Abstract
Gap junction-mediated electrical synapses interconnect diverse types of neurons in the mammalian brain, and they may play important roles in the synchronization and development of neural circuits. Thalamic relay neurons are the major source of input to neocortex. Electrical synapses have not been directly observed between relay neurons in either developing or adult animals. We tested for electrical synapses by recording from pairs of relay neurons in acute slices of developing ventrobasal nucleus (VBN) of the thalamus from rats and mice. Electrical synapses were common between VBN relay neurons during the first postnatal week, and then declined sharply during the second week. Electrical coupling was reduced among cells of connexin36 (Cx36) knockout mice; however, some neuron pairs remained coupled. This implies that electrical synapses between the majority of coupled VBN neurons require Cx36 but that other gap junction proteins also contribute. The anatomical distribution of a β-galactosidase reporter indicated that Cx36 was expressed in some VBN neurons during the first postnatal week and sharply declined over the second week, consistent with our physiological results. VBN relay neurons also communicated via chemical synapses. Rare pairs of relay neurons excited one another monosynaptically. Much more commonly, spikes in one relay neuron evoked disynaptic inhibition (via the thalamic reticular nucleus) in the same or a neighbouring relay neuron. Disynaptic inhibition between VBN cells emerged as electrical coupling was decreasing, during the second postnatal week. Our results demonstrate that thalamic relay neurons communicate primarily via electrical synapses during early postnatal development, and then lose their electrical coupling as a chemical synapse-mediated inhibitory circuit matures.
Introduction
The dorsal thalamus conveys information to the neocortex. Activity originating in peripheral receptors is transferred to sensory areas of neocortex through primary thalamic relay nuclei. However, sensory information is not simply relayed by the thalamus; it is also processed and modified by interactions between two intrinsic physiological states of the neurons (tonic or burst firing modes), synaptic connections with GABAergic neurons in the thalamic reticular nucleus, excitatory inputs descending from neocortex, and state-dependent regulation via neuromodulators (Llinas & Steriade, 2006; Huguenard & McCormick, 2007). In the developing brain, thalamic relay neurons play an important role in shaping the activity-dependent features of thalamocortical circuits (Katz & Shatz, 1996; Weliky & Katz, 1999).
Recently, it has become clear that many types of neurons in the mammalian brain communicate through gap junction-mediated electrical synapses (Bennett & Zukin, 2004; Connors & Long, 2004). Gap junctions can produce both electrical and biochemical coupling between neurons, and are thought to play diverse roles in developing and mature neural circuits (Bennett & Zukin, 2004; Connors & Long, 2004; Hestrin & Galarreta, 2005; Sutor & Hagerty, 2005; Bruzzone & Dermietzel, 2006). In the thalamus, electrical synapses have been directly observed between GABAergic inhibitory neurons of the thalamic reticular nucleus (TRN) (Landisman et al. 2002; Long et al. 2004). The strength of this TRN coupling is relatively stable across early postnatal development (Parker et al. 2009) and is likely to exist into adulthood (Blethyn et al. 2008). Consistent with the physiology, studies using in situ hybridization, immunocytochemistry and reporter gene patterns have implied that there is strong expression of the neuronal gap junction protein, connexin36 (Cx36), in TRN (Condorelli et al. 2000; Deans et al. 2001; Landisman et al. 2002; Liu & Jones, 2003; Degen et al. 2004). In contrast, most thalamic relay nuclei show relatively low Cx36 mRNA expression, suggesting that electrical synapses are absent in those nuclei.
Several studies have provided intriguing indirect evidence for the existence of electrical coupling among thalamic relay neurons. First, anatomical data imply that another neuronal connexin, Cx45, is expressed in thalamic relay neurons, and that it persists into adulthood (Maxeiner et al. 2003; Sohl et al. 2005). Second, several types of indirect physiological and pharmacological evidence suggest gap junction-dependent synchronization of thalamic relay neurons (Hughes et al. 2002, 2004).
In this study we directly tested for the presence of electrical synapses in thalamic relay nuclei of developing rodents by making paired whole-cell recordings from acute slices of ventrobasal nucleus (VBN), the primary somatosensory area of the thalamus. Electrical synapses were common between VBN neurons during the first postnatal week, and they declined during the second week as chemical synaptic circuits emerged. Most of the electrical coupling between VBN cells required Cx36. These data suggest that electrical synapses may play a role in the development of thalamic circuits, analogous to that proposed for intracortical circuits (Connors et al. 1983; Peinado et al. 1993; Kandler & Katz, 1995; Roerig & Feller, 2000; Montoro & Yuste, 2004; Sutor & Hagerty, 2005; Dupont et al. 2006; Hanganu et al. 2009).
