Abstract
ATP is a transmitter secreted from taste bud receptor (Type II) cells through ATP-permeable gap junction hemichannels most probably composed of pannexin 1. The elevation of intracellular Ca2+ and membrane depolarization are both believed to be involved in transmitter secretion from receptor cells, but their specific roles have not been fully elucidated. In the present study, we show that taste-evoked ATP secretion from mouse vallate receptor cells is evoked by the combination of intracellular Ca2+ release and membrane depolarization. Unexpectedly, ATP secretion is not blocked by tetrodotoxin, indicating that transmitter release from these cells still takes place in the absence of action potentials. Taste-evoked ATP secretion is absent in receptor cells isolated from TRPM5 knockout mice or in taste cells from wild type mice where current through TRPM5 channels has been eliminated. These findings suggest that membrane voltage initiated by TRPM5 channels is required for ATP secretion during taste reception. Nonetheless, even in the absence of TRPM5 channel activity, ATP release could be triggered by depolarizing cells with KCl. Collectively, the findings indicate that taste-evoked elevation of intracellular Ca2+ has a dual role: (1) Ca2+ opens TRPM5 channels to depolarize receptor cells and (2) Ca2+ plus membrane depolarization opens ATP-permeable gap junction hemichannels.
Introduction
Taste buds are specialized peripheral chemosensory organs that transduce chemical stimuli and transmit gustatory signals to the central nervous system. Gustatory receptor cells excite primary sensory afferent fibres that transmit the output signal from taste buds to the CNS. Several transmitter candidates have been proposed for these synapses, including serotonin (5-HT), noradrenaline (norepinephrine, NA), glutamate, acetylcholine, ATP and peptides. However, only ATP, 5-HT and NA have been unambiguously identified as transmitters and shown to be released when taste buds are stimulated (Finger et al. 2005; Huang et al. 2005, 2007, 2008, 2009; Romanov et al. 2007, 2008; Murata et al. 2008). For instance, ATP was identified as a neurotransmitter between taste cells and primary sensory afferent fibres (Finger et al. 2005). In response to taste stimulation, taste cells secrete ATP via an unconventional synaptic method – gap junction hemichannels composed of pannexin 1 or connexins (Huang et al. 2007; Romanov et al. 2007; Dando & Roper, 2009). The events that trigger gap junction hemichannels to open and release ATP are not known with confidence, though they are believed to include increased intracellular Ca2+, membrane depolarization or a combination of these two factors (Bao et al. 2004; Locovei et al. 2006; Romanov et al. 2007, 2008). The present report begins to address these questions.
It is now widely recognized that there are at least two types of taste cells in the taste bud that are directly involved in taste transduction: ‘receptor’ (Type II) cells and ‘presynaptic’ (Type III) cells (Yee et al. 2001; Clapp et al. 2006; DeFazio et al. 2006). A third class, Type I taste bud cells, may also participate, especially in ion homeostasis during taste reception and in Na+ sensing (Vandenbeuch et al. 2008; Dvoryanchikov et al. 2009). Binding of tastants to apical sweet, bitter and umami G protein-coupled receptors on receptor (Type II) cells activates a signal transduction pathway involving phospholipase C β2 (PLCβ2), production of 1,4,5 inositol triphosphate (IP3), and intracellular Ca2+ release (Huang et al. 1999). Intracellular Ca2+ triggers open a cation channel, TRPM5, expressed in receptor cells (Pérez et al. 2002; Zhang et al. 2007), allowing Na+ influx and depolarizing taste receptor cells (reviewed by Ishimaru & Matsunami, 2009). This depolarization is believed to initiate action potentials and contribute to ATP release from receptor cells (Murata et al. 2008; Vandenbeuch & Kinnamon, 2009).
In the present report, we investigated how receptor cells secrete ATP, and specifically how membrane depolarization and store-released Ca2+ interact to trigger transmitter release. We tested whether TRPM5 is necessary for ATP secretion, whether action potentials are essential for ATP secretion, and if a transient elevation of intracellular Ca2+ alone is able to elicit ATP secretion.
