considered mutually exclusive and are abbreviated as white, black, Hispanic, and Chinese. White participants were recruited from all study sites, whereas blacks were recruited from Forsyth County, North Carolina; Chicago, Illinois; New York, New York; Baltimore, Maryland; and Los Angeles, California. Hispanics were recruited from St. Paul, Minnesota; New York, New York; and Los Angeles, California. Chinese were recruited from Chicago, Illinois; and Los Angeles, California.
Demographic information, such as age, education, and annual income, was queried in the baseline survey.
Anthropometry.
Height was measured by using a stadiometer ((Accu-Hite Measuring Device; Seca GmbH & Company KG, Hamburg, Germany) with level bubble) and weight with a Detecto platform balance scale (Titus Home Health Care, Alhambra, California). Circumferences were measured to the nearest centimeter by using a Gulick II anthropometric tape (Sammons Preston, Chicago, Illinois), waist circumference at the level of the umbilicus, and hip circumference at the maximum circumference of the buttocks. BMI was calculated as weight divided by height squared (kg/m2), waist-to-hip ratio as waist/hips (cm/cm), waist-to-weight ratio as waist/weight (cm/kg), and waist-to-height ratio as waist/height (cm/m).
Incident diabetes.
Participants taking diabetes medications or whose glucose, after a minimum 8-hour fast, was ≥126 mg/dL at any of the follow-up examinations were classified as having incident diabetes. Serum glucose was measured by rate reflectance spectrophotometry using thin film adaptation of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, New York) at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, Minnesota).
Statistical analysis
Race- and sex-stratified means and frequencies of demographic and anthropometric characteristics were computed. The crude incidence of diabetes was calculated with the number of cases of diabetes as the numerator and number of person-years as the denominator. Person-years accrued from the date of the participant's baseline examination until incident diabetes, death, loss to follow-up, or date of the participant's fourth MESA examination.
Cox proportional hazards regression was used to explore whether the relation between anthropometrics and diabetes risk varied by race/ethnicity. Models were adjusted for age (continuous), education (<high school diploma, high school or some college, college diploma), annual income (<$20,000, $20,000–<$50,000, ≥$50,000), and anthropometric measures, including simultaneous adjustment for multiple anthropometric measures in some instances. Tests of interaction were conducted by including race/ethnicity × anthropometry cross-product terms in the models. For all proportional hazards regression analyses, anthropometric measures were standardized per 1 standard deviation based on the distribution in the entire MESA population. This method enables direct comparison of betas across measures of anthropometry, and between racial/ethnic groups, treating all covariates identically across racial/ethnic groups.
Poisson regression was used to calculate absolute rates of incident diabetes by race. These models adjusted for the same covariates as in the Cox proportional hazards analyses, and they also evaluated interaction by including race/ethnicity × anthropometry cross-product terms in the models.
RESULTS
The MESA participants in our sample were on average 61.6 years of age (range: 45–84); 42.3% were white, 25.5% were black, 11.5% were Chinese, and 20.7% were Hispanic. Unadjusted sex- and race-stratified demographic and anthropometric characteristics are reported in Table 1. Racial/ethnic differences were present for all anthropometric characteristics at P < 0.0001. The correlations between BMI and waist circumference were similar between the races/ethnicities: the r value was 0.85 for whites, 0.84 for Chinese, 0.84 for blacks, and 0.85 for Hispanics.
Table 1.
