Abstract
Body fatness at young ages may be related to breast cancer risk independently of adult adiposity. The authors conducted a prospective analysis among 188,860 women (7,582 breast cancer cases) in the Nurses’ Health Study (1988–2004) and Nurses’ Health Study II (1989–2005) who recalled their body fatness at ages 5, 10, and 20 years using a 9-level pictogram (level 1: most lean; level 9: most overweight). Body fatness at young ages was inversely associated with risk of both premenopausal and postmenopausal breast cancer (per 1-unit increase in adolescent body fatness, relative risk (RR) = 0.88 and RR = 0.91, respectively; Ptrend < 0.0001). Among all women, the RR for adolescent body fatness of level 6.5 or higher versus level 1 was 0.57 (per 1-unit increase, RR = 0.90; Ptrend < 0.0001) and was unaffected by adjustment for current body mass index. The association was stronger for women with birth weights under 8.5 pounds (<3.9 kg) than for women with birth weights of 8.5 pounds or more (≥3.9 kg) (per 1-unit increase, RR = 0.89 and RR = 0.94, respectively; Pinteraction = 0.04) and stronger for estrogen receptor-negative tumors than for estrogen receptor-positive tumors (per 1-unit increase, RR = 0.86 and RR = 0.92, respectively; Pheterogeneity = 0.03). Body fatness at young ages has a strong and independent inverse relation to breast cancer risk throughout life.
Keywords: adiposity, adolescent, breast neoplasms, child, obesity
Overweight and obesity during adulthood are related to breast cancer risk (1). In postmenopausal women, greater body mass index (BMI; weight (kg)/height (m)2) and weight gain are associated with increased risk (2–4), probably resulting from higher estrogen levels in heavier women due to the conversion of androgen to estrogen in excess adipose tissue (5, 6). In contrast, greater BMI is associated with decreased risk in premenopausal women (3, 4). Although the mechanisms are not understood, premenopausal women who are overweight or obese may experience more anovulatory cycles and have lower levels of ovarian hormones and insulin-like growth factor (IGF)-I (7, 8), which could explain their lower risk (9).
Recent evidence suggests that body fatness during earlier periods of life, even before adulthood, may be inversely related to breast cancer risk many years later (10–22). Some studies have shown that greater body fatness during childhood and adolescence is associated with lower breast cancer risk in premenopausal women (11, 13, 18–22), independently of adult BMI. Moreover, several studies have observed that greater body fatness in childhood is associated with decreased risk in postmenopausal women (12, 13, 17, 20), despite the well-documented positive association for adult BMI. These findings suggest that greater body fatness at young ages may confer a long-term protective effect on breast tissue that results in a permanent reduction in breast cancer risk. This is consistent with animal data, epidemiologic studies, and biomathematical models showing that breast tissue is particularly susceptible to exposures between menarche and first childbirth (23).
Breast cancer is the most commonly diagnosed cancer in women, except for skin cancer (24), yet its causes are poorly understood. Further investigation of the association between body fatness at young ages and risk of breast cancer throughout life could help clarify the biologic basis of this disease. Most previous studies have focused only on premenopausal or postmenopausal breast cancer and have had limited power to examine very high body fatness at young ages. In addition, few researchers (12, 25) have explored whether the association varies according to other risk factors or by tumor characteristics.
To address these issues, we conducted a prospective analysis of body fatness during childhood and adolescence and risk of breast cancer among women in the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHS II). This analysis expanded on 2 previous studies in these cohorts (11, 13) but included an additional 16 years of combined follow-up and more than 3 times as many breast cancer cases as either individual study.
MATERIALS AND METHODS
Study population
The NHS began in 1976, when 121,700 US female registered nurses aged 30–55 years completed a mailed questionnaire about their lifestyle factors and medical histories. In 1989, NHS II was initiated, including 116,609 female registered nurses aged 25–42 years. Follow-up questionnaires have been sent to participants in both studies every 2 years since enrollment to obtain updated information. The follow-up rate for each 2-year cycle has been greater than 90% of the original cohorts.
Follow-up for this analysis began in 1988 for the NHS and in 1989 for the NHS II, when body fatness at young ages was assessed, and ended in 2004 and 2005, respectively. We excluded women with a previous diagnosis of cancer, other than nonmelanoma skin cancer, and those who were missing information on body fatness at age 5, 10, or 20 years. There were 76,298 women in the NHS (1,096,872 person-years) and 112,562 women in NHS II (1,709,063 person-years) who contributed to the analysis (n = 188,860). The major reason for exclusion was missing information on body fatness at young ages (n = 32,935 in the NHS, n = 2,992 in the NHS II). Participants who were excluded were slightly older than those who were included and were more likely to be postmenopausal, less likely to have used oral contraceptives, less likely to have a family history of breast cancer or a personal history of benign breast disease, and less likely to be using postmenopausal hormones.
This study was approved by the Committee on the Use of Human Subjects in Research at Brigham and Women's Hospital and the Harvard School of Public Health (Boston, Massachusetts). Completion of the self-administered questionnaire was considered to imply informed consent.
Assessment of body fatness
Participants were asked to recall their body fatness (also called “somatotype”) at ages 5, 10, and 20 years using a 9-level figure drawing, where level 1 represents the most lean and level 9 represents the most overweight (Figure 1) (26). We averaged each participant's reported somatotypes at ages 5 and 10 years and ages 10 and 20 years to obtain estimates of childhood and adolescent body fatness, respectively. Body fatness at ages 30 and 40 years also was assessed using the same figure drawing. In a validation study among participants in the Third Harvard Growth Study, Pearson correlations between recalled body fatness using this pictogram and measured BMI at approximately the same ages were 0.60 for age 5 years, 0.65 for age 10 years, and 0.66 for age 20 years (27). Other breast cancer risk factors were assessed on various questionnaires during the course of the study.
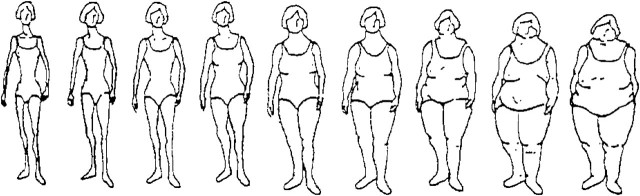
Figure 1.
Figure drawing used to assess body fatness at ages 5, 10, and 20 years in the Nurses’ Health Study (1988) and Nurses’ Health Study II (1989). (Reproduced from Stunkard et al. (26) with permission from Lippincott Williams & Wilkins, Philadelphia, Pennsylvania).
Ascertainment of breast cancer cases
Cases of breast cancer diagnosed between return of the 1988 questionnaire and May 31, 2004, in the NHS and between return of the 1989 questionnaire and May 31, 2005, in NHS II, were identified on the biennial questionnaires, and the National Death Index was searched for nonresponders (28). Investigators reviewed participants’ medical records and pathology reports to confirm the diagnoses and to abstract information on tumor characteristics, including estrogen receptor (ER) and progesterone receptor (PR) status and human epidermal growth factor receptor 2 (HER2) expression. A total of 7,582 cases (4,951 in the NHS and 2,631 in NHS II) were documented.
Statistical analysis
Participants contributed person-time from the return date of the 1988 (NHS) or 1989 (NHS II) questionnaire until the date of breast cancer diagnosis, a report of another cancer (except nonmelanoma skin cancer), death, loss to follow-up, or May 31, 2004 (NHS) or May 31, 2005 (NHS II), whichever occurred sooner. Cox proportional hazards models with joint stratification on age (in months) and 2-year questionnaire cycle were used to calculate relative risks and 95% confidence intervals for categories of body fatness, adjusting for breast cancer risk factors. Although the original pictogram included 9 levels, we collapsed several of the highest categories because of small numbers of cases, which would have decreased the precision of the estimates; this was done prior to estimation of the relative risks. We performed tests for trend by including body fatness in the models as a continuous variable with values ranging from 1 to 9, and we also computed relative risks and 95% confidence intervals for 1-unit increases in body fatness at each age.
