Abstract
Flexion and extension movements are organized reciprocally, so that extensor motoneurones in the spinal cord are inhibited when flexor muscles are active and vice versa. During and just prior to dorsiflexion of the ankle, soleus motoneurones are thus inhibited as evidenced by a depression of the soleus H-reflex. It is therefore surprising that soleus motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) have been found not to be reduced and even facilitated during a voluntary dorsiflexion. The objective of this study was to investigate if MEPs, evoked by TMS, show a similar facilitation prior to and at the onset of contraction of muscles that are antagonists to the muscle in which the MEP is evoked and if so, examine the origin of such a facilitatory motor programme. Eleven seated subjects reacted to an auditory cue by contracting either the tibialis anterior (TA) or soleus muscle of the left ankle. TMS was applied to the hotspot of TA and soleus muscles on separate days. Stimuli were delivered prior to and at the beginning of contraction. Soleus MEPs were significantly facilitated when TMS was applied 50 ms prior to onset of plantar flexion. Surprisingly, soleus MEPs were also facilitated (although to a lesser extent) at a similar time in relation to the onset of dorsiflexion. TA MEPs were facilitated 50 ms prior to onset of dorsiflexion and neither depressed nor facilitated prior to plantar flexion. No difference was found between the facilitation of the soleus MEP and motor evoked responses to cervicomedullary stimulation prior to dorsiflexion, suggesting that the increased soleus MEPs were not caused by changes at a cortical level. This was confirmed by the observation that short-latency facilitation of the soleus H-reflex by subthreshold TMS was increased prior to plantar flexion, but not prior to dorsiflexion. These findings suggest that voluntary contraction at the ankle is accompanied by preceding facilitation of antagonists by a subcortical motor programme. This may help to ensure that the direction of movement may be changed quickly and efficiently during functional motor tasks.
Introduction
It is now established as common textbook knowledge that flexion and extension movements are organized reciprocally, so that extensor motoneurones in the spinal cord are inhibited when flexor muscles are active and vice versa. Parallel control of the respective spinal motoneurones and their corresponding reciprocally organised Ia inhibitory interneurones from descending motor tracts and sensory afferents has been shown in cat, monkey and human experiments to be central for this organization (Hultborn & Lundberg, 1972; Jankowska et al. 1976; Crone et al. 1987; Crone & Nielsen, 1989, 1994). In the cat, Ia inhibitory interneurones projecting to extensor motoneurones are active when their target motoneurones are inactive and stimulation of the Ia inhibitory pathway evokes the largest IPSPs in the target motoneurones in their hyperpolarized phase during locomotion (Pratt & Jordan, 1987). Similarly, in human subjects disynaptic Ia inhibition from ankle dorsiflexors to ankle plantar flexors is largest in the swing phase of gait (Petersen et al. 1999), in the upstroke phase during bicycling (Pyndt et al. 2003) and during a voluntary ankle dorsiflexion in sitting subjects (Crone & Nielsen, 1989). At least partly as a consequence of this inhibition of soleus motoneurones, the soleus H-reflex is reduced at a similar time during these movements (Crone & Nielsen, 1989).
It is therefore surprising that soleus motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) have been found not to be reduced and even facilitated during a voluntary dorsiflexion (Valls-Sole et al. 1994; Goulart & Valls-Sole, 2001). Several possible explanations may be provided. One is that disynaptic reciprocal inhibition is mainly increased at the onset and during the initial part of the dynamic phase of dorsiflexion, whereas it is not or only little increased during static dorsiflexion (Crone et al. 1987; Crone & Nielsen, 1989). The reduction of the H-reflex during static dorsiflexion may thus be mainly caused by presynaptic inhibition of the Ia afferents, which would not affect the MEPs (Nielsen & Petersen, 1994). Another explanation is that the inhibition at the spinal motoneuronal level may be counteracted by increased excitability of the corticospinal neurones projecting to soleus motoneurones during dorsiflexion. This would suggest that the corticospinal neurons are not organized reciprocally and may show more flexibility in their activity than what would be predicted from a strict extension–flexion reciprocal organisation. Experimental evidence for this has been described in monkey (Cheney & Fetz, 1984; Fetz & Cheney, 1987) and human subjects (Capaday et al. 1999; Roy & Gorassini, 2008). A third possibility is that the inhibition of the motoneurones is counteracted by some subcortical facilitatory input to the soleus motoneurones. This could involve for instance a subcortical motor programme for postural (anticipatory) adjustments of the soleus muscle in order to support the dorsiflexion movement (Cordo & Nashner, 1982).
The present project was initiated in order to investigate these possibilities and thus clarify why soleus MEPs are not suppressed during ankle dorsiflexion.
We demonstrate that soleus MEPs, in contrast to soleus H-reflexes, are facilitated prior to the onset of dorsiflexion. Control experiments demonstrated that this was not due to increased cortical excitability and we therefore propose that the facilitation of the MEPs prior to dorsiflexion may involve activation of a subcortical motor programme.
Methods
Participants
Nineteen healthy human subjects (17 men) with an average age of 27 ± 5 years participated in the study. Eleven subjects participated in the first experiment, three in the second and seven in the third. All subjects gave their written, informed consent to the experimental procedures, which were approved by the local ethics committee (j.nr. (KF) 100.1969/1991). The study was performed in accordance with the Declaration of Helsinki.
General organization of the studies
Subjects were comfortably seated in an armchair and the left leg was positioned with the hip semi-flexed (120 deg), the knee flexed (110 deg), and the ankle at a slightly plantar flexed position (140 deg). The left foot was firmly attached to a force pedal using adjustable straps. The torque exerted on the foot plate was recorded by a strain gauge.