Sex- and Race-stratified Demographic and Anthropometric Characteristics,a,b The MESA Study, United States, 2000–2002
| Women |
Men |
|||||||||||||||||||||||
| White (n = 1,236) |
Chinese (n = 333) |
Black (n = 804) |
Hispanic (n = 609) |
White (n = 1,124) |
Chinese (n= 313) |
Black (n = 638) |
Hispanic (n = 546) |
|||||||||||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Demographics | ||||||||||||||||||||||||
| Age, years (SD) | 62.2 (10.3) | 60.8 (10.1) | 61.4 (10.0) | 60.7 (10.3) | 62.5 (10.2) | 61.6 (10.3) | 61.6 (10.3) | 60.4 (10.3) | ||||||||||||||||
| Educationc | ||||||||||||||||||||||||
| <High school diploma | 63 | 5.3 | 90 | 27.8 | 74 | 9.7 | 258 | 43.8 | 44 | 4.0 | 38 | 12.6 | 64 | 10.4 | 200 | 37.3 | ||||||||
| High school or some college | 611 | 51.0 | 138 | 42.6 | 404 | 52.8 | 280 | 47.5 | 390 | 35.4 | 100 | 33.1 | 334 | 54.2 | 260 | 48.5 | ||||||||
| College diploma | 523 | 43.7 | 96 | 29.6 | 287 | 37.5 | 51 | 8.7 | 667 | 60.6 | 164 | 54.3 | 218 | 35.4 | 76 | 14.2 | ||||||||
| Incomec | ||||||||||||||||||||||||
| <$20,000 | 151 | 12.9 | 139 | 43.4 | 161 | 22.4 | 241 | 41.8 | 71 | 6.6 | 103 | 34.1 | 86 | 15.3 | 161 | 30.4 | ||||||||
| $20,000–<$50,000 | 419 | 35.8 | 90 | 28.1 | 312 | 43.5 | 246 | 42.7 | 296 | 27.4 | 94 | 31.1 | 199 | 35.3 | 233 | 44.0 | ||||||||
| ≥$50,000 | 602 | 51.4 | 91 | 28.4 | 245 | 34.1 | 89 | 15.5 | 713 | 66.0 | 105 | 34.8 | 279 | 49.5 | 136 | 25.7 | ||||||||
| Current smokerc | 133 | 11.1 | 5 | 1.5 | 122 | 15.8 | 66 | 11.2 | 116 | 10.5 | 29 | 9.5 | 125 | 20.2 | 88 | 16.4 | ||||||||
| Anthropometry | ||||||||||||||||||||||||
| Weight, kg | 72.0 (15.6) | 58.1 (9.4) | 81.7 (17.2) | 71.7 (14.1) | 86.1 (13.8) | 68.0 (10.3) | 88.5 (16.0) | 81.9 (13.1) | ||||||||||||||||
| Height, cm | 162 (6) | 156 (6) | 162 (7) | 156 (6) | 176 (7) | 168 (6) | 176 (7) | 169 (6) | ||||||||||||||||
| BMI, kg/m2 | 27.3 (5.6) | 23.9 (3.5) | 31.0 (6.4) | 29.5 (5.4) | 27.7 (3.9) | 24.1 (3.1) | 28.4 (4.6) | 28.6 (4.1) | ||||||||||||||||
| Waist, cm | 94.3 (15.7) | 86.1 (10.8) | 100.0 (15.7) | 98.8 (14.1) | 100.3 (11.1) | 87.4 (8.9) | 99.7 (12.3) | 100.2 (10.9) | ||||||||||||||||
| Hip, cm | 107 (12) | 95 (7) | 113 (13) | 107 (11) | 105 (8) | 95 (6) | 106 (9) | 102 (8) | ||||||||||||||||
| Waist-to-hip ratio | 0.88 (0.09) | 0.90 (0.07) | 0.89 (0.08) | 0.92 (0.08) | 0.96 (0.07) | 0.92 (0.05) | 0.94 (0.06) | 0.98 (0.05) | ||||||||||||||||
| Waist/height | 0.58 (0.10) | 0.55 (0.07) | 0.62 (0.10) | 0.63 (0.09) | 0.57 (0.06) | 0.52 (0.05) | 0.57 (0.07) | 0.59 (0.06) | ||||||||||||||||
| Waist/weight | 1.33 (0.15) | 1.50 (0.16) | 1.24 (0.14) | 1.40 (0.17) | 1.18 (0.11) | 1.30 (0.11) | 1.14 (0.11) | 1.24 (0.10) | ||||||||||||||||
Abbreviations: BMI, body mass index; MESA, Multi-Ethnic Study of Atherosclerosis; SD, standard deviation.
All racial/ethnic differences and sex differences were significant at P < 0.0001, except age (women, P = 0.04; men, P = 0.0009). Analysis of variance was used to test racial/ethnic differences and sex differences in continuous traits. For categorical variables, the omnibus χ2 test was used.
Participants with baseline diabetes and those with no follow-up were excluded.
Column percentages are given.