Risk factors assessed more than once during follow-up were treated as time-varying covariates. Because age at menarche and adult BMI could be intermediate variables on the causal pathway, we controlled for them only in secondary analyses. Similarly, BMI at age 18 years was included as a covariate in secondary analyses of body fatness at ages 5 and 10 years, but not at age 20 years or the average of ages 10 and 20 years. Participants classified their pattern of menstrual cycles between ages 18 and 22 years as regular (within 8 days), usually irregular, always irregular, or no periods; we also included this variable in secondary analyses to explore the potential role of anovulation.
We initially conducted analyses separately within each cohort and then evaluated heterogeneity in the estimates for body fatness at each age by cohort (29). To assess whether the association between body fatness at young ages and breast cancer risk varied by menopausal status and other characteristics, we stratified the data by these factors and tested interaction terms between body fatness (continuous) and each potential modifier in multivariate models using the Wald test.
We used competing-risks survival analysis (30, 31) to compare the associations of body fatness at young ages with different types of tumors. This approach uses data augmentation (32) to create a separate observation for each subject for each type of outcome and then stratifies the data on event type, allowing for estimation of separate associations of each risk factor with the relative hazard of each type of outcome (31). We used likelihood ratio tests to compare models that assumed different associations between body fatness and each type of tumor with models that assumed the same association. In these tests for heterogeneity, body fatness was modeled as a continuous variable, with values ranging from 1 to 9. All statistical tests were 2-sided.
RESULTS
The mean ages at the beginning of follow-up were 54.7 years and 34.3 years for NHS and NHS II participants, respectively. In the NHS, participants who were heavy at age 10 years were slightly younger than those who were lean (Table 1). They also had greater BMI at age 18 years and at the beginning of follow-up, were younger at menarche, were more likely to have weighed 8.5 pounds or more (≥3.9 kg) at birth, and were less likely to have a history of benign breast disease. Participants in both the highest and lowest body fatness categories were more likely to have had irregular menstrual cycles between ages 18 and 22 years. These associations were generally similar in the NHS II.
Table 1.
Age and Age-Standardized Characteristics of 76,298 Participants in the Nurses’ Health Study (1988) and 112,562 Participants in Nurses’ Health Study II (1989), According to Self-Reported Body Fatness at Age 10 Years
| Characteristic | Body Fatness at Age 10 Yearsa |
|||||
| 1 | 2 | 3 | 4 | 5 | ≥6 | |
| Nurses’ Health Study | ||||||
| No. of participants | 24,134 | 20,002 | 13,423 | 9,774 | 6,429 | 2,536 |
| Mean age, years | 55.7 | 54.7 | 54.4 | 53.8 | 53.6 | 53.3 |
| Birth weight ≥8.5 pounds (≥3.9 kg), % | 7.1 | 8.6 | 11.0 | 12.5 | 13.3 | 16.6 |
| Mean BMIb at age 18 years | 19.8 | 20.8 | 22.0 | 23.0 | 23.9 | 25.4 |
| Mean current BMI | 24.5 | 25.0 | 26.1 | 27.1 | 27.5 | 28.2 |
| Mean height, inchesc | 64.5 | 64.5 | 64.4 | 64.4 | 64.5 | 64.8 |
| Mean age at menarche, years | 12.8 | 12.6 | 12.4 | 12.3 | 12.2 | 12.2 |
| Irregular menstrual cycles at ages 18–22 years, % | 24.1 | 20.8 | 21.3 | 21.7 | 23.5 | 24.7 |
| Ever use of oral contraceptives, % | 48.4 | 48.3 | 47.6 | 48.1 | 48.2 | 47.9 |
| Parous, % | 92.5 | 93.1 | 93.5 | 93.2 | 92.3 | 90.9 |
| Mean parity, no. of childrend | 3.2 | 3.2 | 3.2 | 3.2 | 3.1 | 3.0 |
| Mean age at first birth, yearsd | 25.2 | 25.1 | 25.1 | 25.1 | 25.2 | 25.2 |
| Premenopausal, % | 26.7 | 27.5 | 27.4 | 28.0 | 27.1 | 27.4 |
| Postmenopausal, % | 73.2 | 72.4 | 72.5 | 71.9 | 72.7 | 72.5 |
| Mean age at menopause, yearse | 48.6 | 48.7 | 48.8 | 48.8 | 48.8 | 48.6 |
| Current postmenopausal hormone use, %e | 32.7 | 32.6 | 31.4 | 30.9 | 29.8 | 28.3 |
| Family history of breast cancer, % | 11.0 | 11.0 | 10.4 | 11.0 | 10.4 | 10.8 |
| History of benign breast disease, % | 39.8 | 38.5 | 36.6 | 35.1 | 34.2 | 33.6 |
| Mean alcohol intake, g/day | 6.2 | 6.3 | 6.2 | 6.2 | 6.3 | 5.9 |
| Nurses’ Health Study II | ||||||
| No. of participants | 21,275 | 34,387 | 25,333 | 17,813 | 10,246 | 3,508 |
| Mean age, years | 34.7 | 34.1 | 34.2 | 34.4 | 34.7 | 34.9 |
| Birth weight ≥8.5 pounds (≥3.9 kg), % | 8.3 | 9.3 | 11.6 | 12.9 | 13.4 | 14.6 |
| Mean BMIb at age 18 years | 19.3 | 20.2 | 21.6 | 22.9 | 23.9 | 25.6 |
| Mean current BMI | 22.0 | 22.8 | 24.5 | 26.1 | 26.9 | 27.9 |
| Mean height, inchesc | 64.9 | 64.9 | 64.8 | 64.8 | 65.0 | 65.2 |
| Mean age at menarche, years | 12.8 | 12.6 | 12.3 | 12.1 | 12.0 | 12.0 |
| Irregular menstrual cycles ages 18–22 years, % | 23.6 | 22.6 | 22.5 | 24.0 | 25.1 | 26.5 |
| Ever use of oral contraceptives, % | 85.0 | 83.7 | 82.6 | 82.6 | 81.8 | 81.1 |
| Parous, % | 69.9 | 70.9 | 70.9 | 68.5 | 65.5 | 61.6 |
| Mean parity, no. of childrend | 2.1 | 2.1 | 2.1 | 2.1 | 2.0 | 2.0 |
| Mean age at first birth, yearsd | 25.3 | 25.5 | 25.5 | 25.5 | 25.4 | 25.4 |
| Premenopausal, % | 96.7 | 97.4 | 97.3 | 96.9 | 96.7 | 96.0 |
| Postmenopausal, % | 2.7 | 2.2 | 2.1 | 2.4 | 2.5 | 3.2 |
| Family history of breast cancer, % | 5.9 | 6.0 | 5.7 | 5.9 | 6.3 | 6.4 |
| History of benign breast disease, % | 30.2 | 28.6 | 28.0 | 27.8 | 27.7 | 29.2 |
| Mean alcohol intake, g/day | 3.2 | 3.1 | 3.1 | 3.1 | 3.1 | 2.9 |
Abbreviation: BMI, body mass index.
Participants were asked to recall their body fatness at ages 5, 10, and 20 years using a 9-level figure drawing, where level 1 represents the most lean and level 9 represents the most overweight (Figure 1) (26).
Weight (kg)/height (m)2.
1 inch = 2.54 cm.
Among parous women only.
Among postmenopausal women only.