Methods
Animals, slice preparation, recording and physiological analysis
All experiments were approved by the Institutional Animal Care and Use Committee of Brown University. Brain slices were prepared from either Sprague−Dawley rats (postnatal day (P)2 to P31, n= 31 animals) or Cx36 knockout (KO, Cx36−/−) or wild-type (WT, Cx36+/+) littermate mice (P4–P15, n= 33 animals). Cx36 KO mice were generated as described previously (Deans et al. 2001), derived from C57BL6-129SvEv mixed background.
Prior to dissection, animals were administered an overdose of thiopental sodium (intraperitoneal injections, 200 mg kg−1). After deep anaesthesia was achieved, animals were decapitated and brains were removed and chilled in ice cold artificial cerebral spinal fluid (ACSF), then sectioned into 280–320 μm thick slices containing the thalamic VBN (thalamocortical plane) (Agmon & Connors, 1991). After sectioning, slices were incubated in ACSF at 32°C for 30 min, and then kept at room temperature until they were transferred to a submersion-type recording chamber perfused with ACSF at 32°C. Cells were visualized using infrared-differential interference contrast (IR-DIC) optics. VBN neurons of <5 μm separation were chosen for paired whole-cell recordings. The ACSF contained (in mm): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 10 dextrose and 2 MgCl2, saturated with 95% O2–5% CO2. Micropipettes were filled with (in mm): 130 potassium gluconate, 0.2 EGTA, 4 KCl, 2 NaCl, 10 Hepes, 4 ATP-Mg, 0.3 GTP-Tris and 14 phosphocreatine-Tris, pH 7.25–7.30 (285–295 mosmol l−1). Recordings were performed in current clamp using Axoprobe or Axoclamp 2B amplifiers (Molecular Devices (Axon Instruments), Sunnyvale, CA, USA). Data were collected and analysed using National Instruments (Austin, TX, USA) and Molecular Devices hardware and software.
To estimate the strength of coupling, 5–40 hyperpolarizing current pulses (600 ms) were first injected into one cell, and the mean steady-state voltage deflection in that cell (ΔV1) and in its coupled neighbour (ΔV2) were measured. A coupling coefficient (CC) was then calculated as ΔV2/ΔV1. This was repeated for current injections into the second cell and the two values were then averaged. Pairs were considered to be electrically coupled if their coupling coefficients were ≥0.01, which was the minimum level that could be reliably detected under the conditions of our experiments. Junctional conductance (GJ) estimates were calculated using values of the injected currents and voltage responses of each cell (Bennett, 1966; Parker et al. 2009). These calculations assume a model of two isopotential neurons coupled directly by a single junction, and do not account for complexities arising from junctions on dendritic or axonal cables, non-linear membrane properties, or effects of additional coupled cells (Bennett, 1966; Amitai et al. 2002). Input resistances were measured from voltage responses to small negative current injections. Resting membrane potentials were measured during the first 5 min of recordings and were corrected for a 14 mV liquid junction potential. For electrophysiological comparisons between Cx36 KO and WT animals, the experimenters were blind to the genotypes of the mice during the recordings. Error values are s.e.m.