Through 6.6 years of follow-up (median = 4.7), 479 incident cases of diabetes accrued: 147 in whites, 150 in blacks, 48 in Chinese, and 134 in Hispanics. The overall crude diabetes incidence rate was 2.00 per 100 person-years, and there was substantial variation by race/ethnicity and sex. Among women, the incidence rate (per 100 person-years) for whites was 1.25, whereas the rates for Chinese, blacks, and Hispanics were 2.01, 2.56, and 2.67, respectively. Likewise, these incidence rates among men were 1.61 for whites, 1.40 for Chinese, 2.50 for blacks, and 2.84 for Hispanics.
Interaction analyses
Interactions between race/ethnicity and select anthropometric measures (i.e., waist circumference and BMI) were evaluated by using proportional hazards regression (Table 2). Significant interactions were observed in the relation of race/ethnicity to diabetes risk by BMI, waist, waist adjusted for BMI, and BMI adjusted for waist. In most instances, a 1 standard deviation difference in these anthropometric measures was associated with a significantly lower diabetes risk increment for blacks relative to whites. Although the risk did not always reach the threshold for statistical significance, Chinese participants, compared with whites and blacks, tended to be at greater risk of incident diabetes per 1 standard deviation increase in anthropometry. There were no significant pairwise differences in the slope of diabetes incidence across anthropometric measures between Hispanics and any other racial/ethnic group.
Table 2.
Interactionsa in the Relation of Race/Ethnicity and Select Anthropometric Factors (per 1 SD)b to Diabetes Incidence, The MESA Study, United States, 2000–2007
| White (147 Events) |
Black (150 Events) |
Chinese (48 Events) |
Hispanic (134 Events) |
Overall Interaction P Value | |||||||||
| Model | Betac | 95% CI | HR per 1 SDd | Betac | 95% CI | HR per 1 SDd | Betac | 95% CI | HR per 1 SDd | Betac | 95% CI | HR per 1 SDd | |
| BMI × race | 0.69 | 0.55, 0.84 | 1.99 | −0.28 | −0.48, −0.08 | 1.51 | 0.36 | −0.02, 0.75 | 2.86 | −0.10 | −0.30, 0.11 | 1.80 | 0.002 |
| Waist × race | 0.64 | 0.51, 0.76 | 1.90 | −0.19 | −0.39, 0.00 | 1.57 | 0.29 | −0.08, 0.65 | 2.53 | −0.04 | −0.23, 0.16 | 1.82 | 0.05 |
| Waist × race adjusted for BMI | 0.44 | 0.25, 0.63 | 1.55 | −0.27 | −0.48, −0.06 | 1.19 | 0.24 | −0.13, 0.60 | 1.97 | −0.13 | −0.33, 0.08 | 1.36 | 0.01 |
| BMI × race adjusted for waist | 0.40 | 0.19, 0.61 | 1.49 | −0.28 | −0.48, −0.07 | 1.13 | 0.32 | −0.07, 0.70 | 2.05 | −0.12 | −0.32, 0.09 | 1.32 | 0.005 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; MESA, Multi-Ethnic Study of Atherosclerosis; SD, standard deviation.
Adjusted for age, sex, race/ethnicity, education, and income.
SDs were based on the entire MESA population. Therefore, betas, based on a constant absolute increment, can be compared directly between races/ethnicities. The SD for BMI is 5.5 kg/m2; the SD for waist is 14.4 cm.
Standardized beta coefficients. Pairwise differences from whites are statistically significant if the 95% CI does not include 0.
HR per 1 SD increase in anthropometry. For whites, the impact of a 1 SD increase in anthropometry was calculated by exponentiating the standardized beta coefficient. For example, a 1 SD increase in BMI is associated with an HR of 1.99 (i.e., exp(0.69)). For nonwhites, the risk associated with a 1 SD difference in anthropometry was calculated by exponentiating the sum of the main effect in whites and the nonwhite group of interest. For instance, for BMI, the impact of a 1 SD increase in BMI among blacks was calculated as [exp(0.69 + −0.28)], resulting in an HR of 1.51.