Methods
Ethical approval
Mice were killed following National Institutes of Health guidelines, as approval by the University of Miami Animal Care and Use Committee. All experiments were conducted following the guidelines of these two regulatory bodies.
Experimental animals
Two lines of mice were used in these experiments: C57BL/6J mice (wild type) of both sexes (n= 44) and TRPM5 knockout mice (n= 5) (Zhang et al. 2003). Mice were killed by exposure to CO2 followed by cervical dislocation. This procedure minimizes distress (NIH Office of Animal Care and Use, http://oacu.od.nih.gov/ARAC/EuthCO2.pdf). Tongues were then removed for further dissection.
Isolated taste cells
We removed the lingual epithelium containing taste papillae from the tongue and isolated vallate taste bud cells as described in Huang et al. (2007). Briefly, we injected 1 mg ml−1 collagenase A (Roche), 2.5 mg ml−1 dispase II (Roche) and 1 mg ml−1 trypsin inhibitor (Sigma, St Louis, MO, USA) directly under the epithelium surrounding taste papillae. The peeled epithelium was bathed in Ca2+-free solution for 20 min at room temperature and vallate taste cells were drawn into fire-polished glass micropipettes with gentle suction. Taste cells were transferred to a shallow recording chamber having a glass coverslip base and then loaded with 5 μm Fura 2-AM. The coverslip was coated with Cell-Tak (BD Biosciences, San Jose, CA, USA) to hold taste cells firmly attached. Taste cells were superfused with Tyrode solution (in mm; 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose, 10 sodium pyruvate, 5 NaHCO3, pH 7.2, 310–320 mosmol l−1). For nominally Ca2+-free Tyrode solution, MgCl2 was substituted for CaCl2 (in mm; 140 NaCl, 5 KCl, 3 MgCl2, 10 Hepes, 10 glucose, 10 sodium pyruvate, 5 NaHCO3, 2 BAPTA, 2 EGTA, pH 7.2, 310–320 mosmol l−1). In Tyrode solution where we varied K+ concentration, KCl was exchanged for an equimolar concentration of NaCl. In Tyrode solution where Na+ was substituted with NMDG+, NMDG·Cl was exchanged for equimolar NaCl.
Biosensor cells
Chinese hamster ovary (CHO) cells expressing P2X2/P2X3 receptors (hereafter called ‘ATP biosensor cells’) were prepared and loaded with 5 μm Fura 2-AM as described in Huang et al. (2007, 2009). Read-out of ATP biosensor cell activation was accomplished by imaging intracellular Ca2+. We verified that Ca2+ signals in ATP biosensor cells were not evoked by bath-applied KCl (up to 140 mm) or by taste mix (cycloheximide, 10 μm; saccharin, 2 mm; SC45647, 0.1 mm; denatonium, 1 mm) (see Huang et al. 2007, 2008, 2009). Moreover, none of the procedures used in the present studies, including applying tetrodotoxin (TTX) or substituting Na+ with N-methyl-d-glucamine (NMDG+), affected Ca2+ responses in ATP biosensor cells. TTX and NMDG·Cl were purchased from Sigma.
Ca2+ imaging
Conventional Ca2+ imaging was carried out using Indec Workbench v.5 software. Namely, Fura 2-loaded cells were excited at 340 nm and 380 nm, and emission images were collected at ≥510 nm (e.g. Huang et al. 2007). The ratio of F340/F380 was converted to approximate [Ca2+]i as described by Grynkiewicz et al. (1985). The fluorescence ratios of free and Ca2+-bound Fura 2 at 340 nm and the fluorescence of free and Ca2+-bound Fura 2 at 380 nm were determined using a Fura 2 Calcium Imaging Calibration Kit (Invitrogen, USA). The average baseline (resting) Ca2+ in these experiments was 118 ± 53 nm (N= 75 cells), in good correspondence with values reported by others (Hacker & Medler, 2008).
Our criteria for accepting Ca2+ responses for analysis were described in our previous publication (Huang et al. 2009). In brief, responses were quantified as peak minus baseline [Ca2+] (i.e. Δ[Ca2+]). We accepted Ca2+ responses only if they could be elicited repetitively in the same cell by the same stimulus, and control/washout responses were at least 2× baseline fluctuation. All experiments were conducted at room temperature (25°C).