At the beginning of each experiment, subjects were instructed to perform a maximal dorsiflexion (1–2 s) to measure their maximal voluntary contraction (MVC) strength. Subjects were verbally encouraged to produce maximal torque. The torque was displayed as a moving line on the monitor. At least three trials were performed separated by 30 s rest periods and the peak torque was used as the dorsiflexion MVC. Subsequently, the same procedure was used to measure the plantar flexor MVC.
Subjects performed a reaction time task where an auditory warning cue (Cwarning) prepared the subjects to be ready. They were instructed to dorsiflex their ankle to 30% MVC as fast as possible (the dynamic phase lasted around 100–200 ms) when a second auditory ‘go’ cue (Cgo) was delivered 2.8–3.2 s after Cwarning. Prior to the experiment, subjects trained the reaction time task in order to reduce the variability of the reaction time and the effect of learning. After about 5 min training subjects could complete the task with little variation in their reaction time.
EMG measurements
EMG activity was recorded from the anterior tibial (TA) and soleus muscles by non-polarizable bipolar electrodes (diameter 0.5 cm; Blue Sensor, Ambu, Ølstykke, Denmark) placed over the belly of the muscles with an interelectrode distance of 2 cm. The EMG signals were amplified (500–5000×), filtered (band pass, 5 Hz to 1 kHz), sampled at 2 kHz, and stored on a PC for offline analysis (CED 1401+ with Signal 4.05 software, Cambridge Electronic Design, Cambridge, UK). In three subjects, EMG was measured from the vastus lateralis of the quadriceps muscle (Q) with the same procedure.
Study 1. Motor evoked potentials (MEPs)
In study 1, we investigated the modulation of flexor and extensor MEPs in the time period preceding either an agonist or antagonist contraction. The following experiments were done for TA and soleus on separate days in random order. Magnetic stimuli were delivered by a Magstim 200 stimulator connected to a figure-of-eight shaped bat-wing coil with an individual wing diameter of 90 mm (The Magstim Co., Whitland, Dyfed, UK). The site where stimuli of slightly suprathreshold intensity consistently produced the largest MEPs in the resting muscle (referred to as ‘motor hot spot’) was marked in BrainSight 1.7 (Rogue Research Inc., Montreal, Quebec, Canada) navigation software. The coil was then secured in place and the resting motor threshold was found by reducing the stimulus intensity to a level that elicited an MEP in 3 out of 5 trials (66 ± 8% and 72 ± 7% of maximal stimulator output for TA and soleus, respectively). Single stimuli at 1.2 × resting motor threshold were applied to the hotspot of the TA and soleus muscles, respectively, at different intervals prior to and at the beginning of contraction in random order. The intervals used were: 1 s prior to Cwarning (<Cwarning), 100 ms prior to Cgo (<Cgo) and 0, 25, 50, 75, 100, 125 and 150 ms after Cgo (Fig. 1). At least 12 MEPs were measured at each interval. Control trials in which the cues were presented but no TMS applied were interspersed 40% of the time. Thereby it was possible to measure reaction times, the time between Cgo and the onset of contraction (onset of agonist EMG), during the course of the experiment to ensure the validity of the estimates. The average reaction time was calculated offline for each subject and the onset of EMG activity in the agonist muscle was set as time 0. The MEP data of each subject were grouped into 25 ms bins based on the time of stimulation relative to the onset of contraction. Peak-to-peak amplitudes of the MEPs were then averaged and compared to MEPs elicited at <Cgo. It was checked that similar results were obtained when measuring the MEP area.
Figure 1. Overview of experimental design.
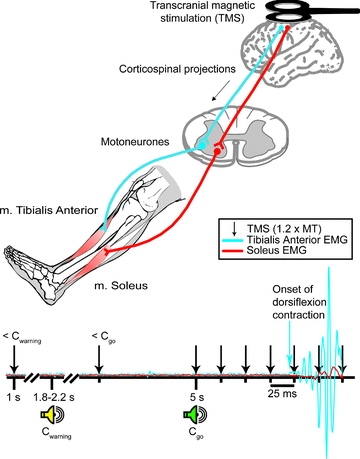
TMS was applied to the hotspot of TA or soleus at different time points prior to and at the onset of contraction. Single stimuli were delivered randomly prior to the first (Cwarning) and second (Cgo) auditory cue and at 25 ms intervals between 0 and 150 ms after Cgo (Fig. 1). Control trials were also included in which the auditory cues were presented, but TMS was not applied.
Trials in which the subject made a ‘false start’ or ‘missed’ Cgo were omitted from the analyses.
In separate control experiments, the soleus MEP size at 25 ms prior to onset of dorsiflexion and at <Cgo were compared under different conditions. In one condition, subjects were required to react to a visual cue instead of the auditory cue (Cgo). This was done to investigate whether the modulation of the MEPs prior to dorsiflexion could be related to the nature of the go signal and reflect a possible startle reaction. In another condition, the subjects were requested to perform the dorsiflexion non-isometrically (the foot was not strapped to the force pedal) and in a third condition the subjects were requested to react as quickly as possible, but to perform the actual dorsiflexion contraction non-ballistically, so that the torque increased slowly (around 1 s). Finally, in order to investigate the muscle specificity of the modulation, we tested if MEPs from vastus lateralis (Q) were modulated prior to dorsiflexion. As in the experiments with TA and soleus, we found the hotspot and threshold for eliciting Q MEPs at rest in 3 of 5 trials. We then stimulated with 1.2 × resting motor threshold and compared the MEP-size at 25 ms prior to onset of dorsiflexion and at <Cgo.
Solues H-reflexes
The modulation of soleus H-reflexes prior to plantar- and dorsiflexion was investigated for comparison to the soleus MEP modulation. Soleus H-reflexes were induced by stimulation (1 ms rectangular pulses; model DS7A, Digitimer, Welwyn Garden City, UK) of the posterior tibial nerve using a ball-shaped monopolar electrode (Simon electrode) placed in the popliteal fossa. The anode was placed proximal to the patella. The reflex size at <Cgo was adjusted to 15% of Mmax, since reflexes of this size have previously been shown to be most sensitive to facilitatory and inhibitory inputs (Crone et al. 1990). However, since the soleus MEPs were much smaller, the modulation of H-reflexes that were adjusted to 2% of Mmax at <Cgo was also investigated.