Body fatness at ages 5, 10, and 20 years and average body fatness during childhood (ages 5–10 years) and adolescence (ages 10–20 years) were both significantly inversely associated with breast cancer risk in the NHS, with the greatest decrease in risk being observed for adolescent body fatness (for level 5.5 or higher vs. level 1, multivariate relative risk (RR) = 0.69, 95% confidence interval (CI): 0.55, 0.86; Ptrend < 0.0001). The associations were slightly stronger in NHS II (RR = 0.57, 95% CI: 0.41, 0.78; Ptrend < 0.0001). Because there were no significant differences between the 2 cohorts (all Pheterogeneity values > 0.11), the data were combined for all subsequent analyses.
In the pooled analyses, body fatness at each age was significantly inversely associated with risk of both premenopausal and postmenopausal breast cancer (Table 2). The multivariate relative risks for adolescent body fatness of level 5.5 or higher (vs. level 1) were 0.54 (95% CI: 0.37, 0.78; Ptrend < 0.0001) for premenopausal breast cancer and 0.66 (95% CI: 0.53, 0.83; Ptrend < 0.0001) for postmenopausal breast cancer (per 1-unit increase, RR = 0.88 and RR = 0.91, respectively); there were no significant interactions with menopausal status. The associations for premenopausal breast cancer were slightly attenuated after adjustment for current BMI, whereas the associations for postmenopausal breast cancer became stronger. Adjustment for age at menarche and menstrual cycle patterns between ages 18 and 22 years did not affect the observed associations (data not shown). The multivariate relative risks for body fatness of level 6 or higher (vs. level 1) at age 30 years were 0.49 (95% CI: 0.33, 0.74; Ptrend < 0.0001) and 0.79 (95% CI: 0.64, 0.98; Ptrend = 0.002) for premenopausal and postmenopausal breast cancer, respectively; after adjustment for adolescent body fatness, however, the association for premenopausal breast cancer was substantially attenuated (RR = 0.67, 95% CI: 0.43, 1.03; Ptrend = 0.04) and the direction of the association for postmenopausal breast cancer was reversed (RR = 1.18, 95% CI: 0.93, 1.50; Ptrend = 0.01). Body fatness at age 40 years was not associated with risk in either group (data not shown).
Table 2.
Relative Risk of Breast Cancer According to Self-Reported Body Fatness at Young Ages and Menopausal Status Among 188,860 Participants in the Nurses’ Health Study (1988–2004) and Nurses’ Health Study II (1989–2005)a
| Body Fatnessb | Premenopausal Women (2,188 Cases) |
Postmenopausal Women (4,974 Cases) |
|||||||||||
| No. of Cases | Age-Adjusted RR | MV-Adjusted RRc | 95% CI | MV-Adjusted RR + Current BMId | 95% CI | No. of Cases | Age-Adjusted RR | MV-Adjusted RRc | 95% CI | MV-Adjusted RR + Current BMId | 95% CI | ||
| At age 5 years | |||||||||||||
| 1 | 614 | 1.0 | 1.0 | 1.0 | 2,218 | 1.0 | 1.0 | 1.0 | |||||
| 2 | 713 | 0.98 | 0.98 | 0.87, 1.09 | 0.98 | 0.88, 1.09 | 1,210 | 0.99 | 0.98 | 0.91, 1.05 | 0.98 | 0.91, 1.05 | |
| 3 | 481 | 0.87 | 0.87 | 0.77, 0.98 | 0.89 | 0.79, 1.00 | 773 | 0.84 | 0.84 | 0.77, 0.91 | 0.83 | 0.76, 0.90 | |
| 4 | 277 | 0.91 | 0.91 | 0.79, 1.05 | 0.95 | 0.82, 1.10 | 455 | 0.79 | 0.79 | 0.72, 0.88 | 0.77 | 0.70, 0.85 | |
| 5 | 79 | 0.61 | 0.60 | 0.47, 0.76 | 0.63 | 0.50, 0.80 | 244 | 0.80 | 0.81 | 0.71, 0.93 | 0.79 | 0.69, 0.90 | |
| ≥6 | 24 | 0.61 | 0.59 | 0.38, 0.89 | 0.63 | 0.41, 0.96 | 74 | 0.76 | 0.77 | 0.61, 0.97 | 0.74 | 0.58, 0.93 | |
| Per 1-unit increase | 0.93 | 0.92 | 0.89, 0.96 | 0.94 | 0.90, 0.97 | 0.93 | 0.94 | 0.92, 0.96 | 0.93 | 0.91, 0.95 | |||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Pinteraction = 0.56e | |||||||||||||
| At age 10 years | |||||||||||||
| 1 | 476 | 1.0 | 1.0 | 1.0 | 1,720 | 1.0 | 1.0 | 1.0 | |||||
| 2 | 716 | 0.99 | 1.00 | 0.89, 1.12 | 1.01 | 0.90, 1.13 | 1,443 | 1.01 | 1.02 | 0.95, 1.09 | 1.01 | 0.94, 1.08 | |
| 3 | 481 | 0.89 | 0.90 | 0.80, 1.03 | 0.93 | 0.82, 1.06 | 813 | 0.84 | 0.86 | 0.79, 0.93 | 0.83 | 0.77, 0.91 | |
| 4 | 324 | 0.84 | 0.85 | 0.74, 0.98 | 0.89 | 0.77, 1.03 | 546 | 0.79 | 0.80 | 0.73, 0.88 | 0.77 | 0.69, 0.85 | |
| 5 | 144 | 0.64 | 0.64 | 0.53, 0.77 | 0.68 | 0.56, 0.82 | 325 | 0.73 | 0.74 | 0.66, 0.84 | 0.71 | 0.63, 0.80 | |
| ≥6 | 47 | 0.60 | 0.59 | 0.43, 0.80 | 0.63 | 0.46, 0.86 | 127 | 0.73 | 0.75 | 0.62, 0.90 | 0.70 | 0.58, 0.84 | |
| Per 1-unit increase | 0.91 | 0.91 | 0.88, 0.94 | 0.92 | 0.89, 0.95 | 0.92 | 0.93 | 0.91, 0.95 | 0.92 | 0.90, 0.94 | |||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Pinteraction = 0.31e | |||||||||||||
| At age 20 years | |||||||||||||
| 1 | 139 | 1.0 | 1.0 | 1.0 | 650 | 1.0 | 1.0 | 1.0 | |||||
| 2 | 663 | 0.93 | 0.94 | 0.78, 1.13 | 0.95 | 0.79, 1.14 | 1,611 | 0.99 | 0.99 | 0.90, 1.08 | 0.98 | 0.90, 1.08 | |
| 3 | 810 | 0.82 | 0.83 | 0.69, 1.00 | 0.86 | 0.71, 1.03 | 1,625 | 0.90 | 0.91 | 0.83, 0.99 | 0.88 | 0.80, 0.96 | |
| 4 | 409 | 0.75 | 0.76 | 0.63, 0.93 | 0.80 | 0.66, 0.98 | 744 | 0.79 | 0.80 | 0.72, 0.89 | 0.76 | 0.68, 0.84 | |