Stimulation
Isolated taste cells were stimulated by bath perfusion of taste mix (cycloheximide, 10 μm; saccharin, 2 mm; SC45647, 0.1 mm; denatonium, 1 mm). Alternatively, taste cells were depolarized by KCl (50, 100, 120 and 140 mm). All stimuli were made up in Tyrode solution and applied at pH 7.2. Membrane potentials were approximated using the Nernst equation for K+ and assuming intracellular [K+] is 155 mm. As detailed in Huang et al. (2009), we applied stimuli for 30 s followed by return to Tyrode solution. The recording chamber was perfused with Tyrode solution for a minimum of 3–5 min between trials.
Results
It has long been recognized that taste bud cells generate action potentials. However, the significance of excitatory impulses in peripheral gustatory sensory receptor cells is not well understood (reviewed in Vandenbeuch & Kinnamon, 2009). One notion is that taste cell action potentials are key for synaptic neurotransmitter release, especially the secretion of ATP from taste receptor (Type II) cells during gustatory stimulation (Murata et al. 2008; Romanov et al. 2008). We tested the dependence of transmitter release on impulse activity by measuring taste-evoked ATP secretion from taste receptor (Type II) cells and determining whether blocking action potentials affected this release. ATP secreted from individual receptor cells was monitored with biosensor cells as described previously (Huang et al. 2007, 2009). Remarkably, bathing the preparation in a relatively high concentration of tetrodotoxin (TTX, 1 μm), a toxin known to block taste cell impulses at this concentration (Ohtubo et al. 2009; Gao et al. 2009) had little to no effect on taste-evoked ATP release (Fig. 1). We conclude that action potentials may be sufficient to evoke ATP release from receptor cells (Romanov et al. 2008; Murata et al. 2008), but they are not necessary for this release.
Figure 1. TTX does not block taste-evoked transmitter (ATP) release from isolated receptor (Type II) cells.
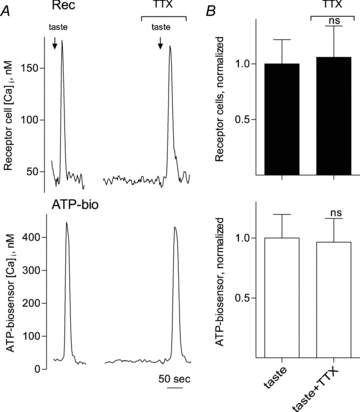
A, simultaneous recordings of Ca2+ responses from an isolated receptor cell (Rec, top) and an apposed ATP biosensor (ATP-bio, bottom) to monitor transmitter secretion during taste stimulation. The arrows above the recordings indicate application of taste mix (see Methods). Taste-evoked Ca2+ responses in the receptor cell and ATP secretion were unaffected by the presence of TTX (1 μm). B, summary of data. Bars (mean ±s.e.m.) show taste-evoked responses in receptor cells (filled bars, top) and taste-evoked ATP secretion (biosensor response, open bars, below) (N= 11 cells). Individual responses were normalized to the average of the taste-evoked responses in receptor cells (upper) or ATP-biosensor cells (lower) for all experiments in this series. No significant differences (ns) were obtained by applying 1 μm TTX. Student's paired t test.