Mmax
All MEP and H-reflex data were normalized to the maximal M-response (Mmax) in the respective muscles. In TA and soleus, Mmax was evoked by stimulation of the common peroneal nerve and the posterior tibial nerve, respectively. The posterior tibial nerve was stimulated as described above and the common peroneal nerve was stimulated through bipolar surface electrodes (diameter 0.5 cm; Blue Sensor, Ambu, Ølstykke, Denmark) placed 1–3 cm distal to the neck of the fibula. In these measurements, the intensity of stimulation was increased from a subliminal level until there was no further increase in the peak-to-peak amplitude of the M-response with increasing stimulation intensity.
Study 2. Cervicomedullary motor evoked potentials (CMEPs)
To examine whether the observed facilitation in soleus MEPs was of cortical origin, we compared responses to TMS and brainstem stimulation. Electrical stimulation through electrodes fixed over the mastoid processes can evoke motor-evoked responses (CMEPs) in target muscles (Ugawa et al. 1991, 1995). The electrical stimulator was a Digitimer D180A with a maximal output of 1500 V. A high-voltage electrical pulse (50–100 μs duration, up to 900 V) was passed between cup electrodes placed at the posterior edge of each mastoid process.
The stimulus strength of the electrical stimulation was adjusted so as to produce MEPs in soleus of a comparable size to those produced by TMS at 1.2 × motor threshold. As in study 1, the subjects had to dorsiflex their ankle to 30% MVC while MEPs and CMEPs were randomly evoked at <Cgo or 25 ms prior to onset of contraction (measured individually for each subject). Non-stimulation control trials were randomly interspersed. Peak-to-peak amplitudes of MEP and CMEP responses were then averaged for each condition in each subject.
Study 3: TMS conditioning of the H-reflex
We further investigated the origin of the observed changes by looking at the modulation of TMS-conditioned soleus H-reflex responses prior to contraction.
Soleus H-reflexes were evoked as described above. The unconditioned test reflex size was kept at 15% of Mmax while the reflex was conditioned by TMS at different conditioning–test intervals (see details under study 3). Reflexes with and without conditioning stimulation were randomly alternated.
A time course of the TMS-conditioning effect on the test H-reflex was obtained for each subject (Nielsen et al. 1993). The coil was placed over the soleus motor hotspot and the intensity of the conditioning stimulation was adjusted to 2–3% below the threshold for eliciting an MEP in soleus during a slight tonic contraction (10% MVC). The conditioning pulse was given at 1 ms intervals after the test H-reflex (−5 to 0 ms) and 10 ms before the test H-reflex during tonic plantar- and dorsiflexion at 10% MVC and at rest. The following time intervals were used for the experiment: (1) the earliest interval where the TMS pulse had a facilitatory effect on the H-reflex; (2) The earliest interval yielding an inhibitory effect; and (3) a conditioning–test interval of 10 ms, at which a long-latency facilitatory effect is seen (Nielsen et al. 1993; Nielsen & Petersen, 1995a,b;).
Similar to study 1, the subjects were instructed to dorsiflex or plantar flex their ankle to 30% MVC as fast as possible after Cgo. Dorsiflexion and plantar flexion were investigated in separate session. In one dorsiflexion session, stimulations were applied about 25–50 ms prior to onset of dorsiflexion and in another the stimulations were applied at <Cgo. The same procedure was used in plantar flexion sessions.
Statistics
Individual MEP, CMEP and H-reflex amplitudes computed within the window from 30 to 60 ms after stimulation were averaged for each condition in every subject. The average value of the peak-to-peak amplitudes for each condition was then divided (normalized) by the value of the average control amplitude (<Cgo) and this ratio was multiplied by 100 and graphed on a common log scale. The peak-to-peak amplitude of conditioned H-reflexes was expressed relative to the unconditioned H-reflexes.
The statistical significance of differences obtained during the different conditions was tested off-line using Student's t test for paired data in each subject. Statistical analysis was done using SigmaStat 2.03 (Systat Software Inc., San Jose, CA, USA). Before statistical comparison, all data sets were tested for normal distribution by a Kolmogorov–Smirnov test.
The modulation of MEPs was investigated by comparing MEP size at <Cgo with MEPs evoked at different times leading up to onset of contraction using repeated measures ANOVA. For multiple-comparison analysis, Tukey's post hoc test was used for all pairwise comparisons between the group mean responses. The same was used for TMS conditioning of the H-reflex.
Data are presented as means ± standard error of the mean (s.e.m.) unless reported otherwise.
Results
Soleus MEPs are facilitated prior to plantar and dorsiflexion
The average reaction time to Cgo was similar for dorsiflexion (128.3 ± 13.7 ms) and plantar flexion (130.4 ± 14.2 ms; P= 0.73).
Figure 2 shows the different behaviour of the soleus H-reflex and MEPs prior to agonist and antagonist contraction in one subject. At 25 ms prior to dorsiflexion (−25 ms in Fig. 2), the soleus H-reflex was clearly depressed (34 ± 3% of the H-reflex size at <Cgo) whereas the MEP was facilitated (231 ± 24% of the MEP size at <Cgo). Control experiments in three subjects showed that reflexes of a similar small size as the MEPs at <Cgo were still inhibited prior to dorsiflexion.
Figure 2. Modulation of soleus H-reflexes and MEPs prior to plantar- and dorsiflexion.