β-Galactosidase histochemistry
P3–20 Cx36 knockout (Cx36−/−), heterozygote (Cx36+/−), and wild-type (Cx36+/+) littermate mice were deeply anaesthetized with intraperitoneal injections of thiopental sodium (200 mg kg−1). Vibrating blade microtome sections of 300–400 μm were prepared at the thalamocortical slice angle, then immediately fixed by submersion for 3 h in 0.5% glutaraldehyde/2.0 mm MgCl2/1.25 mm EGTA (pH 7.4). After fixation, slices were thoroughly rinsed in phosphate-buffered saline (PBS) consisting of 100 mm sodium phosphate + 150 mm sodium chloride (pH 7.4), cryo-protected overnight in 30% sucrose/PBS, then re-sectioned at 60 μm using a freezing microtome. A modified X-gal staining procedure was followed (Mombaerts et al. 1996). Sections were washed twice (first at room temperature, then at 37°C) for 10 min each in X-gal wash buffer (100 mm phosphate buffer at pH 7.4, 2 mm MgCl2-hydrate, 0.01% sodium desoxycholate, and 0.02% (octylphenoxy)polyethoxyethanol (IGEPAL). An 18–20 h incubation in staining solution followed, done at 37°C in darkness. The staining solution contained X-gal wash buffer plus 5 mm potassium ferricyanide, 5 mm potassium ferrocyanide and 1 mg ml−1 X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). After staining, sections were rinsed twice for 10 min each with pre-warmed (37°C) X-gal wash buffer to retard crystal formation, then rinsed in PBS followed by 100 mm phosphate buffer (PB). Sections were transferred to 50 mm PB, mounted onto glass slides, coverslipped with glycerol, then imaged using a Nikon E600 light microscope and a digital camera (SPOT, Diagnostic Instruments, Inc., Sterling Heights, MI, USA). Histological processing, camera settings and exposure times were identical across mouse ages and genotypes.
Results
Electrical synapses between rodent VBN relay neurons
To determine the extent of electrical coupling in the ventrobasal nucleus (VBN) of the thalamus, we made dual whole-cell recordings of relay neuron pairs in acute thalamocortical slices obtained from rats and mice aged postnatal day (P)2 to P31 (Fig. 1D). Closely spaced cell pairs (<5 μm between somata) were deliberately chosen in order to maximize the probability that they would be coupled (Amitai et al. 2002; Long et al. 2004). We observed clear electrical coupling in 21–24% of tested cell pairs during the first two postnatal weeks (24/102 pairs from rat and 15/70 from wild-type mice). Figure 1A–C shows an example from a P8 rat. Injection of depolarizing or hyperpolarizing current pulses produced direct voltage responses in the injected neuron and attenuated voltage changes, due to coupling, in the paired neuron. Electrical transmission was bidirectional and relatively symmetrical in magnitude, as reported previously for neurons in other mammalian brain regions (Connors & Long, 2004). The mean coupling coefficients (defined as ΔV2/ΔV1, see Methods) among all connected VBN cell pairs were 0.024 ± 0.002 for rats and 0.029 ± 0.007 for mice.
Figure 1. Electrical synapses between rat VBN neurons.
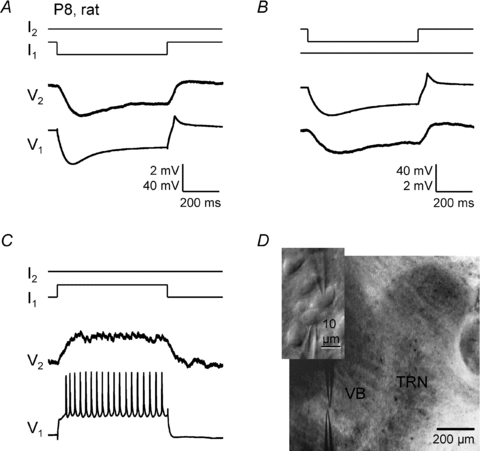
Electrical coupling recorded in current clamp from a pair of closely spaced VBN neurons (P8 rat). A, negative intracellular current steps (−150 pA) in cell 1 evoked a large voltage hyperpolarzation in cell 1 and a smaller hyperpolarization, due to coupling, in cell 2 (coupling coefficient ∼0.04). B, the same cell pair, but in this case negative current steps (−100 pA) were delivered to cell 2. C, positive current steps (+340 pA) delivered to cell 1 produced depolarization and spiking in cell 1 and smaller depolarization in cell 2. Calibration bars in A also apply to C. D, IR-DIC image of the paired whole-cell recording in the ventrobasal nucleus (VBN) of a thalamocortical slice. Inset shows a higher power view of the recorded cells.