| 5 | 124 | 0.65 | 0.65 | 0.51, 0.83 | 0.70 | 0.54, 0.90 | 253 | 0.74 | 0.75 | 0.65, 0.87 | 0.69 | 0.59, 0.80 | |
| ≥6 | 43 | 0.55 | 0.53 | 0.38, 0.75 | 0.60 | 0.42, 0.86 | 91 | 0.70 | 0.72 | 0.57, 0.89 | 0.64 | 0.51, 0.79 | |
| Per 1-unit increase | 0.90 | 0.89 | 0.86, 0.93 | 0.91 | 0.87, 0.95 | 0.92 | 0.93 | 0.90, 0.95 | 0.91 | 0.88, 0.93 | |||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Pinteraction = 0.23e | |||||||||||||
| Average during childhood (ages 5–10 years) | |||||||||||||
| 1 | 451 | 1.0 | 1.0 | 1.0 | 1,655 | 1.0 | 1.0 | 1.0 | |||||
| 1.5–2 | 710 | 0.97 | 0.97 | 0.86, 1.09 | 0.98 | 0.87, 1.10 | 1,498 | 1.01 | 1.02 | 0.95, 1.09 | 1.01 | 0.94, 1.08 | |
| 2.5–3 | 522 | 0.85 | 0.86 | 0.76, 0.98 | 0.88 | 0.78, 1.01 | 858 | 0.82 | 0.83 | 0.76, 0.90 | 0.81 | 0.74, 0.88 | |
| 3.5–4 | 347 | 0.83 | 0.83 | 0.72, 0.96 | 0.87 | 0.76, 1.01 | 559 | 0.78 | 0.79 | 0.71, 0.87 | 0.76 | 0.69, 0.83 | |
| 4.5–5 | 125 | 0.66 | 0.65 | 0.53, 0.79 | 0.70 | 0.57, 0.85 | 299 | 0.75 | 0.76 | 0.67, 0.86 | 0.73 | 0.64, 0.82 | |
| ≥5.5 | 33 | 0.55 | 0.53 | 0.37, 0.76 | 0.58 | 0.40, 0.83 | 105 | 0.74 | 0.76 | 0.62, 0.93 | 0.71 | 0.58, 0.87 | |
| Per 1-unit increase | 0.91 | 0.91 | 0.87, 0.94 | 0.92 | 0.89, 0.96 | 0.92 | 0.93 | 0.90, 0.95 | 0.91 | 0.89, 0.94 | |||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Pinteraction = 0.36e | |||||||||||||
| Average during adolescence (ages 10–20 years) | |||||||||||||
| 1 | 108 | 1.0 | 1.0 | 1.0 | 587 | 1.0 | 1.0 | 1.0 | |||||
| 1.5–2 | 649 | 0.98 | 0.99 | 0.80, 1.21 | 1.00 | 0.81, 1.22 | 1,687 | 1.00 | 1.00 | 0.91, 1.10 | 0.99 | 0.90, 1.09 | |
| 2.5–3 | 759 | 0.88 | 0.90 | 0.73, 1.10 | 0.92 | 0.75, 1.13 | 1,481 | 0.93 | 0.95 | 0.86, 1.04 | 0.92 | 0.83, 1.01 | |
| 3.5–3 | 489 | 0.84 | 0.85 | 0.69, 1.05 | 0.89 | 0.72, 1.10 | 776 | 0.79 | 0.81 | 0.72, 0.90 | 0.76 | 0.68, 0.85 | |
| 4.5–5 | 142 | 0.58 | 0.58 | 0.45, 0.74 | 0.62 | 0.48, 0.80 | 348 | 0.75 | 0.76 | 0.66, 0.87 | 0.70 | 0.61, 0.81 | |
| ≥5.5 | 41 | 0.55 | 0.54 | 0.37, 0.78 | 0.59 | 0.41, 0.86 | 95 | 0.65 | 0.66 | 0.53, 0.83 | 0.60 | 0.48, 0.74 | |
| Per 1-unit increase | 0.88 | 0.88 | 0.84, 0.91 | 0.90 | 0.86, 0.93 | 0.91 | 0.91 | 0.89, 0.93 | 0.89 | 0.87, 0.92 | |||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Pinteraction = 0.16e | |||||||||||||
Abbreviations: BMI, body mass index; CI, confidence interval; MV, multivariate; RR, relative risk.
Women who were missing data on menopausal status were excluded from this analysis.
Participants were asked to recall their body fatness at ages 5, 10, and 20 years using a 9-level figure drawing, where level 1 represents the most lean and level 9 represents the most overweight (Figure 1) (26).
Multivariate RRs for premenopausal women were adjusted for age, time period, parity/age at first birth, family history of breast cancer, personal history of benign breast disease, height, alcohol intake, oral contraceptive use, and birth weight. Multivariate RRs for postmenopausal women were adjusted for the same factors plus age at menopause and postmenopausal hormone use.
Multivariate RRs were adjusted for the factors listed above, plus current BMI (weight (kg)/height (m)2) as a continuous variable.
Tests for interaction compared trends for body fatness from multivariate models without adjustment for current BMI.
The same inverse associations for body fatness at young ages were observed in analyses combining all women, regardless of menopausal status, and the larger number of cases allowed examination of higher levels of body fatness (Table 3). Breast cancer risk continued to decline with increasing body fatness; for adolescent body fatness, the multivariate relative risk was 0.66 (95% CI: 0.55, 0.80) for levels 5.5–6 and 0.57 (95% CI: 0.37, 0.87) for level 6.5 or higher (per 1-unit increase, RR = 0.90; Ptrend < 0.0001). Adjustment for current BMI had little impact on the associations, although the associations for body fatness at ages 5 and 10 years individually and average body fatness in childhood were somewhat attenuated when adjusted for BMI at age 18 years. Body fatness at age 30 years also was inversely associated with breast cancer risk, although this was no longer apparent after adjustment for adolescent body fatness (RR = 1.07, 95% CI: 0.81, 1.42; Ptrend = 0.39).
Table 3.
Relative Risk of Breast Cancer According to Self-Reported Body Fatness at Young Ages Among 188,860 Participants in the Nurses’ Health Study (1988–2004) and Nurses’ Health Study II (1989–2005)
| Body Fatnessa | No. of Cases | No. of Person-Years | Age-Adjusted RR | MV-Adjusted RRb | 95% CI | MV-Adjusted RR + Current BMIc | 95% CI | MV-Adjusted RR + BMI at Age 18 Yearsd | 95% CI |
| At age 5 years | |||||||||
| 1 | 2,959 | 879,954 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| 2 | 2,061 | 792,863 | 1.00 | 0.98 | 0.93, 1.04 | 0.98 | 0.93, 1.04 | 1.00 | 0.94, 1.06 |
| 3 | 1,338 | 594,737 | 0.85 | 0.85 | 0.80, 0.91 | 0.85 | 0.79, 0.90 | 0.89 | 0.83, 0.95 |