We noted that, although the slope for blacks was statistically lower than the slope for whites, as hypothesized, the hazard ratios for incident diabetes slopes over anthropometric variables were not greatly different across whites, blacks, and Hispanics. This observation permitted analyses directly testing the primary hypothesis: that Chinese would be different from other racial/ethnic groups. Thus, we compared Chinese participants with pooled non-Chinese MESA participants, and we evaluated whether interactions were present between Chinese versus non-Chinese race/ethnicity and anthropometric measures. Significant interactions in diabetes risk were observed between Chinese race/ethnicity (yes vs. no) and BMI (P = 0.01), waist circumference (P = 0.04), waist adjusted for BMI (P = 0.03), and BMI adjusted for waist (P = 0.02).
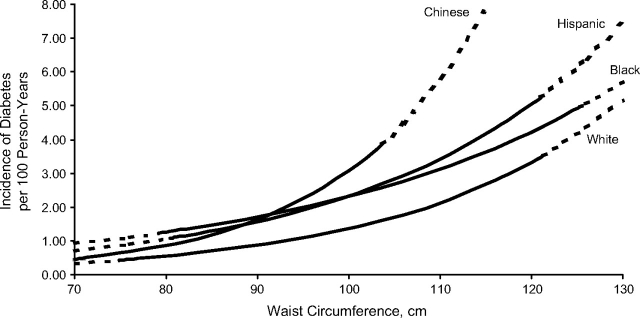
Race-stratified incident diabetes rates by waist circumference, adjusted for age, sex, education, and income, are presented in Figure 1. At a small waist circumference, the incidence of diabetes was low and similar across racial/ethnic groups. As waist circumference increased, the curves diverged, with Chinese having the steepest curve, followed by Hispanics, blacks, and then whites. Chinese participants at the 95th percentile of their race-specific waist circumference distribution (103.7 cm) had a diabetes incidence rate of 3.87 per 100 person-years. Despite less-steep curves for blacks and Hispanics, the incidence rates at their respective 95th waist percentiles were highest among Hispanics (5.27 per 100 person-years at 121.1 cm) followed by blacks (4.95 per 100 person-years at 125.3 cm). Among whites, at their 95th percentile (121.3 cm), the incidence rate was 3.52 per 100 person-years. Modeling anthropometry as BMI yielded results similar to the waist circumference results presented in this paper (data not shown).
Figure 1.
Absolute incidence rates of diabetes by waist circumference, stratified by race/ethnicity, The Multi-Ethnic Study of Atherosclerosis, United States, 2000–2007. Solid lines pertain to values between the race-specific 5th and 95th percentiles of waist circumference. Dotted lines are extrapolated values outside the aforementioned race-specific ranges. Lines are curved because Poisson regression calculates incidence on the log scale. Adjusted for age, sex, education, and income.
Additional analyses
Given the large variation in anthropometry by sex (Table 1), we assessed whether sex should be modeled as a covariate or as an effect modifier in our analyses. Sex was identified as a confounder of the associations between anthropometry and incident diabetes, but it was not an effect modifier. Thus, we did not stratify our analyses by sex.
We also evaluated the impact of using standard deviations that were race specific, sex specific, and race and sex specific. Regardless of the origin of the standard deviations, the results were similar (data not shown).
Additional analyses concerning other anthropometric measures (e.g., waist-to-hip ratio and waist/height) were also conducted. In general, interactions between these measures of anthropometry and race/ethnicity in relation to risk of incident diabetes were similar to the BMI and waist circumference results we reported (data not shown).
Lastly, in Appendix Table 1 we report race/ethnicity main-effect analyses (i.e., adjusting for anthropometric measures but omitting anthropometric measure × race/ethnicity terms). Given the presence of significant interactions, the main-effect analyses are of limited value. However, they may be of interest to those wanting to directly compare the main effects observed in MESA with those reported in the literature by others.
DISCUSSION
In this large, multiethnic cohort of middle-aged and older adults, the relation of race/ethnicity to diabetes risk varied by adiposity. Relative to whites, a 1 standard deviation increase in most anthropometric measures was associated with a lower risk of incident diabetes for blacks but a greater risk of diabetes for Chinese. Hispanics were similar to whites. In absolute terms, although all racial/ethnic groups were at low diabetes risk at low levels of adiposity, Chinese experienced the steepest incline in risk as anthropometry increased. However, because of their lower overall level of adiposity, they never attained the absolute risks observed at the upper end of the distributions of the more obese black and Hispanic samples.