Developmental regulation of electrical synapses in VBN
For some other areas of the mammalian central nervous system, there is a consensus that electrical synapses exist early in development and are down-regulated during maturation (Connors et al. 1983; Peinado et al. 1993; Arumugam et al. 2005; Sutor & Hagerty, 2005; Bruzzone & Dermietzel, 2006; Dupont et al. 2006). We tested this possibility for thalamic relay cells. In the rat VBN, we compared levels of electrical coupling from P2 through P31. The probability of observing electrical synaptic connections between closely spaced VBN cells was comparable from P2 to P9 (range: 28–44%), but steeply decreased after P10 (≤8%; Fig. 2A). We found no electrically coupled VBN cell pairs from rats older than P13 (n= 13 tested). The functional strength of electrical coupling among connected pairs (i.e. the CC) also decreased with development (P < 0.02, linear regression; Fig. 2B). Thus, both of these electrical coupling measures decreased sharply with age, leading to undetectable levels after about P13 in the rat VBN (Fig. 2A and B). We found similar developmental changes in the coupling between mouse VBN relay neurons, with strong decreases in coupling from the first to the second postnatal weeks (Fig. 3).
Figure 2. Developmental change in electrical coupling between rat VBN neurons.

A, the probability of observing electrical coupling between adjacent VBN cells decreased with postnatal age (P2–P31). Values in parentheses are the number of pairs recorded for the respective age groups. B, coupling coefficients of electrically coupled pairs also decreased with postnatal age (P < 0.02, linear regression). Circles represent individual pairs and dashes represent group means. C, estimated junctional conductances of connected pairs from P2 to P14 (P= 0.051, linear regression).
Figure 3. Electrical synapses between VBN neurons of WT mice.
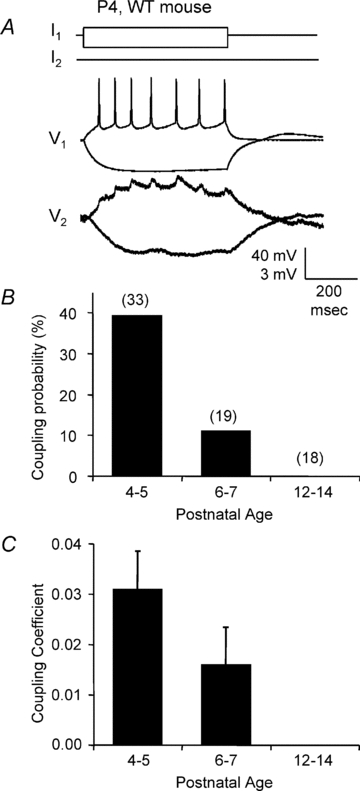
A, recording of an electrically coupled VBN neuronal pair from a P4 wild-type mouse. Current pulses of 25 pA and −50 pA were injected into the first cell. Note the postsynaptic ‘spikelets’ in the strongly coupled paired cell (CC = 0.079). B and C, coupling probabilities and coefficients of VBN neurons in wild-type mice at different postnatal ages. Values in parentheses in B are the number of pairs recorded for the respective age groups.
The intrinsic membrane properties of VBN neurons also underwent large changes during the tested period. First, input resistances were very high at perinatal ages and decreased steeply with age. For example, in the rat VBN, input resistances were greater than 1000 MΩ at P2–3 and decreased to about 200 MΩ by P14. Similar developmental changes in input resistance were observed in mice (Table 1). Second, resting membrane potentials generally hyperpolarized as animals matured (Table 1). Third, intrinsic excitability changed. Mature VBN neurons are well known to generate rebound spike bursts, which are mediated by low-threshold calcium currents, following offset from hyperpolarization (Contreras, 2006; Llinas & Steriade, 2006). Rebound bursting also underwent developmental changes; bursts were absent during the first postnatal week, then appeared around P9–10, as observed in previous studies (Perez Velazquez & Carlen, 1996; Warren & Jones, 1997). After P12, all rat and mouse VBN neurons exhibited rebound burst spiking (see Figs 6B and 7C).
Table 1.
Postnatal development of input resistance and resting membrane potential of VBN cells
| Postnatal age (days)* |
||||||||
|---|---|---|---|---|---|---|---|---|
| 2–3 | 4–5 | 6–7 | 8–9 | 10–11 | 12–14 | 15–19 | ||
| Rin | Rat | 1042 ± 38 | 721 ± 33 | 519 ± 44 | 414 ± 20 | 267 ± 18 | 203 ± 7 | 155 ± 9 |
| Mouse (WT) | — | 758 ± 26 | 626 ± 30 | — | — | 267 ± 12 | — | |
| Mouse (KO) | — | 700 ± 25 | 749 ± 26 | — | — | 230 ± 16 | — | |
| Vm | Rat | −67 ± 1 | −66 ± 1 | −65 ± 1 | −69 ± 1 | −73 ± 1 | −74 ± 1 | −74 ± 1 |
| Mouse (WT) | — | −68 ± 1 | −69 ± 1 | — | — | −74 ± 1 | — | |
| Mouse (KO) | — | −70 ± 1 | −68 ± 2 | — | — | −74 ± 1 | — | |
Values reported for each age group are means ±s.e.m.; Rin, input resistance in MΩ; Vm, membrane potential in mV; the number of neurons in each age group was ≥7.