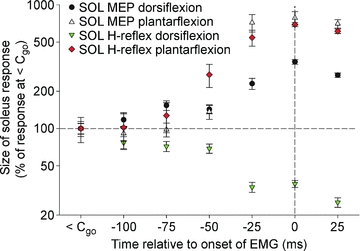
The amplitude of soleus MEPs was increased prior to both dorsiflexion and plantar flexion whereas the amplitude of the soleus H-reflex was only increased prior to plantar flexion. Prior to dorsiflexion, the soleus H-reflex was strongly depressed as has been shown by Crone & Nielsen (1989). Data from one subject.
Figure 3 shows the size of soleus and TA MEPs prior to dorsiflexion and plantar flexion relative to the MEP size at <Cgo. There was a gradual increase in soleus MEP size from around 75–100 ms prior to both plantar flexion and dorsiflexion (Fig. 3A), but this did not reach significance until 50 ms prior to onset of contraction (P= 0.001). In all subjects, the size of the soleus MEPs increased significantly 25 ms prior to onset of plantar flexion (810 ± 105% of the MEP size at <Cgo). At 25 ms prior to dorsiflexion, soleus MEPs were also significantly increased in 10 out of 11 subjects (233 ± 34% of the MEP size at <Cgo). There was no significant difference between the control MEP size in the dorsiflexion and plantar flexion session for soleus (P= 0.84) or TA (P= 0.53).
Figure 3. Modulation of MEPs prior to dorsiflexion and plantar flexion.
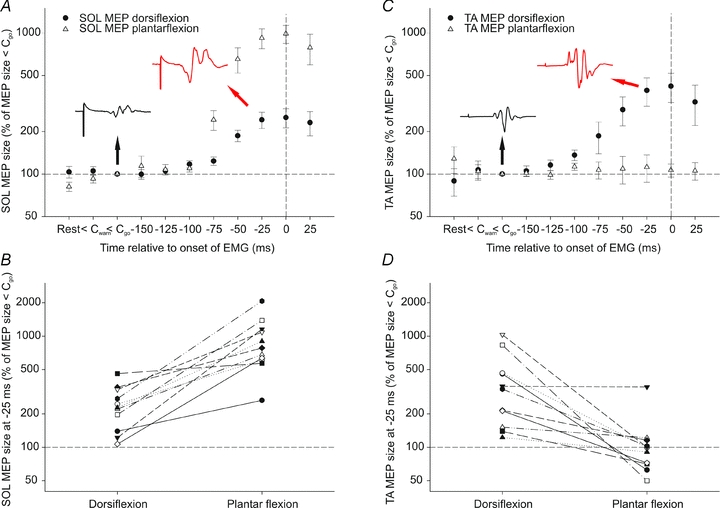
A, average soleus MEP size at rest, < Cwarning, <Cgo and at 25 ms intervals leading up to plantar flexion (▵) and dorsiflexion (•) as a percentage of MEP size at <Cgo (n= 11). The black and red insets are average responses to TMS measured in soleus in one subject at <Cgo (1.9% of Mmax) and at 25 ms prior to dorsiflexion (4.3% of Mmax), respectively. B, size of soleus MEP at 25 ms prior to dorsiflexion and plantar flexion for each subject as a percentage of MEP size at <Cgo. C, like in A, but here TA MEP data are plotted. The black and red insets are average responses to TMS measured in TA in one subject in a soleus experiment at <Cgo (17.8% of Mmax) and at 25 ms prior to dorsiflexion (34.3% of Mmax), respectively. Note the different shape of TA responses compared to the soleus responses in A. These responses were evoked by the same stimulus in the same session. D, size of TA MEP at 25 ms prior to dorsiflexion and plantar flexion for each subject as a percentage of MEP size at <Cgo.
Separate experiments in three subjects demonstrated that there was no increase in the size of Q MEPs at 25 ms prior to onset of dorsiflexion suggesting that the facilitation was specific for soleus MEPs.
TA MEPs increased gradually from around 100 ms prior to onset of dorsiflexion and reached statistical significance at 50 ms (P < 0.001). However, there were no significant changes in the TA MEPs prior to plantar flexion (Fig. 3C). In 9 out of 11 subjects, TA MEPs were significantly increased 25 ms prior to dorsiflexion contraction (327 ± 77% of the MEP size at <Cgo). The modulation of soleus MEPs at 25 ms prior to dorsiflexion and plantar flexion in relation to <Cgo is shown in Fig. 3B and for TA MEPs in Fig. 3D.
Since the test MEP size was much larger in TA than in soleus at a stimulus intensity of 1.2 × resting motor threshold, we performed a control experiment in three subjects where we matched the size of TA MEPs to that of soleus MEPs. To do this we had to reduce the stimulus intensity to about threshold for evoking an MEP in TA. These small TA MEPs (2–3% of Mmax) were similarly modulated as the larger TA MEPs.
In three subjects, we performed control experiments where soleus MEPs were tested under different conditions to make sure that our observations were not due to startle responses or the mode of contraction. These experiments showed that the time course and extent of soleus MEP facilitation prior to dorsiflexion was similar when the subjects had to react to a visual cue instead of the auditory Cgo. This was also the case when the contraction was non-isometric (the foot was not strapped to the force pedal) and when the movement was performed slowly (non-ballistic).
Since MEP size depends on the excitability of spinal motoneurones as well as the amplitude of the descending corticospinal volley it is possible that some of the changes in the MEP were caused by spinal rather than cortical excitability changes. This led us to the following experiments where we investigated the nature of this facilitation.
Soleus CMEPs also increased prior to dorsiflexion
Transmastoid brainstem stimulation is thought to activate the same axons in the corticospinal tract as those activated by cortical stimulation. MEPs evoked by stimulation at the two sites are therefore likely to be similarly influenced by excitability changes at a spinal level, whereas only responses to TMS may be influenced by cortical excitability changes (Ugawa et al. 1991; Taylor et al. 2002). If the increase in soleus MEPs prior to dorsiflexion was of cortical origin, we would thus expect to see a different modulation of CMEPs and MEPs. With comparable sizes of the control MEP and CMEP at <Cgo (0.8 ± 0.4 and 0.6 ± 0.3% of Mmax, respectively), both MEPs and CMEPs were facilitated 25 ms prior to onset of dorsiflexion in all 3 investigated subjects (2.3 ± 1.6 and 2.3 ± 1.8% of Mmax, respectively) (Fig. 4). This suggests that the facilitation is in all likelihood caused by a subcortical mechanism. A statistical analysis was not performed due to the low number of subjects. Notice that the shape of the MEP and the CMEP was similar at <Cgo and that all parts of the responses increased similarly in amplitude and duration when evoked prior to dorsiflexion (see insert, Fig. 4).