In line with the thinking of Geoffery Rose (17), small changes in the distributions of and risks associated with continuous traits can have a substantial impact on the pervasiveness of subsequent common disease outcomes. As demonstrated in Figure 1, although low adiposity is associated with uniformly low risk for all racial/ethnic groups, even at modest levels of obesity, Chinese participants have a risk of diabetes similar to that observed in other racial/ethnic groups at much greater degrees of adiposity. This finding is highly relevant from a public health perspective, in that it describes an elevated risk of diabetes associated with obesity in Chinese compared with other groups. These results may provide insight into present discussions concerning the possible need for race/ethnicity-specific anthropometric cutpoints (18–21), particularly in relation to screening for risk of diabetes. Furthermore, our findings portray the excess risk of incident diabetes conferred at levels of obesity well above established cutpoints but common among blacks, Hispanics, and whites in today's society.
Typically, the association of race/ethnicity in diabetes risk has been examined by controlling for adiposity as a confounder or conducting stratified analyses. Prior studies have shown elevated diabetes prevalence and/or serum markers of diabetes propensity among Asians (22–26), blacks (22, 23), and Hispanics (22, 23, 27, 28) relative to whites, even after accounting for level of adiposity. Taking into account BMI, both the MultiEthnic Cohort (22) and the Nurses’ Health Study (23) found that, compared with the risk for whites, risk of diabetes was elevated for Asians, blacks, and Hispanics. Similar Hispanic-white differences were observed in the San Antonio Heart Study (27) and the Massachusetts Hispanic Elders Study (28). In a cross-sectional Canadian-based study, uniform cutpoints for the classification of obesity using BMI, waist-to-hip ratio, and waist circumference resulted in marked variation in hemoglobin A1c levels, with Chinese having higher median levels compared with participants of European ancestry (24). Similarly, a matched study of young, lean, healthy subjects from multiple ethnic groups reported that Chinese displayed marked postprandial hyperglycemia and hyperinsulinemia compared with matched European white subjects (25), indicating that pathophysiologic differences may exist between races. Filipinas have also been identified as having a higher prevalence of type 2 diabetes than whites or blacks, even after controlling for computed tomography–assessed visceral adipose tissue (26).
In accord with these previous studies, and as demonstrated in the data presented in Appendix Table 1, in MESA, racial/ethnic differences in the risk of incident diabetes remained even after adjustment for anthropometric measures. The statistical evidence, presented in this paper, of interactions between race/ethnicity and anthropometry on diabetes risk contributes to the accumulating body of literature on this topic and is highly relevant from a public health perspective.
Heterogeneity in the composition of anthropometric circumferences due to racial/ethnic variation in the comparative volumes of tissues (particularly bone, skeletal muscle, subcutaneous adipose tissue, and visceral adipose tissue) provides one possible explanation for our findings (19, 29). For instance, after adjustment for various measures of total adiposity, Chinese have greater visceral adipose tissue than do whites (30), who have greater visceral adipose tissue than do blacks (3, 31, 32). Nevertheless, simple anthropometric measurements remain reasonable markers of obesity in large studies, are relatively easy and inexpensive to measure, and should continue to be used extensively in clinical settings as an initial proxy for body composition and possible health risks. Genetic differences among racial/ethnic groups could also provide some explanation for the interactions we observed. Genetic polymorphisms have been identified that influence both anthropometry and diabetes risk, and, for some, allele frequencies/variants are known to differ by race/ethnicity (33–36). A further explanatory factor could be differences in lifestyle, particularly diet and physical activity, which can lead to obesity and differ across racial/ethnic groups.
Limitations and strengths
Several limitations of this study should be acknowledged. First, for some racial/ethnic groups, the number of incident events was relatively small; thus, confidence intervals were wide, and we may have been underpowered to detect differences between races/ethnicities in some instances. This possibility was particularly poignant for Chinese; relative to other racial/ethnic groups, the proportion of Chinese enrolled at baseline was low. Similarly, Hispanics are a heterogeneous group, and although there may be racial differences and genetic variation among Hispanics, we lacked a sufficient number of incident diabetes cases in Hispanic subgroups to justify stratification. Second, anthropometry provides only proxy measures of body fat, fat type, and fat distribution. It is possible that more sophisticated measures of body fat, such as those obtained through magnetic resonance imaging and/or computed tomography, could have yielded different results. Last, at baseline, MESA participants were aged 45–84 years, and findings may not be generalizable to other age ranges.