Figure 6. Excitatory chemical synaptic transmission between VBN neurons.
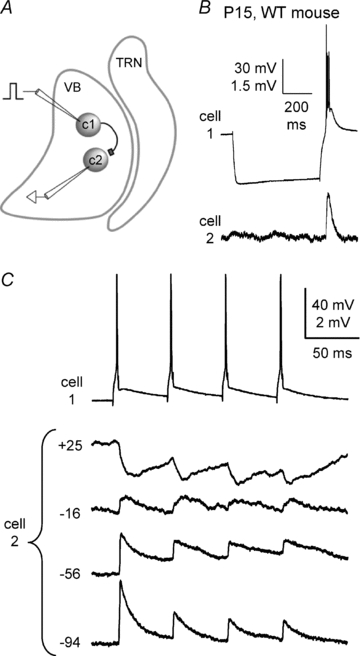
Recordings from a pair of VBN cells from a P15 mouse. A, schematic diagram of protocol and presumed connections for the example in B and C. Cell 1 makes an excitatory synapse with cell 2. Intracellular current steps were injected into cell 1 (c1) evoking spikes. Cell 1 projected to cell 2 (c2), producing monosynaptic EPSPs. B, initially, long negative current pulses (600 ms) were injected into cell 1, producing hyperpolarization followed by offset bursts. The spike bursts in cell 1 evoked EPSPs in cell 2. This cell pair was not electrically coupled. C, reversal potential of the EPSPs was tested by inducing spike trains in cell 1 (4 spikes, 20 Hz) and recording EPSPs in cell 2 while its steady-state membrane potential was adjusted across a wide range (−94 to +25 mV) with intracellular current. The EPSPs reversed near 0 mV, consistent with glutamatergic synapses.
Figure 7. Disynaptic inhibitory transmission between VBN neurons.
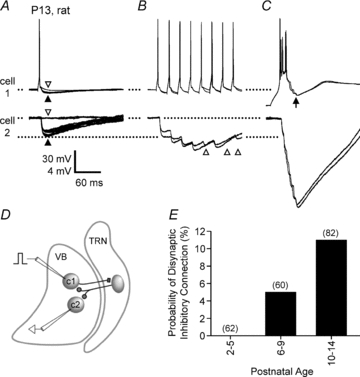
A–D, disynaptic inhibition in a pair of VBN cells from a P13 rat. Spikes were evoked in cell 1 (with intracellular current steps) while disynaptic IPSPs were recorded in cell 2 and in cell 1. Cell 2 was maintained at a resting potential of −66 mV. A, single spikes in cell 1 evoked 3–4 mV IPSPs in cell 2 (filled arrowhead), although failures occurred occasionally (2 failures in 15 trials shown; open arrowhead). Spikes in cell 1 also evoked IPSPs in cell 1 (filled arrowhead); these are evident by comparison with trials where the IPSP failed (open arrowhead). The IPSP failures in cell 1 occurred on the same trials as those in cell 2. B, trains of spikes at 40 Hz resulted in IPSP summation. Two example trains are shown. IPSPs were evoked in cell 2 following most spikes in cell 1 (IPSP failures are evident after spikes 5, 7 and 8: open arrowheads). The individual IPSPs depressed in amplitude during the train, but summation caused peak voltages to be nearly 2-fold larger than single spike-evoked IPSPs. C, bursts were most effective at evoking disynaptic inhibition. Two bursts are shown (4 spikes per burst). Cell 1 was first stepped to −90 mV with hyperpolarizing current (>500 ms), then current was removed, allowing for depolarization and bursting. Peak burst-evoked IPSPs recorded in cell 2 were larger than peak train-evoked IPSPs in B. Note the presence of IPSPs also in cell 1 following the burst (arrow). D, schematic of the presumed connections underlying the example of A–C. Cell 1 (c1) in VBN makes excitatory synapses with at least one inhibitory cell in TRN (not recorded). The TRN cell makes inhibitory synapses with cells 1 and 2 in the VBN, leading to IPSPs. E, the probability of observing disynaptic inhibition between adjacent VBN cells across early postnatal development in rats. Values in parentheses are the number of connections tested for the respective age groups (2 connections tested per pair).