Figure 4. Modulation of MEPs and CMEPs.
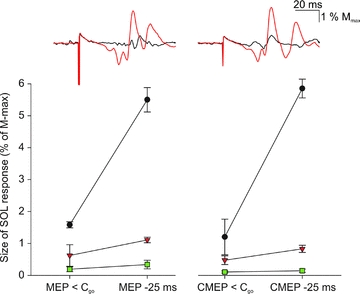
In three subjects, the responses to TMS and brainstem stimulation were measured at <Cgo and 25 ms prior to dorsiflexion (see Fig. 1). The average responses to TMS (left inset) and brainstem stimulation (right inset) at <Cgo (black) and 25 ms prior to dorsiflexion (red) are illustrated from a single subject. The graphs below show the size of the MEP (left) and CMEP (right) responses as a percentage of Mmax at <Cgo and 25 ms prior to dorsiflexion. Each symbol represents one subject. Vertical lines represent s.e.m.
Short-latency facilitation of soleus H-reflexes
The MEP is a compound response, which is influenced by transmission in direct and indirect facilitatory and inhibitory pathways to the spinal motoneurones. To obtain a more specific evaluation of transmission in some of these pathways we conditioned H-reflexes with subthreshold TMS at different conditioning–test intervals prior to plantar flexion and dorsiflexion. In the beginning of each experiment a time course of the TMS-conditioning effect on the test H-reflex was made in order to determine the onset of the earliest (presumed monosynaptic) facilitation and the earliest (presumed disynaptic) inhibition (Nielsen et al. 1993; Nielsen & Petersen, 1995a,b;). Figure 5 shows a time course of effect of TMS on the H-reflex in a single subject. The short-latency facilitation had an onset around −4 ms (P < 0.05) whereas the short-latency inhibition was most clearly seen at −2 ms (P < 0.001). A conditioning–test interval of 10 ms (P= 0.07) was used in all subjects to examine long-latency facilitation (Nielsen et al. 1993; Nielsen & Petersen, 1995a,b;) which is believed to be caused by different descending pathways than the short-latency facilitation (Nielsen & Petersen, 1995b).
Figure 5. Time course of TMS conditioning of the H-reflex.
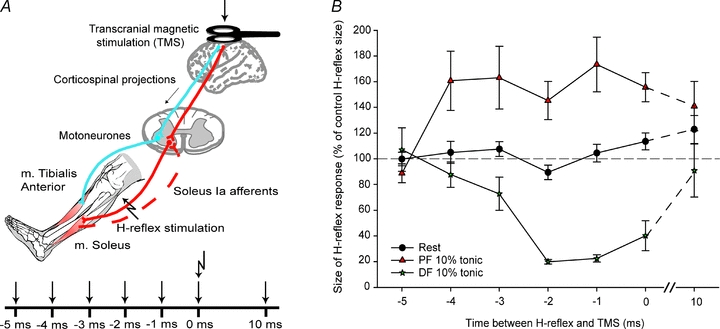
A, an H-reflex was evoked at time ‘0 ms’ by stimulation of the posterior tibial nerve. A conditioning TMS pulse was applied at different times before and after the H-reflex stimulation and the size of the conditioned H-reflex response was then calculated for each conditioning–test interval. B, example of the time course of conditioned H-reflex response as a percentage of the control H-reflex response that was obtained in each subject at rest and during tonic plantar flexion and dorsiflexion. In this subject, the earliest facilitation during tonic planter flexion is observed at a conditioning–test interval of −4 ms. During tonic dorsiflexion, inhibition is seen at −2 ms. These intervals were then used for the following experiments.
Changes in the short-latency facilitation before plantar flexion and dorsiflexion are shown for each subject in Fig. 6A. The amount of short-latency facilitation just prior to plantar flexion (−25 ms) was significantly larger than at <Cgo (167 ± 11%vs. 127 ± 7% of control H-reflex size, P < 0.05) and prior to dorsiflexion (122 ± 6% of control H-reflex size, P < 0.05). However, there was no significant difference between the amount of short-latency facilitation measured prior to dorsiflexion and at <Cgo (P= 0.33).
Figure 6. Modulation of short-latency facilitation, short-latency inhibition and long-latency facilitation prior to contraction.
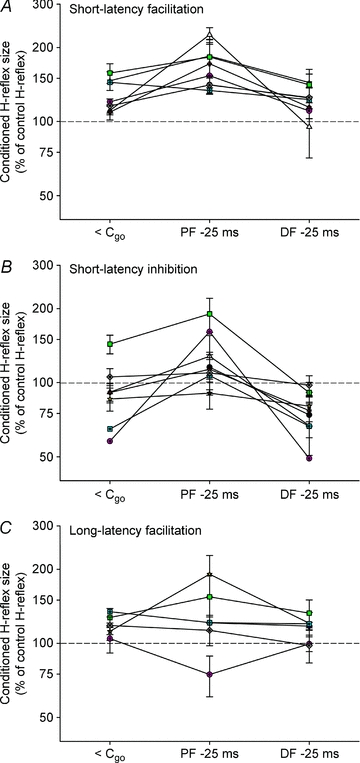
The soleus H-reflex was conditioned by subthreshold TMS at <Cgo and 25 ms prior to dynamic plantar flexion (PF −25 ms) and dorsiflexion (DF −25 ms) contraction to 30% MVC. The size of the conditioned reflex is presented as a percentage of the control reflex size. A, for each subject, the conditioning–test interval eliciting the earliest facilitation in the time course (Fig. 4) was used for measuring short-latency facilitation. B, for short-latency inhibition, the earliest conditioning–test interval giving inhibition during tonic dorsiflexion was used. C, a conditioning–test interval of 10 ms was used to measure long-latency facilitation in all subjects.