Next, we investigated the role of graded membrane depolarization in transmitter secretion from receptor cells. Taste stimulation is believed to trigger TRPM5 channels by releasing intracellular Ca2+. TRPM5 channels, when opened by intracellular Ca2+ (Pérez et al. 2002; Zhang et al. 2003, 2007), allow a graded influx of Na+, thereby depolarizing the membrane (Zhang et al. 2007):
We tested whether TRPM5 channels are necessary for taste-evoked ATP secretion by interfering with TRPM5 channels. To reduce or eliminate TRPM5 channel activity, we substituted NMDG+ for Na+ in the bathing medium. Replacing Na+ with NMDG+ effectively eliminates TRPM5 function because these channels only poorly permeate NMDG+ (Hofmann et al. 2003). Parenthetically, we attempted to block TRPM5 channels pharmacologically by using triphenylphosphine oxide (TPPO) (Shah et al. 2009). However, TPPO non-selectively activated ATP biosensor cells used to monitor ATP secretion and thus could not be employed in these experiments. Figure 2 shows that when Na+ was replaced with NMDG+, taste stimulation still evoked normal Ca2+ mobilization in receptor cells but completely eliminated their ability to secrete ATP. Even so, ATP secretion from receptor cells was restored in the absence of TRPM5 function (i.e. NMDG+ substitution) by combining taste stimulation with depolarization produced by 50 mm KCl (Fig. 2).
Figure 2. Blocking TRPM5 current by replacing extracelluar Na+ with NMDG+ abolishes taste-evoked ATP secretion.
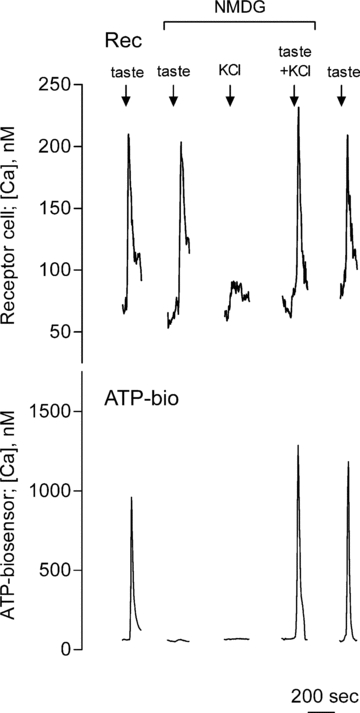
Traces show concurrent recordings from an isolated receptor cell (Rec, top) and from an ATP biosensor (ATP-bio, bottom) that was closely apposed to the receptor cell. Arrows above the recordings indicate application of taste mix, 50 mm KCl or both. Bath-applied taste mix evoked Ca2+ responses in the receptor cell (top trace) and in the biosensor cell (bottom trace), indicating taste excitation and ATP secretion. Taste-evoked ATP secretion (bottom trace), but not taste-evoked responses in the receptor cell, were abolished. TRPM5 activity was eliminated by replacing Na+ with NMDG+ in the bathing medium. As shown previously (DeFazio et al. 2006; Huang et al. 2007), KCl depolarization (50 mm) alone does not evoke Ca2+ mobilization in receptor cells nor ATP secretion. However, taste-evoked ATP secretion from receptor cells was rescued when Ca2+ mobilization (taste mix) was combined with depolarization (KCl, 50 mm). The final pair of responses shows full recovery of taste-evoked ATP secretion when Na+ was re-introduced to the bath and NMDG+ eliminated. These results indicate that when TRPM5 function is eliminated by Na+ substitution, taste-evoked ATP secretion can still take place in the absence of TRPM5 currents provided that receptor cells are depolarized by an alternative mechanism, KCl.
These findings are consistent with the role of TRPM5 during taste stimulation being to generate a depolarizing Na+ current. In short, intracellular Ca2+ mobilization and TRPM5 channels are sufficient, but not necessary, for ATP secretion. By-passing TRPM5 channels by directly depolarizing the membrane (high K+) rescues transmitter secretion.
Our findings that taste receptor cells could secrete neurotransmitter in the absence of action potentials or in the absence of TRPM5-mediated depolarization led us to examine the roles of graded membrane depolarization and intracellular Ca2+ mobilization more closely. Romanov et al. (2007) patch-clamped taste receptor cells and reported that cells could secrete ATP in the absence of increased [Ca2+]. We repeated these experiments using a different approach. Namely, we depolarized isolated receptor cells by increasing K+ in the bath still higher than in our above experiments, i.e. 50 to 140 mm. We calculated the approximate depolarization at each point based on the Nernst potential for K+. These experiments were conducted with NMDG+-substituted buffer to eliminate TRPM5 channel activity. We found that sufficient depolarization (100 mm KCl, membrane potential ∼−11 mV) triggered ATP secretion without mobilizing intracellular Ca2+ (Fig. 3; Supplemental Fig. S1). This result is close to the value (−10 to ∼0 mV) that Romanov et al. (2007) reported to evoke ATP secretion, also in the absence of an increase in [Ca2+]i. Further depolarization to ∼−6 mV (120 mm KCl) or ∼−3 mV (140 mm KCl) in the presence of NMDG+-substituted buffer enhanced ATP secretion even more (Fig. 3A and B). However, our methodology only allows us to derive an approximate voltage–release relationship; our estimated membrane potentials are only as valid as the assumed values for [K+]i.