| 4 | 775 | 335,909 | 0.83 | 0.83 | 0.77, 0.90 | 0.82 | 0.76, 0.89 | 0.89 | 0.82, 0.96 |
| 5 | 344 | 154,075 | 0.75 | 0.75 | 0.67, 0.84 | 0.74 | 0.66, 0.83 | 0.82 | 0.73, 0.92 |
| 6 | 83 | 39,752 | 0.70 | 0.70 | 0.56, 0.87 | 0.69 | 0.56, 0.86 | 0.79 | 0.63, 0.98 |
| ≥7 | 22 | 8,645 | 0.82 | 0.81 | 0.53, 1.23 | 0.79 | 0.52, 1.21 | 0.93 | 0.61, 1.41 |
| Per 1-unit increase | 0.93 | 0.93 | 0.92, 0.95 | 0.93 | 0.91, 0.95 | 0.95 | 0.94, 0.97 | ||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| At age 10 years | |||||||||
| 1 | 2,292 | 667,307 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| 2 | 2,304 | 810,187 | 1.02 | 1.02 | 0.96, 1.08 | 1.02 | 0.96, 1.08 | 1.04 | 0.98, 1.10 |
| 3 | 1,376 | 578,964 | 0.87 | 0.88 | 0.82, 0.94 | 0.87 | 0.81, 0.93 | 0.92 | 0.85, 0.98 |
| 4 | 923 | 412,314 | 0.81 | 0.82 | 0.76, 0.88 | 0.80 | 0.74, 0.87 | 0.87 | 0.80, 0.94 |
| 5 | 498 | 247,605 | 0.70 | 0.71 | 0.64, 0.78 | 0.69 | 0.62, 0.76 | 0.76 | 0.69, 0.84 |
| 6 | 160 | 74,291 | 0.73 | 0.73 | 0.62, 0.86 | 0.71 | 0.61, 0.84 | 0.81 | 0.69, 0.96 |
| ≥7 | 29 | 15,268 | 0.63 | 0.63 | 0.44, 0.91 | 0.61 | 0.42, 0.88 | 0.72 | 0.50, 1.04 |
| Per 1-unit increase | 0.92 | 0.92 | 0.91, 0.94 | 0.92 | 0.90, 0.93 | 0.94 | 0.92, 0.96 | ||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| At age 20 years | |||||||||
| 1 | 816 | 205,324 | 1.0 | 1.0 | 1.0 | ||||
| 2 | 2,390 | 770,336 | 0.98 | 0.98 | 0.90, 1.06 | 0.98 | 0.90, 1.06 | ||
| 3 | 2,585 | 1,009,016 | 0.88 | 0.89 | 0.83, 0.97 | 0.88 | 0.81, 0.96 | ||
| 4 | 1,239 | 542,624 | 0.80 | 0.81 | 0.74, 0.89 | 0.79 | 0.72, 0.86 | ||
| 5 | 408 | 196,208 | 0.73 | 0.73 | 0.65, 0.83 | 0.70 | 0.62, 0.79 | ||
| 6 | 119 | 62,436 | 0.68 | 0.68 | 0.56, 0.83 | 0.65 | 0.53, 0.79 | ||
| ≥7 | 25 | 19,991 | 0.52 | 0.52 | 0.35, 0.78 | 0.48 | 0.32, 0.72 | ||
| Per 1-unit increase | 0.92 | 0.92 | 0.90, 0.94 | 0.91 | 0.89, 0.93 | ||||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | ||||||
| Average during childhood (ages 5–10 years) | |||||||||
| 1 | 2,193 | 630,338 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| 1.5–2 | 2,356 | 820,617 | 1.01 | 1.01 | 0.95, 1.07 | 1.01 | 0.95, 1.07 | 1.03 | 0.97, 1.10 |
| 2.5–3 | 1,475 | 638,865 | 0.84 | 0.85 | 0.79, 0.91 | 0.84 | 0.79, 0.90 | 0.89 | 0.83, 0.95 |
| 3.5–4 | 959 | 433,852 | 0.80 | 0.80 | 0.74, 0.87 | 0.79 | 0.73, 0.86 | 0.86 | 0.79, 0.93 |
| 4.5–5 | 450 | 212,978 | 0.72 | 0.72 | 0.65, 0.80 | 0.71 | 0.64, 0.79 | 0.79 | 0.71, 0.88 |
| 5.5–6 | 123 | 57,476 | 0.70 | 0.71 | 0.59, 0.85 | 0.69 | 0.58, 0.83 | 0.79 | 0.66, 0.96 |
| ≥6.5 | 26 | 11,810 | 0.71 | 0.70 | 0.48, 1.03 | 0.68 | 0.46, 1.00 | 0.81 | 0.55, 1.20 |
| Per 1-unit increase | 0.92 | 0.92 | 0.90, 0.94 | 0.92 | 0.90, 0.93 | 0.94 | 0.92, 0.96 | ||
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Average during adolescence (ages 10–20 years) | |||||||||
| 1 | 720 | 177,507 | 1.0 | 1.0 | 1.0 | ||||
| 1.5–2 | 2,454 | 777,451 | 0.99 | 0.99 | 0.91, 1.08 | 0.99 | 0.91, 1.08 | ||
| 2.5–3 | 2,383 | 911,516 | 0.92 | 0.93 | 0.86, 1.02 | 0.92 | 0.85, 1.00 | ||
| 3.5–4 | 1,355 | 595,126 | 0.82 | 0.83 | 0.76, 0.91 | 0.81 | 0.74, 0.89 | ||
| 4.5–5 | 520 | 263,320 | 0.69 | 0.69 | 0.62, 0.78 | 0.67 | 0.59, 0.75 | ||
| 5.5–6 | 128 | 66,774 | 0.65 | 0.66 | 0.55, 0.80 | 0.62 | 0.51, 0.75 | ||
| ≥6.5 | 22 | 14,243 | 0.57 | 0.57 | 0.37, 0.87 | 0.53 | 0.35, 0.81 | ||
| Per 1-unit increase | 0.90 | 0.90 | 0.89, 0.92 | 0.89 | 0.87, 0.91 | ||||
| Ptrend | <0.0001 | <0.0001 | <0.0001 |
Abbreviations: BMI, body mass index; CI, confidence interval; MV, multivariate; RR, relative risk.
Participants were asked to recall their body fatness at ages 5, 10, and 20 years using a 9-level figure drawing, where level 1 represents the most lean and level 9 represents the most overweight (Figure 1) (26).
Multivariate RRs were adjusted for age, time period, parity/age at first birth, family history of breast cancer, personal history of benign breast disease, height, alcohol intake, oral contraceptive use, birth weight, menopausal status, age at menopause, and postmenopausal hormone use.
Multivariate RRs were adjusted for the factors listed above, plus current BMI (weight (kg)/height (m)2) as a continuous variable.
Multivariate RRs were adjusted for the factors listed above, plus BMI at age 18 years as a continuous variable.
The inverse association for adolescent body fatness was stronger for women who weighed less than 8.5 pounds (<3.9 kg) at birth than for those who weighed 8.5 pounds or more (≥3.9 kg) (per 1-unit increase, RR = 0.89 and RR = 0.94, respectively; Pinteraction = 0.04) (Table 4). The inverse association for adolescent body fatness also was stronger for past and current postmenopausal hormone users (per 1-unit increase, RR = 0.89 and RR = 0.90, respectively) than for never users (per 1-unit increase, RR = 0.97; Pinteraction = 0.05). There was no significant variation according to any of the other factors that were examined, including current BMI.
Table 4.