Figure 6B shows changes in the short-latency inhibition prior to plantar flexion and dorsiflexion. The amount of short-latency inhibition was 92 ± 9% of control H-reflex size at <Cgo. Prior to plantar flexion, the inhibition was significantly reduced and actually turned in to a facilitation (127 ± 12%, P < 0.05). Prior to dorsiflexion, the short-latency inhibition significantly increased to 75 ± 5% of the control H-reflex size (P < 0.05), which was also significantly larger than prior to plantar flexion (P < 0.01). This is similar to what was observed by Nielsen et al. (1993) and as discussed by them the increase of the inhibition prior to dorsiflexion is likely to reflect facilitation of Ia reciprocal inhibitory interneurones in the spinal cord.
The long-latency facilitation was of similar size at <Cgo and prior to dorsiflexion as well as plantar flexion for all subjects (Fig. 6C; 119 ± 12%vs. 129 ± 16% for plantar flexion and 115 ± 5% for dorsiflexion).
Discussion
The main finding in this study is that soleus MEPs, in contrast to soleus H-reflexes, were facilitated prior to the onset of dorsiflexion.
As the amplitude of soleus MEPs was small, one could fear that the facilitation was simply due to cross-talk from the larger TA MEPs (Capaday, 1997), but this is not very likely, since the shape of TA and soleus MEPs to the same stimuli was clearly different both in the control situation and just prior to the onset of dorsiflexion (see insets in Fig. 3). Furthermore, it has been shown by cross-correlation analysis that there is hardly any cross-talk between the TA and soleus recordings with similar electrode arrangements and amplification as in the present study (Hansen et al. 2005). It should also be noted that all recordings were made prior to the onset of contraction. The EMG from the contracting dorsiflexors therefore appeared later than the time of the MEP recordings and did not contaminate the MEP recordings in either the TA or the soleus muscles.
A similar facilitation of soleus MEPs as we have observed here prior to the onset of dorsiflexion has been reported in previous studies during static dorsiflexion (Valls-Sole et al. 1994). Facilitation of extensor carpi radialis MEPs has also been observed during wrist flexion (Izumi et al. 2000). In these studies, it was proposed that the facilitation of the antagonist MEP was due to a widespread, non-specific increase of motoneuronal excitability to corticospinal inputs. If so, the facilitation of soleus MEPs prior to dorsiflexion may relate to other observations of non-specific modulation of MEPs in relation to a variety of movements and may thus not be specific for the agonist–antagonist pair studied here and in the studies by Valls-Sole et al. (1994) and Izumi et al. (2000). Increased MEPs have been observed in finger muscles during face movements (Andersen et al. 1999) and teeth clenching (Furubayashi et al. 2003), in arm muscles during contralateral elbow movements (Zijdewind et al. 2006) and in arm muscles during ispilateral foot movements (Baldissera et al. 2002).
However, the observation that Q MEPs were unchanged prior to dorsiflexion suggests that the facilitation of the soleus MEPs does not reflect a simple widespread non-specific excitation, but that it is rather a specific part of the motor programme for dorsiflexion.
We cannot say from our data whether a similar facilitation of TA is also a part of the command for plantar flexion. We did not observe any facilitation of the TA MEPs prior to plantar flexion and this could suggest a specific effect on soleus MEPs in relation to dorsiflexion. However, it should be pointed out that the TA H-reflex has been found to be reduced at the onset of and during plantar flexion at least partly due to increased reciprocal inhibition (Crone et al. 1987). The excitability of TA motoneurones must therefore be relatively reduced prior to and during plantar flexion and a reduction of the MEPs – similar to the depression of the TA H-reflexes – would therefore have been expected. The fact that this was not observed suggests that there is a similar facilitatory drive to TA motoneurones prior to plantar flexion as there is for soleus prior to dorsiflexion. Although somewhat speculative, a plausible explanation why this facilitatory drive was not revealed as an actual facilitation of the TA MEP, but only as a lack of the expected inhibition, is that disynaptic reciprocal inhibition of TA motoneurones has been found to be larger than for soleus motoneurones (Crone et al. 1987; Crone & Nielsen, 1994).
In previous studies, it has been assumed that cortical excitatory changes could be at least a contributing factor to the increase of the MEPs in the antagonist muscles. However, our data suggest that a large part of the facilitation takes place at a subcortical site. Firstly, a similar facilitation of CMEPs evoked by cervicomedullary stimulation was observed. Since one subject showed slightly more and the other two slightly less facilitation, we cannot exclude that there is also some cortical component to the facilitation of soleus MEPs prior to dorsiflexion, but the findings indicate that subcortical mechanisms at least contribute to the observed modulation. CMEPs are evoked by activation of the axons of corticospinal neurons at a site far away from the soma and are therefore not likely to be susceptible to changes in cortical excitability (Taylor et al. 2002). Secondly, it could also be ruled out that the facilitation of the MEPs was due to changes in excitability of spinal interneurones activated by the corticospinal volleys evoked by TMS, since no change in any of the facilitatory or inhibitory effects of TMS on soleus H-reflexes was observed prior to dorsiflexion. These facilitatory and inhibitory effects have been shown to be insensitive to changes in spinal motoneuronal excitability and to provide a good estimate of transmission through spinal interneurones contacted by corticospinal pathways (Iles & Pisini, 1992; Nielsen et al. 1993; Nielsen, 1994). Not all corticospinal pathways were activated by the conditioning pulse, so there may be corticospinal pathways that we did not investigate with this technique. Nevertheless, it may with some certainty be concluded that the increased spinal motoneuronal excitability is not mediated by the particular corticospinal tract pathways we investigated, but must originate from some other source. This suggests that corticospinal activation of spinal motoneurones at the onset of voluntary movement is accompanied by triggering of a facilitatory drive to antagonist muscles from subcortical centres.