Figure 3. Intracellular Ca2+ mobilization is not required for receptor cells to secrete ATP.
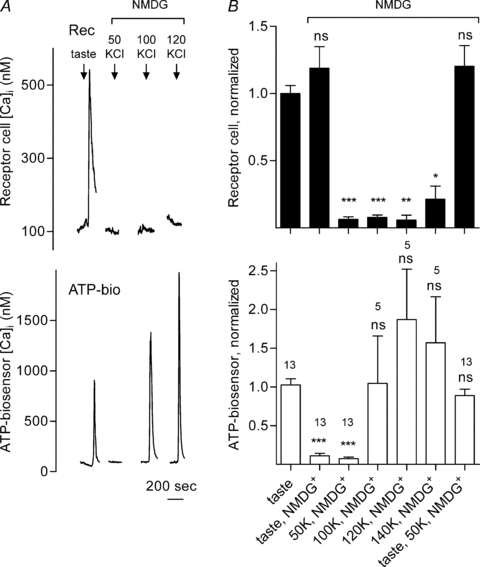
A, traces show concurrent recordings of Ca2+ responses in a receptor cell (Rec, top) and ATP secretion (biosensor responses, ATP-bio, bottom). The arrows above the recordings indicate application of taste mix or KCl. Bath-applied taste mix evoked Ca2+ responses in the receptor cell and in the biosensor cell, indicating ATP secretion. Depolarizing the receptor cell with increasing concentrations of KCl (50 to 120 mm) did not evoke Ca2+ mobilization in the receptor cell (top traces). However, depolarizing the receptor cell with KCl at 100 mm and above evoked ATP secretion (bottom traces). B, summary of data from several experiments. Bars show mean ±s.e.m. of Ca2+ responses in receptor cells (filled bars, top) and ATP secretion (i.e. biosensor responses, open bars, below). Numbers above bars indicate the number of cells. Individual responses were normalized to the average of the taste-evoked responses for all experiments in the series (i.e. initial bar). *P < 0.05; **P < 0.01; ***P < 0.001; Student's paired t test; ns, not significantly different. These data show that even in the absence of TRPM5 and the absence of intracellular Ca2+ mobilization, strong depolarization of receptor cells can activate ATP secretion.
In a final test of the role of TRPM5 in taste, we examined ATP secretion in TRPM5-null mice (TRPM5 knockout (KO)) (Zhang et al. 2003). TRPM5 KO mice have a pronounced reduction in ability to respond to sweet, bitter and umami tastes (Zhang et al. 2003; Damak et al. 2006). Taste stimuli evoked normal Ca2+ mobilization in receptor cells from TRPM5 KO mice, but failed to secrete ATP (Fig. 4). However, ATP secretion was rescued in TRPM5 KO mice if receptor cells were sufficiently depolarized with KCl, even in the absence of intracellular Ca2+ mobilization (Fig. 4). This finding parallels results from experiments in wild type mice where TRPM5 had been inactivated by NMDG+ substitution and yet still secreted ATP in response to KCl depolarization (Fig. 3). The findings reinforce the notion that, under certain experimental conditions, TRPM5 is not necessary for receptor cells to secrete ATP. However, under physiological conditions, of course, TRPM5 is key for taste-evoked ATP secretion.
Figure 4. Receptor (Type II) cells from TRPM5 knockout mice do not secrete ATP in response to taste stimulation, but do so when sufficiently depolarized.