Multivariate-Adjusted Relative Riska of Breast Cancer According to Self-Reported Average Adolescent Body Fatness and Other Characteristics Among Participants in the Nurses’ Health Study (1988–2004) and Nurses’ Health Study II (1989–2005)
| Characteristic | No. of Cases | RR by Average Adolescent Body Fatnessb (Ages 10–20 Years) |
Ptrend | |||||||
| 1 (Referent) | 1.5–2 | 2.5–3 | 3.5–4 | ≥4.5 |
Per 1-Unit Increase |
|||||
| RR | 95% CI | RR | 95% CI | |||||||
| Birth weight, pounds (kg) | ||||||||||
| <8.5 (<3.9) | 4,992 | 1.0 | 0.97 | 0.91 | 0.80 | 0.64 | 0.56, 0.73 | 0.89 | 0.87, 0.92 | <0.0001 |
| ≥8.5 (≥3.9) | 809 | 1.0 | 1.13 | 1.07 | 0.96 | 0.90 | 0.64, 1.27 | 0.94 | 0.89, 1.00 | 0.05 |
| Pinteraction = 0.04 | ||||||||||
| Height, inches (cm) | ||||||||||
| ≤63 (≤161) | 2,263 | 1.0 | 1.22 | 1.08 | 0.97 | 0.89 | 0.73, 1.09 | 0.93 | 0.89, 0.96 | <0.0001 |
| 64–65 (162–166) | 2,346 | 1.0 | 0.89 | 0.85 | 0.73 | 0.65 | 0.54, 0.79 | 0.90 | 0.86, 0.93 | <0.0001 |
| ≥66 (≥167) | 2,969 | 1.0 | 0.92 | 0.91 | 0.81 | 0.58 | 0.49, 0.69 | 0.89 | 0.87, 0.92 | <0.0001 |
| Pinteraction = 0.20 | ||||||||||
| Current body mass indexc | ||||||||||
| Premenopausal women | ||||||||||
| <22 | 649 | 1.0 | 0.98 | 0.86 | 0.86 | 0.63 | 0.37, 1.07 | 0.90 | 0.83, 0.98 | 0.02 |
| 22–24.9 | 643 | 1.0 | 0.88 | 0.83 | 0.74 | 0.55 | 0.34, 0.87 | 0.88 | 0.82, 0.96 | 0.002 |
| ≥25 | 895 | 1.0 | 1.20 | 1.14 | 1.11 | 0.73 | 0.43, 1.22 | 0.88 | 0.83, 0.94 | <0.0001 |
| Pinteraction = 0.38 | ||||||||||
| Postmenopausal women | ||||||||||
| <22 | 889 | 1.0 | 0.93 | 0.94 | 0.81 | 0.53 | 0.36, 0.77 | 0.90 | 0.84, 0.96 | 0.002 |
| 22–24.9 | 1,339 | 1.0 | 1.05 | 0.91 | 0.79 | 0.74 | 0.57, 0.97 | 0.90 | 0.85, 0.95 | <0.0001 |
| 25–29.9 | 1,668 | 1.0 | 1.02 | 0.99 | 0.77 | 0.73 | 0.58, 0.91 | 0.91 | 0.87, 0.94 | <0.0001 |
| ≥30 | 1,076 | 1.0 | 0.86 | 0.76 | 0.65 | 0.61 | 0.46, 0.80 | 0.88 | 0.83, 0.92 | <0.0001 |
| Pinteraction = 0.55 | ||||||||||
| Family history of breast cancer | ||||||||||
| No family history | 6,214 | 1.0 | 0.98 | 0.90 | 0.81 | 0.68 | 0.60, 0.76 | 0.90 | 0.88, 0.92 | <0.0001 |
| Family history | 1,368 | 1.0 | 1.09 | 1.09 | 0.96 | 0.71 | 0.54, 0.92 | 0.91 | 0.87, 0.96 | 0.0003 |
| Pinteraction = 0.29 | ||||||||||
| Parity | ||||||||||
| Nulliparous | 890 | 1.0 | 0.96 | 1.01 | 0.97 | 0.62 | 0.46, 0.85 | 0.91 | 0.86, 0.96 | 0.001 |
| Parous | 6,479 | 1.0 | 1.00 | 0.92 | 0.82 | 0.71 | 0.63, 0.80 | 0.91 | 0.89, 0.93 | <0.0001 |
| Pinteraction = 0.83 | ||||||||||
| Age at menarche, years | ||||||||||
| <13 | 3,902 | 1.0 | 1.07 | 0.95 | 0.88 | 0.74 | 0.63, 0.86 | 0.90 | 0.88, 0.93 | <0.0001 |
| ≥13 | 3,631 | 1.0 | 0.93 | 0.93 | 0.77 | 0.60 | 0.51, 0.71 | 0.90 | 0.87, 0.92 | <0.0001 |
| Pinteraction = 0.50 | ||||||||||
| Menstrual cycle pattern at ages 18–22 years | ||||||||||
| Regular | 5,775 | 1.0 | 1.01 | 0.94 | 0.84 | 0.70 | 0.62, 0.79 | 0.91 | 0.89, 0.93 | <0.0001 |
| Irregular | 1,704 | 1.0 | 0.94 | 0.91 | 0.79 | 0.62 | 0.50, 0.77 | 0.89 | 0.85, 0.93 | <0.0001 |
| Pinteraction = 0.41 | ||||||||||
| Postmenopausal hormone use (postmenopausal women only) | ||||||||||
| Never use | 1,247 | 1.0 | 0.95 | 0.89 | 0.86 | 0.89 | 0.71, 1.12 | 0.97 | 0.92, 1.01 | 0.15 |
| Past use | 960 | 1.0 | 0.98 | 1.05 | 0.75 | 0.67 | 0.50, 0.89 | 0.89 | 0.84, 0.95 | <0.0001 |
| Current use | 2,454 | 1.0 | 1.06 | 0.95 | 0.86 | 0.71 | 0.59, 0.86 | 0.90 | 0.87, 0.93 | <0.0001 |
| Pinteraction = 0.05 | ||||||||||
Abbreviations: CI, confidence interval; RR, relative risk.
Multivariate RRs were adjusted for age, time period, parity/age at first birth, family history of breast cancer, personal history of benign breast disease, height, alcohol intake, oral contraceptive use, birth weight, menopausal status, age at menopause, and postmenopausal hormone use; models stratifying on an individual factor included all variables except that factor.
Participants were asked to recall their body fatness at ages 5, 10, and 20 years using a 9-level figure drawing, where level 1 represents the most lean and level 9 represents the most overweight (Figure 1) (26).
Weight (kg)/height (m)2.
The inverse association for adolescent body fatness was stronger for ER-negative (ER−) than for ER-positive (ER+) tumors (per 1-unit increase, RR = 0.86 and RR = 0.92, respectively; Pheterogeneity = 0.03) (Table 5). These estimates were not affected by adjustment for menstrual cycle patterns between ages 18 and 22 years (data not shown). In contrast, PR status was not an important factor (Pheterogeneity = 0.15). The association appeared stronger for tumors that overexpressed HER2 compared with those that did not, although this difference was not statistically significant (per 1-unit increase, RR = 0.85 and RR = 0.93, respectively; Pheterogeneity = 0.07). The associations did not differ on the basis of invasiveness or ductal/lobular origin of the tumors.
Table 5.
Multivariate-Adjusted Relative Riska of Breast Cancer of Different Tumor Subtypes According to Self-Reported Average Adolescent Body Fatness Among Participants in the Nurses’ Health Study (1988–2004) and Nurses’ Health Study II (1989–2005)
| Tumor Subtype | No. of Cases | RR by Average Adolescent Body Fatnessb (Ages 10–20 Years) |
Ptrend | |||||||
| 1 (Referent) | 1.5–2 | 2.5–3 | 3.5–4 | ≥4.5 |
Per 1-Unit Increase |
|||||
| RR | 95% CI | RR | 95% CI | |||||||
| Invasive | 6,176 | 1.0 | 0.98 | 0.94 | 0.81 | 0.69 | 0.61, 0.77 | 0.90 | 0.88, 0.92 | <0.0001 |
| In situ | 1,406 | 1.0 | 1.04 | 0.92 | 0.93 | 0.66 | 0.51, 0.85 | 0.91 | 0.87, 0.95 | <0.0001 |
| Pheterogeneity = 0.82 | ||||||||||
| ER+/PR+ | 3,191 | 1.0 | 0.92 | 0.91 | 0.79 | 0.72 | 0.61, 0.84 | 0.92 | 0.89, 0.95 | <0.0001 |
| ER−/PR− | 880 | 1.0 | 1.09 | 0.93 | 0.85 | 0.56 | 0.40, 0.79 | 0.85 | 0.80, 0.90 | <0.0001 |
| ER+/PR− | 696 | 1.0 | 1.02 | 0.90 | 0.80 | 0.72 | 0.51, 1.01 | 0.90 | 0.85, 0.96 | 0.002 |
| Pheterogeneity = 0.08 | ||||||||||
| All ER+ | 4,031 | 1.0 | 0.93 | 0.91 | 0.78 | 0.72 | 0.63, 0.83 | 0.92 | 0.89, 0.94 | <0.0001 |
| All ER− | 1,077 | 1.0 | 1.09 | 0.94 | 0.87 | 0.56 | 0.42, 0.76 | 0.86 | 0.81, 0.91 | <0.0001 |
| Pheterogeneity = 0.03 | ||||||||||
| Ductal | 4,671 | 1.0 | 0.96 | 0.91 | 0.79 | 0.69 | 0.60, 0.79 | 0.91 | 0.88, 0.93 | <0.0001 |
| Lobular | 615 | 1.0 | 1.16 | 1.13 | 0.76 | 0.75 | 0.51, 1.10 | 0.88 | 0.82, 0.95 | 0.001 |
| Pheterogeneity = 0.46 | ||||||||||
| HER2+c | 391 | 1.0 | 0.90 | 0.74 | 0.63 | 0.57 | 0.35, 0.90 | 0.85 | 0.77, 0.93 | 0.0004 |
| HER2−c | 1,517 | 1.0 | 1.01 | 0.98 | 0.85 | 0.82 | 0.65, 1.04 | 0.93 | 0.89, 0.97 | 0.002 |
| Pheterogeneity = 0.07 | ||||||||||
Abbreviations: CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; RR, relative risk.