The generation of voluntary movement is thought to begin with preparatory activity in higher motor areas, propagating through the premotor and primary motor cortex to the spinal cord. However, this sequential activation may be more parallel than previously thought thereby allowing movement-related activity to appear simultaneously in many motor areas. In a study on monkeys, Prut & Fetz (1999) observed pre-movement activity in spinal interneurones in an instructed delay task. This could indicate that even early in movement preparation, the motor cortical areas may interact continuously with subcortical networks (Prut & Fetz, 1999). Since MEPs may reflect excitability changes also in such networks, this may explain the observed facilitation of soleus MEPs prior to dorsiflexion.
Auditory startle reactions are motor responses evoked by a sudden sound of adequate intensity and are believed to be mediated through reticulo-spinal pathways (Davis et al. 1982; Delwaide & Schepens, 1995). It has been proposed that such brainstem–spinal pathways may be involved in releasing the motor programme in startle responses as well as voluntary movements (Rothwell et al. 2002; Valls-Sole et al. 2008). Such connections might explain the subcortical facilitation of soleus MEPs observed in our study. However, it should be pointed out that in the studies by Valls-Sole and colleagues the facilitatory effect was seen in relation to the warning signal and prior to the ‘go’ signal (Valls-Sole, 2004; Kumru & Valls-Sole, 2006). In our study, we observed a similar small facilitatory effect in a few subjects, but we corrected for this effect by comparing all MEPs recorded after the ‘go’ signal to the size of the MEP recorded 100 ms before the ‘go’ signal (at <Cgo), which was almost 3 s after the warning signal. The facilitatory effect we have observed is thus tightly linked to the actual initiation of movement and is thus different from the generalized ‘preparatory’ facilitation described by Valls-Sole et al. (2008).
Earlier studies have also suggested that there is a subcortical contribution to the MEP in antagonist muscles in relation to fast wrist movements. MacKinnon & Rothwell (2000) investigated the corticospinal contribution to the agonist and antagonist EMG bursts in the triphasic ballistic movement pattern accompanying rapid flexion/extension of the wrist. They found that the agonist MEP amplitude began to increase about 10–20 ms before onset of EMG in the agonist muscle, whereas there was no increase in the amplitude of MEPs in the antagonist muscle prior to the antagonist EMG burst. They therefore suggested that the corticospinal tract is not involved in generating the antagonist EMG burst (MacKinnon & Rothwell, 2000), raising the possibility that subcortical structures could be involved (Rothwell et al. 2002).
The exact nature of the facilitatory mechanism cannot be elucidated further at the present time, but possibilities such as changes in the setting of spinal networks, changes in recruitment properties of the motoneuronal pool and changes in membrane properties of the spinal motoneurones may all be considered.
What is the functional significance?
It seems somewhat contradictory that part of the supraspinal control of agonist–antagonist pairs appears to involve a parallel activation pattern and another part the classical reciprocal inhibitory activation pattern. Why this apparent competition of facilitatory and inhibitory influences? One possibility is that the facilitation of the antagonist may help to ensure that quick transitions from dorsiflexion into plantar flexion may be made more efficiently than if the plantar flexors would need first to be strongly depolarized from a hyperpolarized state before becoming active. In this way only small changes in the descending corticospinal drive would be necessary to switch from one movement direction to another. An organization pattern somewhat like this has been described for finger motoneurones where motoneurones to several finger muscles are depolarized in relation to movement of any of the fingers (Bremner et al. 1991). Because of the organization of the corticospinal innervation of the finger motoneurones only the motoneurones to one particular finger are depolarized sufficiently to reach threshold whereas the other motoneurones are maintained right below firing threshold (Bennett & Lemon, 1996). A small change in the descending drive may thus switch the activation from one finger to the other, thereby making very quick finger movements possible. We propose that our observations may reflect that the control of antagonistic ankle muscles is organized to ensure a similar quick switch between activation of the muscles. Such quick switches between antagonistic muscle activities may especially be of importance in relation to high performance sports.
Where does this leave reciprocal inhibition and the problem that stretch of the antagonist muscle at the onset of agonist contraction may easily evoke stretch reflexes when the antagonist motoneurones are not suppressed? First, a very significant part of reciprocal inhibition is caused by presynaptic inhibition of the antagonist Ia afferents (Crone & Nielsen, 1989; Nielsen & Kagamihara, 1993). In this way, the stretch reflex may be efficiently suppressed while maintaining a high excitability of the antagonist motoneurones. Secondly, it may be argued that the increased (postsynaptic) reciprocal inhibition at the onset of movement, which has been demonstrated now in numerous studies (and also evidenced from the present study from the increase of TMS-induced inhibition of the soleus H-reflex at the onset of dorsiflexion) is still of importance to ensure that the antagonist motoneurones do not reach threshold and thus prevent the agonist movement from taking place. Indeed, it is notable that very little EMG activity is generally seen in the soleus muscle even at the onset of very quick dorsiflexion (Geertsen et al. 2008).
The findings in the study may potentially have relevance for rehabilitation strategies in patients with stroke and other lesions of the brain and spinal cord. Most interventions tend to focus on the possibility of promoting reorganization at the cortical level, but if subcortical motor programmes, as we suggest, are involved in the control of voluntary movement there might be some benefit in targeting such programmes also. One way may be to take advantage of a possible overlap with some of the circuitry involved in startle reactions.