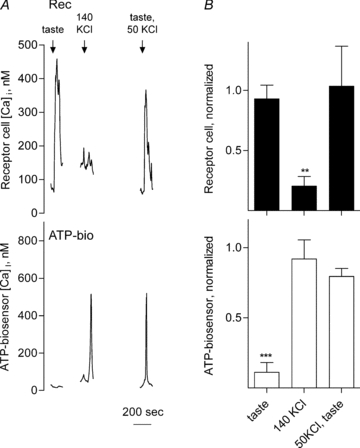
A, simultaneous recordings of Ca2+ responses of an isolated receptor cell (Rec, top) isolated from a TRPM5 knockout mouse and a closely apposed ATP biosensor (ATP-bio, bottom). The arrows above the traces indicate application of taste mix, KCl or both. Applying taste stimuli evoked a response in the receptor cell but failed to elicit ATP secretion. However, when depolarized with 140 mm KCl, the receptor cell secreted ATP even in the absence of Ca2+ mobilization in the receptor cell. ATP secretion was rescued when taste mix and 50 mm KCl were co-applied (50 mm KCl alone did not trigger ATP secretion, data not shown). B, summary of data from TRPM5 knockout mice. Bars show mean ±s.e.m. of Ca2+ responses in receptor cells (filled bars, top) and concurrent ATP secretion (biosensor cell responses, open bars, bottom). Individual responses were normalized to the average of the responses evoked by a control stimulus of 1 μm ATP. N= 5 experiments, 10 cells. **P < 0.01; ***P < 0.001; Student's paired t test.
Discussion
Upon gustatory stimulation, taste receptor (Type II) cells secrete ATP as a paracrine and neurocrine transmitter, probably via pannexin 1 gap junction hemichannels (although connexon-based hemichannels have also been suggested) (Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007; Dando & Roper, 2009). Our findings here indicate that taste-evoked ATP secretion is elicited by the combination of (a) membrane depolarization from Na+ influx through TRPM5 channels, and (b) Ca2+ released from intracellular stores. Moreover, regenerative impulse activity is not required for this release: taste receptor cells can secrete ATP even in the absence of action potentials. Our findings do not indicate, however, that regenerative activity in receptor cells is inconsequential for taste-evoked transmitter release. Action potentials evoked by taste stimulation probably augment and shape transmitter secretion initiated by taste-evoked receptor potentials and subsequent Ca2+ mobilization (Murata et al. 2008; Romanov et al. 2008). Specifically, Murata et al. (2008) showed that ATP secretion increases with the number of action potentials produced by receptor cells during taste stimulation. Our findings, instead, suggest that store-released Ca2+ decreases the level of depolarization required to activate gap junction hemichannels and secrete ATP (Supplemental Fig. S1). Testing this idea more quantitatively could be accomplished better with heterologous expression of pannexin 1 (or connexins) and inside-out patch recording.
TRPM5, the ion channel that appears to be responsible for taste-evoked membrane depolarization in receptor cells, is expressed specifically in taste bud cells (Pérez et al. 2002). TRPM5, PLCβ2 and 1,4,5 inositol triphosphate receptor type 3 (IP3R3) are the signal transduction components immediately downstream of G protein-coupled taste receptors (Clapp et al. 2001; Pérez et al. 2002). TRPM5 channels are activated by intracellular Ca2+ (Hofmann et al. 2003; Liu & Liman, 2003; Prawitt et al. 2003). TRPM5 channels are obligatory for taste transduction under physiological conditions; mutant mice lacking TRPM5 show a marked deficit in taste responses (Zhang et al. 2003; Damak et al. 2006). As the resting input resistance of taste cells is somewhat above 1 GΩ (mouse: Bigiani, 2001; rat: Gilbertson et al. 2001), even small inward current through TRPM5 channels opened by Ca2+ mobilization during taste stimulation would be expected to produce relatively large receptor potentials. These receptor potentials presumably initiate action potentials in receptor cells (Gao et al. 2009; Vandenbeuch & Kinnamon, 2009). Action potentials lead to transmitter release (Murata et al. 2008; Romanov et al. 2008). However, as the present findings indicate, TRPM5 and receptor cell action potentials are not absolutely essential for transmitter secretion. If TRMP5-mediated depolarizing current is eliminated by replacing Na+ with an impermeant cation or by genetic manipulation, receptor cells will still secrete transmitter if they are depolarized by other means such as elevated K+. Moreover, if sufficiently depolarized, receptor cells will release transmitter even in the absence of intracellular Ca2+, as shown in the present report and by Romanov et al. (2008).