Multivariate RRs were adjusted for age, time period, parity/age at first birth, family history of breast cancer, personal history of benign breast disease, height, alcohol intake, oral contraceptive use, birth weight, menopausal status, age at menopause, and postmenopausal hormone use.
Participants were asked to recall their body fatness at ages 5, 10, and 20 years using a 9-level figure drawing, where level 1 represents the most lean and level 9 represents the most overweight (Figure 1) (26).
Analyses for HER2+ and HER2− tumors were from 1998–2004 in the Nurses’ Health Study and from 1999–2005 in Nurses’ Health Study II, because HER2 status was not evaluated before the 1998 and 1999 follow-up cycles, respectively.
DISCUSSION
In this large prospective study, greater body fatness at young ages, particularly during adolescence, was associated with a substantial decrease in breast cancer risk. The inverse association was fairly linear, with no apparent threshold. Similar associations were observed for premenopausal and postmenopausal breast cancer, and both were independent of current BMI. The associations were stronger for women who weighed less than 8.5 pounds at birth and for past and current postmenopausal hormone users but did not vary significantly by other factors. Inverse associations were observed for all tumor subtypes, although they appeared stronger for ER− tumors than for ER+ tumors and possibly stronger for HER2-positive (HER2+) tumors than for HER2-negative (HER2−) tumors.
Our findings confirm those from previous studies that have shown inverse associations between body fatness at young ages and risk of premenopausal breast cancer (11, 33). Results for postmenopausal breast cancer are not as well-documented but are consistent with several prior studies (13, 33). When we adjusted for current BMI, the inverse associations for postmenopausal breast cancer became stronger and more similar to those for premenopausal breast cancer, providing further evidence that greater body fatness at young ages may confer a lasting protective effect.
The mechanisms by which greater body fatness during childhood and adolescence may reduce breast cancer risk are not understood. Girls who are overweight at young ages may experience slower pubertal growth and sexual maturation (13), despite having earlier menarche; rapid adolescent growth has been associated with increased breast cancer risk (13, 23). Obesity in preadolescent and adolescent girls also is associated with higher levels of insulin (34) and androgens (35, 36), greater frequency of anovulatory cycles (35), and reduced fertility later in life (37). In previous analyses in the NHS and NHS II, higher BMI at age 18 years was associated with increased risk of irregular and long menstrual cycles between ages 18 and 22 years and increased risk of ovulatory infertility (38). However, a recent study in NHS II found that these factors were unlikely to explain the relation between BMI in early adulthood and premenopausal breast cancer (19). Similarly, in the present study, the associations of body fatness with risk of all breast cancers and with risk of ER+ and ER− tumors separately were unchanged after adjustment for menstrual cycle patterns between ages 18 and 22 years, suggesting that other factors may be involved.
Girls who are heavier at young ages might experience earlier differentiation of breast tissue due to higher levels of estrogens and other sex hormones (36, 39), and terminally differentiated cells are less susceptible to malignant transformation (40). Experiments in rats have shown that prepubertal or pubertal administration of sex hormones leads to differentiation of cells of the mammary gland and a reduction in the incidence of mammary tumors (41, 42). However, a longitudinal study in girls showed no differences in estrogen or progesterone concentrations according to BMI between ages 8 and 10 years (36), and body fatness at ages 5 and 10 years was not associated with levels of sex hormones among premenopausal women in the NHS (8). Body fatness during childhood and adolescence could act through other hormonal pathways, such as IGF-I/IGF binding protein 3 (7, 43), although this merits further exploration.
Few studies have had sufficient power to examine whether the inverse association for body fatness at young ages is modified by other factors. In 1 study, women with above-average weight at age 12 years who were relatives of breast cancer cases were at increased risk of breast cancer, whereas the association was inverse among women not related to the cases (25). In contrast, a prospective analysis of postmenopausal breast cancer among participants in the Iowa Women's Health Study found no interaction between relative weight at age 12 years and family history (12), similarly to our study.
Our findings suggest that the inverse association for adolescent body fatness may be restricted to women who weighed less than 8.5 pounds at birth. To our knowledge, this is the first study to have documented such an interaction. Birth weight has been positively associated with risk of breast cancer, especially premenopausal breast cancer, in multiple studies (44). Greater birth weight also has been linked to elevated levels of IGF-I and decreased levels of IGF binding protein 3 in premenopausal women (7). Women with greater birth weight may be at higher breast cancer risk due to permanent reprogramming of the growth hormone/IGF axis (45) or genetic variation in the IGF pathway (46). As a result, greater body fatness at later ages may no longer be protective among these women.
The observed interaction between adolescent body fatness and postmenopausal hormone use, with a stronger inverse association among past and current users than among never users, was unexpected and possibly due to chance. Previous studies of adult BMI and weight gain in the NHS have shown stronger positive associations for postmenopausal breast cancer among women who have never used postmenopausal hormones (47). We did not observe this for adolescent body fatness, providing further evidence that the mechanism does not operate through current BMI and probably is not mediated by sex hormones, at least in postmenopausal women.
To our knowledge, only 1 prior study has examined associations between body fatness at young ages and risk of different breast cancer tumor subtypes. In this study, which included only postmenopausal women (2,503 cases), the inverse association for relative weight at age 12 years was strongest for PR-negative (PR−) tumors and weakest for PR-positive (PR+) tumors, although no formal tests of heterogeneity were conducted (12). In contrast, we observed a stronger inverse association for ER− tumors than for ER+ tumors, with significant heterogeneity, while PR status did not seem to be important. Both our study and the previous study, however, indicated that the association for body fatness at young ages was not stronger for risk of ER+/PR+ breast cancer as compared with other subtypes, again suggesting a pathway not mediated by sex hormones.
In addition, although the test for heterogeneity was not significant at the 0.05 level, our data suggest that the inverse association may be stronger for tumors that overexpress HER2 than for those that do not. To our knowledge, this has not been explored in previous studies, although Borgquist et al. (48) found differences in the associations of adult BMI and other anthropometric characteristics with HER2+ and HER2− tumors. In future studies, researchers should explore the relation between body fatness at young ages and risks of different breast cancer molecular subtypes that incorporate expression of ER, PR, and HER2, as well as other markers.