There are also key strengths of this study. These strengths expand upon previous research of this topic in a variety of ways: 1) the prospective design, which reduces bias and allows for use of a clinically relevant outcome; 2) direct comparison of relatively large samples of participants representing 4 racial/ethnic groups; and 3) highly standardized anthropometric measurements, serum processing, and covariate assessment across study centers.
Conclusions
In this large, multiethnic cohort, the relation of adiposity to diabetes risk varied by race/ethnicity, with Chinese tending to be at greatest risk per anthropometric unit, followed by whites and Hispanics at lesser risk, and then blacks, who had the lowest risk per unit. Nevertheless, Chinese did not reach the same degree of obesity observed in other populations. Consequently, at the upper end of their race-/ethnic-specific adiposity distributions, the greatest absolute risk was observed for blacks and Hispanics. Whites achieved levels of obesity similar to those for blacks and Hispanics, but their diabetes risk was lower at those levels of obesity than in the other racial/ethnic groups. The findings from this study enhance current discussions concerning the utility of race-/ethnicity-specific anthropometric cutpoints (18–21), particularly in relation to screening for risk of diabetes among individuals of Chinese ancestry.
Acknowledgments
Author affiliations: Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, Minnesota (Pamela L. Lutsey, Mark A. Pereira, David R. Jacobs, Jr.); Department of Public Health Sciences, Wake Forest University, Winston-Salem, North Carolina (Alain G. Bertoni); Division of General Internal Medicine, Northwestern University, Chicago, Illinois (Namratha R. Kandula); and Department of Nutrition, University of Oslo, Oslo, Norway (David R. Jacobs, Jr.).
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (Bethesda, Maryland).
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
A full list of participating MESA investigators and institutions can be found at the following Web site: http://www.mesa-nhlbi.org.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- MESA
Multi-Ethnic Study of Atherosclerosis
Appendix Table 1.
Impact of Anthropometric Adjustment on the Relation of Race/Ethnicity to Incident Diabetes, The MESA Study, United States, 2000–2007
| White (147 Events) |
Black (150 Events) |
Chinese (48 Events) |
Hispanic (134 Events) |
||||
| HR | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Demographic model (no anthropometric adjustments)a,b | 1.00 | 1.78 | 1.40, 2.26 | 1.07 | 0.76, 1.50 | 1.70 | 1.30, 2.23 |
| BMI | 1.00 | 1.38 | 1.09, 1.77 | 1.74 | 1.22, 2.46 | 1.54 | 1.18, 2.02 |
| Waist | 1.00 | 1.66 | 1.31, 2.11 | 1.87 | 1.32, 2.66 | 1.74 | 1.33, 2.28 |
| Hip | 1.00 | 1.53 | 1.20, 1.95 | 1.87 | 1.31, 2.67 | 1.87 | 1.43, 2.45 |
| Waist-to-hip ratio | 1.00 | 1.90 | 1.49, 2.42 | 1.17 | 0.83, 1.65 | 1.65 | 1.26, 2.17 |
| Waist/height | 1.00 | 1.66 | 1.30, 2.11 | 1.57 | 1.11, 2.22 | 1.47 | 1.12, 1.92 |
| Waist/weight | 1.00 | 1.58 | 1.23, 2.02 | 1.39 | 0.97, 1.98 | 1.90 | 1.45, 2.50 |
| Waist and hips | 1.00 | 1.65 | 1.29, 2.10 | 1.89 | 1.33, 2.70 | 1.75 | 1.33, 2.29 |
| BMI and waist-to-hip ratio | 1.00 | 1.48 | 1.16, 1.89 | 1.74 | 1.23, 2.47 | 1.51 | 1.15, 1.98 |
| BMI and waist | 1.00 | 1.51 | 1.18, 1.94 | 1.89 | 1.33, 2.69 | 1.64 | 1.25, 2.15 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; MESA, Multi-Ethnic Study of Atherosclerosis.
Adjusted for age, sex, race/ethnicity, education, and income.