Taste receptor cells secrete transmitter, ATP, through gap junction hemichannels, most probably composed of pannexin 1 (Huang et al. 2007; Romanov et al. 2007; Dando & Roper, 2009). Gap junction hemichannels are downstream of TRPM5 and are opened by depolarization and by intracellular Ca2+ (Locovei et al. 2006). The Ca2+ sensitivity of pannexin 1 hemichannels distinguishes them from connexon gap junction channels. Connexon channels generally are closed by an elevation of intracellular Ca2+ (Li et al. 1996). In contrast, pannexin 1 channels are opened by membrane depolarization or by the elevation of the intracellular Ca2+ (Bao et al. 2004; Locovei et al. 2006). We conclude that the conjunction of PLCβ2/IP3-mediated Ca2+ release, combined with TRPM5-mediated membrane depolarization in receptor cells, allows ATP secretion through pannexin 1 hemichannels when taste buds are excited by sweet, bitter and umami taste stimuli.
An alternative explanation for the action of KCl on opening gap junction hemichannels is that K+ is, itself, acting as a ligand for pannexin 1, independent of its ability to depolarize the cell membrane. This intriguing observation was recently reported for caspase-1 activation following pannexin 1 activation in primary neurons and astrocytes (Silverman et al. 2009). Without voltage clamp measurements, one cannot rule out this possibility.
Acknowledgments
We would like to thank Drs. S. Simon and C. Zuker for providing the TRPM5 knockout mice used in these experiments. We also thank Dr. Yutaka Maruyama who generated the ATP biosensor cells used in this study using constructs provided by Dr. A. Surprenant. Funded by grants to S.D.R. from NIH/NIDCD 5R01DC000374 and 5R01DC007630.
Supplemental material
Supplemental Material Figure 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bigiani A. Mouse taste cells with glialike membrane properties. J Neurophysiol. 2001;85:1552–1560. doi: 10.1152/jn.2001.85.4.1552. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signalling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Sinclair M, Perea-Martinez I, Wang T, Chaudhari N. The inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009;517:1–14. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signalling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Gao N, Lu M, Echeverri F, Laita B, Kalabat D, Williams ME, Hevezi P, Zlotnik A, Moyer BD. Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 2009;10:20. doi: 10.1186/1471-2202-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson TA, Boughter JD, Jr, Zhang H, Smith DV. Distribution of gustatory sensitivities in rat taste cells: whole-cell responses to apical chemical stimulation. J Neurosci. 2001;21:4931–4941. doi: 10.1523/JNEUROSCI.21-13-04931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hacker K, Medler KF. Mitochondrial calcium buffering contributes to the maintenance of basal calcium levels in mouse taste cells. J Neurophysiol. 2008;100:2177–2191. doi: 10.1152/jn.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is co-released with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J Dent Res. 2009;88:212–218. doi: 10.1177/0022034508330212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Murata Y, Yoshida R, Yasuo T, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Firing rate-dependent ATP release from mouse fungiform taste cells with action potentials. Chem Senses. 2008;33:S128. [Google Scholar]
- Ohtubo Y, Hashiba Y, Kimura K, Kumazawa T, Yoshii K. Voltage-gated Na+ currents of each cell type in mouse taste buds. Chem Senses. 2009;34:E59. [Google Scholar]
- Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BP, Liu P, Yu T, Hansen DR, Gilbertson TA. Direct evidence of the role of TRPM5 in bitter transduction in enteroendocrine cells. Chem Senses. 2009;34:A85. [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Kinnamon SC. Why do taste cells generate action potentials? J Biol. 2009;8:42. doi: 10.1186/jbiol138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. ‘Type III’ cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signalling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