This study had several important strengths. By combining data from the NHS and NHS II and including premenopausal and postmenopausal women, we were able to examine higher levels of body fatness, interactions with other factors, and variation by tumor characteristics. In addition, we had detailed information on many breast cancer risk factors, permitting careful adjustment for these factors. A limitation is that we relied on participants’ recall of their body fatness at young ages, although the somatotype pictogram used has been validated (27) and has been associated with risk of breast cancer (11, 13) and other related endpoints (49) in this population. In the analysis of HER2+ and HER2− tumors, we could not include cases diagnosed before the 1998/1999 follow-up cycles because HER2 status was not assessed in earlier years. Future studies in this population that utilize tissue microarrays to evaluate marker expression will help address these issues.
In summary, this study showed strong and significant inverse associations between body fatness during childhood and adolescence and risk of breast cancer throughout life. These findings confirm those from previous studies, and they suggest that body fatness at young ages acts through a biologic pathway that is not mediated by adult BMI or endogenous sex hormones. Elucidating the mechanisms that explain the inverse relation of body fatness at early stages of life with risk of breast cancer may contribute to understanding of the causes of this important disease.
Acknowledgments
Author affiliations: Division of General Medicine and Primary Care, Brigham and Women’s Hospital, Boston, Massachusetts (Heather J. Baer); Department of Medicine, Harvard Medical School, Boston, Massachusetts (Heather J. Baer, Shelley S. Tworoger, Susan E. Hankinson, Walter C. Willett); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Heather J. Baer, Shelley S. Tworoger, Susan E. Hankinson, Walter C. Willett); Channing Laboratory, Brigham and Women’s Hospital, Boston, Massachusetts (Shelley S. Tworoger, Susan E. Hankinson, Walter C. Willett); and Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Walter C. Willett).
This work was supported by the National Cancer Institute (grants P01 CA87969 and R01 CA50385).
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- IGF
insulin-like growth factor
- NHS
Nurses’ Health Study
- NHS II
Nurses’ Health Study II
- PR
progesterone receptor
- RR
relative risk
References
- 1.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 2.Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10(1):15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 4.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 5.Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 6.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 7.Schernhammer ES, Tworoger SS, Eliassen AH, et al. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer. 2007;14(3):721–732. doi: 10.1677/ERC-06-0080. [DOI] [PubMed] [Google Scholar]
- 8.Tworoger SS, Eliassen AH, Missmer SA, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2494–2501. doi: 10.1158/1055-9965.EPI-06-0671. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 10.Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351(16):1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 11.Baer HJ, Colditz GA, Rosner B, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7(3):R314–R325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardia A, Vachon CM, Olson JE, et al. Relative weight at age 12 and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):374–378. doi: 10.1158/1055-9965.EPI-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkey CS, Frazier AL, Gardner JD, et al. Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400–2409. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Brinton LA, Swanson CA. Height and weight at various ages and risk of breast cancer. Ann Epidemiol. 1992;2(5):597–609. doi: 10.1016/1047-2797(92)90004-a. [DOI] [PubMed] [Google Scholar]
- 15.Hilakivi-Clarke L, Forsén T, Eriksson JG, et al. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85(11):1680–1684. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Marchand L, Kolonel LN, Earle ME, et al. Body size at different periods of life and breast cancer risk. Am J Epidemiol. 1988;128(1):137–152. doi: 10.1093/oxfordjournals.aje.a114936. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson C, Baron J, Persson I, et al. Body size in different periods of life and breast cancer risk in post-menopausal women. Int J Cancer. 1998;76(1):29–34. doi: 10.1002/(sici)1097-0215(19980330)76:1<29::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Magnusson CM, Roddam AW, Pike MC, et al. Body fatness and physical activity at young ages and the risk of breast cancer in premenopausal women. Br J Cancer. 2005;93(7):817–824. doi: 10.1038/sj.bjc.6602758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166(21):2395–2402. doi: 10.1001/archinte.166.21.2395. [DOI] [PubMed] [Google Scholar]
- 20.Palmer JR, Adams-Campbell LL, Boggs DA, et al. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1795–1802. doi: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]
- 21.Verla-Tebit E, Chang-Claude J. Anthropometric factors and the risk of premenopausal breast cancer in Germany. Eur J Cancer Prev. 2005;14(4):419–426. doi: 10.1097/00008469-200508000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1121–1127. [PubMed] [Google Scholar]
- 23.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4(5):567–571. [PubMed] [Google Scholar]
- 24.American Cancer Society. Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 25.Cerhan JR, Grabrick DM, Vierkant RA, et al. Interaction of adolescent anthropometric characteristics and family history on breast cancer risk in a historical cohort study of 426 families (USA) Cancer Causes Control. 2004;15(1):1–9. doi: 10.1023/B:CACO.0000016566.30377.4e. [DOI] [PubMed] [Google Scholar]
- 26.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety SS, Rowland LP, Sidman RL, et al., editors. The Genetics of Neurological and Psychiatric Disorders. New York, NY: Raven Press; 1983. pp. 115–120. [PubMed] [Google Scholar]
- 27.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 28.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Glynn RJ, Rosner B. Methods to evaluate risks for composite end points and their individual components. J Clin Epidemiol. 2004;57(2):113–122. doi: 10.1016/j.jclinepi.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 32.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 33.Ruder EH, Dorgan JF, Kranz S, et al. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer. 2008;8(4):334–342. doi: 10.3816/CBC.2008.n.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269(1):E118–E126. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 35.Stoll BA. Teenage obesity in relation to breast cancer risk. Int J Obes Relat Metab Disord. 1998;22(11):1035–1040. doi: 10.1038/sj.ijo.0800769. [DOI] [PubMed] [Google Scholar]
- 36.Baer HJ, Colditz GA, Willett WC, et al. Adiposity and sex hormones in girls. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1880–1888. doi: 10.1158/1055-9965.EPI-07-0313. [DOI] [PubMed] [Google Scholar]
- 37.Apter D, Vihko R. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab. 1990;71(4):970–974. doi: 10.1210/jcem-71-4-970. [DOI] [PubMed] [Google Scholar]
- 38.Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 39.Hilakivi-Clarke L, Cabanes A, Olivo S, et al. Do estrogens always increase breast cancer risk? J Steroid Biochem Mol Biol. 2002;80(2):163–174. doi: 10.1016/s0960-0760(01)00184-4. [DOI] [PubMed] [Google Scholar]
- 40.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 41.Nagasawa H, Yanai R, Shodono M, et al. Effect of neonatally administered estrogen or prolactin on normal and neoplastic mammary growth and serum estradiol-17 beta level in rats. Cancer Res. 1974;34(10):2643–2646. [PubMed] [Google Scholar]
- 42.Shellabarger CJ, Soo VA. Effects of neonatally administered sex steroids on 7,12-dimethylbenz(a)anthracene-induced mammary neoplasia in rats. Cancer Res. 1973;33(7):1567–1569. [PubMed] [Google Scholar]
- 43.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 44.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8(12):1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 45.Holt RI. Fetal programming of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab. 2002;13(9):392–397. doi: 10.1016/s1043-2760(02)00697-5. [DOI] [PubMed] [Google Scholar]
- 46.Johnston LB, Dahlgren J, Leger J, et al. Association between insulin-like growth factor I (IGF-I) polymorphisms, circulating IGF-I, and pre- and postnatal growth in two European small for gestational age populations. J Clin Endocrinol Metab. 2003;88(10):4805–4810. doi: 10.1210/jc.2003-030563. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 48.Borgquist S, Jirström K, Anagnostaki L, et al. Anthropometric factors in relation to different tumor biological subgroups of postmenopausal breast cancer. Int J Cancer. 2009;124(2):402–411. doi: 10.1002/ijc.23850. [DOI] [PubMed] [Google Scholar]
- 49.Baer HJ, Schnitt SJ, Connolly JL, et al. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2889–2897. doi: 10.1158/1055-9965.EPI-05-0525. [DOI] [PubMed] [Google Scholar]