Each row that follows is for a separate model, in which the anthropometric factor(s) listed were added to the demographic-adjusted model.
References
- 1.CDC. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 2.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a National Health Survey. Diabetes Care. 2004;27(1):66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Bouchard C. Handbook of Obesity: Etiology and Pathophysiology. New York, NY: Marcel Dekker, Inc; 2004. [Google Scholar]
- 4.Okosun IS, Chandra KM, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960–2000. Prev Med. 2004;39(1):197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Hill JO, Sidney S, Lewis CE, et al. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) Study. Am J Clin Nutr. 1999;69(3):381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer FX. The epidemiology of central fat distribution in relation to disease [see comment] Nutr Rev. 2004;62(7 pt 2):S120–S126. doi: 10.1111/j.1753-4887.2004.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 7.Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19(5):457–466. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes Metab Rev. 1997;13(1):3–13. doi: 10.1002/(sici)1099-0895(199703)13:1<3::aid-dmr178>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Peiris AN, Sothmann MS, Aiman EJ, et al. The relationship of insulin to sex hormone-binding globulin: role of adiposity. Fertil Steril. 1989;52(1):69–72. doi: 10.1016/s0015-0282(16)60791-4. [DOI] [PubMed] [Google Scholar]
- 10.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 11.Day JC. Current Population Reports. Washington, DC: Government Printing Office; 1996. Population projections of the United States by age, sex, race, and Hispanic origin: 1995 to 2050. US Bureau of the Census. 25-1130. (P25-1130) [Google Scholar]
- 12.Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 13.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 14.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030 [see comment] Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 15.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14(1):32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 18.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies [see comment; erratum in Lancet. 2004;363(9412):902] Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19.Misra A, Wasir JS, Vikram NK. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition. 2005;21(9):969–976. doi: 10.1016/j.nut.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.International Diabetes Federation Consensus Group. Brussels: Belgium; The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation; 2006. ( http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf) [Google Scholar]
- 21.Zhu S, Heymsfield SB, Toyoshima H, et al. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81(2):409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Grandinetti A, Matsuura G, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis. 2009;19(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29(7):1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 24.Razak F, Anand S, Vuksan V, et al. Ethnic differences in the relationships between obesity and glucose-metabolic abnormalities: a cross-sectional population-based study. Int J Obes (Lond) 2005;29(6):656–667. doi: 10.1038/sj.ijo.0802937. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson S, Colagiuri S, Faramus E, et al. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr. 2002;132(9):2574–2579. doi: 10.1093/jn/132.9.2574. [DOI] [PubMed] [Google Scholar]
- 26.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and White women. Obes Res. 2005;13(8):1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 27.Stern MP, Gaskill SP, Hazuda HP, et al. Does obesity explain excess prevalence of diabetes among Mexican Americans? Results of the San Antonio Heart Study. Diabetologia. 1983;24(4):272–277. doi: 10.1007/BF00282712. [DOI] [PubMed] [Google Scholar]
- 28.Bermudez OI, Tucker KL. Total and central obesity among elderly Hispanics and the association with Type 2 diabetes. Obes Res. 2001;9(8):443–451. doi: 10.1038/oby.2001.58. [DOI] [PubMed] [Google Scholar]
- 29.Deurenberg P, Deurenberg-Yap M. Validity of body composition methods across ethnic population groups. Acta Diabetol. 2003;40(suppl 1):S246–S249. doi: 10.1007/s00592-003-0077-z. [DOI] [PubMed] [Google Scholar]
- 30.Park YW, Allison DB, Heymsfield SB, et al. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9(7):381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 31.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 32.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91(1):7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Li Y, Tollefsbol TO. Gene-environment interactions and epigenetic basis of human diseases. Curr Issues Mol Biol. 2008;10(1-2):25–36. [PMC free article] [PubMed] [Google Scholar]
- 34.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. doi: 10.1371/journal.pgen.0030115. (doi:10.1371/journal.pgen.0030115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry JR, Frayling TM. New gene variants alter type 2 diabetes risk predominantly through reduced beta-cell function. Curr Opin Clin Nutr Metab Care. 2008;11(4):371–377. doi: 10.1097/MCO.0b013e32830349a1. [DOI] [PubMed] [Google Scholar]