Concluding remarks
We have demonstrated that soleus MEPs, in contrast to soleus H-reflexes, are facilitated prior to the onset of dorsiflexion. We have not been able to find the origin of this facilitation, but we propose that it may involve activation of a subcortical motor programme. We propose that the apparent parallel activation of agonists and antagonists may be of importance for making quick switches in movement direction.
Acknowledgments
This work was supported by grants from the Danish Medical Research Council and the Ludvig and Sara Elsass Foundation.
Glossary
Abbreviations
- Cgo
auditory go cue
- Cwarning
auditory warning cue
- CMEP
cervicomedullary motor evoked potential
- EMG
electromyography
- Mmax
maximal M-response
- MEP
motor evoked potential
- MVC
maximal voluntary contraction
- TA
tibialis anterior
- TMS
transcranial magnetic stimulation
Author contributions
All authors contributed to the concept and design of the experiments as well as to the collection, analysis and interpretation of data. The work was done at the Panum Institute, University of Copenhagen. S.S.G. and J.B.N. drafted the manuscript and all authors critically revised the manuscript and approved the final version for publication.
References
- Andersen B, Rosler KM, Lauritzen M. Nonspecific facilitation of responses to transcranial magnetic stimulation. Muscle Nerve. 1999;22:857–863. doi: 10.1002/(sici)1097-4598(199907)22:7<857::aid-mus7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Borroni P, Cavallari P, Cerri G. Excitability changes in human corticospinal projections to forearm muscles during voluntary movement of ipsilateral foot. J Physiol. 2002;539:903–911. doi: 10.1113/jphysiol.2001.013282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KM, Lemon RN. Corticomotoneuronal contribution to the fractionation of muscle activity during precision grip in the monkey. J Neurophysiol. 1996;75:1826–1842. doi: 10.1152/jn.1996.75.5.1826. [DOI] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol. 1991;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol. 1989;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand. 1994;152:351–363. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Schepens B. Auditory startle (audio-spinal) reaction in normal man: EMG responses and H reflex changes in antagonistic lower limb muscles. Electroencephalogr Clin Neurophysiol. 1995;97:416–423. doi: 10.1016/0924-980x(95)00136-9. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Functional relations between primate motor cortex cells and muscles: fixed and flexible. Ciba Found Symp. 1987;132:98–117. doi: 10.1002/9780470513545.ch7. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Sugawara K, Kasai T, Hayashi A, Hanajima R, Shiio Y, Iwata NK, Ugawa Y. Remote effects of self-paced teeth clenching on the excitability of hand motor area. Exp Brain Res. 2003;148:261–265. doi: 10.1007/s00221-002-1299-y. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Lundbye-Jensen J, Nielsen JB. Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol. 2008;105:915–922. doi: 10.1152/japplphysiol.01155.2007. [DOI] [PubMed] [Google Scholar]
- Goulart F, Valls-Sole J. Reciprocal changes of excitability between tibialis anterior and soleus during the sit-to-stand movement. Exp Brain Res. 2001;139:391–397. doi: 10.1007/s002210100771. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sorensen F, Nielsen JB. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol. 2005;94:934–942. doi: 10.1152/jn.00082.2005. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Lundberg A. Reciprocal inhibition during the stretch reflex. Acta Physiol Scand. 1972;85:136–138. doi: 10.1111/j.1748-1716.1972.tb05243.x. [DOI] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Cortical modulation of transmission in spinal reflex pathways of man. J Physiol. 1992;455:425–446. doi: 10.1113/jphysiol.1992.sp019309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi S, Koyama Y, Furukawa T, Ishida A. Effect of antagonistic voluntary contraction on motor responses in the forearm. Clin Neurophysiol. 2000;111:1008–1014. doi: 10.1016/s1388-2457(00)00283-2. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976;258:467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Valls-Sole J. Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp Brain Res. 2006;169:427–432. doi: 10.1007/s00221-005-0156-1. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol. 2000;528:633–645. doi: 10.1111/j.1469-7793.2000.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. Further evidence of increased motor cortex excitability during tonic plantar flexion in humans. Acta Physiol Scand. 1994;152:341–343. doi: 10.1111/j.1748-1716.1994.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Changes in the effect of magnetic brain stimulation accompanying voluntary dynamic contraction in man. J Physiol. 1995a;484:777–789. doi: 10.1113/jphysiol.1995.sp020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. J Physiol. 1995b;486:779–788. doi: 10.1113/jphysiol.1995.sp020853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt CA, Jordan LM. Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J Neurophysiol. 1987;57:56–71. doi: 10.1152/jn.1987.57.1.56. [DOI] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 1999;401:590–594. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Pyndt HS, Laursen M, Nielsen JB. Changes in reciprocal inhibition across the ankle joint with changes in external load and pedaling rate during bicycling. J Neurophysiol. 2003;90:3168–3177. doi: 10.1152/jn.00444.2003. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, MacKinnon CD, Valls-Sole J. Role of brainstem-spinal projections in voluntary movement. Mov Disord. 2002;17(Suppl 2):S27–29. doi: 10.1002/mds.10054. [DOI] [PubMed] [Google Scholar]
- Roy FD, Gorassini MA. Peripheral sensory activation of cortical circuits in the leg motor cortex of man. J Physiol. 2008;586:4091–4105. doi: 10.1113/jphysiol.2008.153726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Genba-Shimizu K, Kanazawa I. Electrical stimulation of the human descending motor tracts at several levels. Can J Neurol Sci. 1995;22:36–42. doi: 10.1017/s0317167100040476. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J. Contribution of subcortical motor pathways to the execution of ballistic movements. Suppl Clin Neurophysiol. 2004;57:554–562. doi: 10.1016/s1567-424x(09)70394-0. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Alvarez R, Tolosa ES. Responses of the soleus muscle to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:421–427. doi: 10.1016/0168-5597(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006;175:526–535. doi: 10.1007/s00221-006-0570-z. [DOI] [PubMed] [Google Scholar]