Junctional conductances of VBN electrical synapses were estimated from the values of input resistances and coupling coefficients (see Methods). These conductances appeared to trend upward from P2–3 to P12–13 (Fig. 2C). However, it is quite possible that we were unable to distinguish very weak junctions among older VBN pairs because of the inherently low input resistances of mature neurons; test current pulses would have yielded very small postsynaptic responses below our detection threshold. Thus, it is likely that the average junctional conductance estimates from older neurons are overestimates. Nevertheless, high conductance (>0.08 nS) coupling, which was observed occasionally at P8–9 and P12–13, was absent at P2–7, suggesting a real increase in junctional conductances between some neurons during early development (cf. Parker et al. 2009).
Connexin36 is a major component of VBN electrical synapses
Current evidence indicates that at least two neuronal connexins, Cx36 and Cx45, are expressed in VBN at early postnatal ages (Belluardo et al. 2000; Maxeiner et al. 2003). To test for the involvement of Cx36 in VBN electrical synapses, we estimated levels of coupling in Cx36 knockout (KO) mice and their wild-type (WT) littermates (Deans et al. 2001). Similar to rats, WT mice had significant electrical coupling in the VBN during the first postnatal week (Fig. 3A), which decreased to undetectable levels by the end of the second week (Fig. 3B and C). We thus made WT vs. KO comparisons from P4–5 animals, which was an age that exhibited robust coupling in WT mice. The probability of observing electrical coupling between pairs of cells in VBN was significantly reduced in Cx36 KO mice (Fig. 4A; P < 0.03, chi square; 39.4% probability of coupling in WT, 14.3% in Cx36 KO). In addition, the most strongly coupled pairs in the WT group had about 3-fold greater coupling coefficients than the strongest in the KO group (Fig. 4B). These differences in coupling probability and strength suggest that Cx36 is a major component of electrical synapses in the young VBN. Even so, the presence of some clearly coupled pairs in the Cx36 KO (4/28 tested pairs; Fig. 4B and C) indicates that gap junction proteins other than Cx36 are also involved, though their contribution appears to be minor.
Figure 4. Most electrical coupling between young mouse VBN neurons depends on Cx36.

A, coupling probabilities in VBN nuclei of KO vs. WT mice at P4–5. Coupling probability was reduced in Cx36 KO. Values in parentheses are the number of pairs recorded for each genotype. B, coupling coefficients of VBN cell pairs from WT and Cx36 KO mice. Shading demarcates coupling coefficients below threshold (CC < 0.01, see Methods). C, example traces from one of the 4 electrically coupled VBN pairs from the Cx36 KO group (P4, CC = 0.023). Negative current pulses (−80 pA) were injected into cell 1, causing a large hyperpolarization in that cell and a smaller hyperpolarization, due to coupling, in cell 2.
Cx36 reporter expression in VBN
Our electrophysiological data imply that Cx36 is necessary for much of the electrical coupling between neurons in the immature VBN. To estimate the expression profile of the Cx36 gene in the immature VBN, we examined the level of β-galactosidase (β-gal) reporter from brains of Cx36 heterozygote (Cx36+/−) mice in which the β-gal gene was inserted in place of the Cx36 coding sequence (Deans et al. 2001). We studied P4, P7, P12 and P20 brains and we observed clear β-gal activity in the VBN at P4 and P7 (Fig. 5A), though the signal intensity was weaker than in the TRN. The β-gal signals could be seen more clearly in Cx36 KO (homozygous) animal brains, which presumably had twice as many copies of the β-gal gene (Fig. 5B). Reporter expression was absent in WT tissue (Fig. 5C). The β-gal signals appeared to be located in cell bodies, broadly scattered within the VBN. Interestingly, expression was not uniform across VBN. For example, posterior regions showed substantially greater expression than more central/anterior regions (Fig. 5A and B).
Figure 5. β-Galactosidase reporter expression for Cx36 in mouse VBN at different postnatal ages.
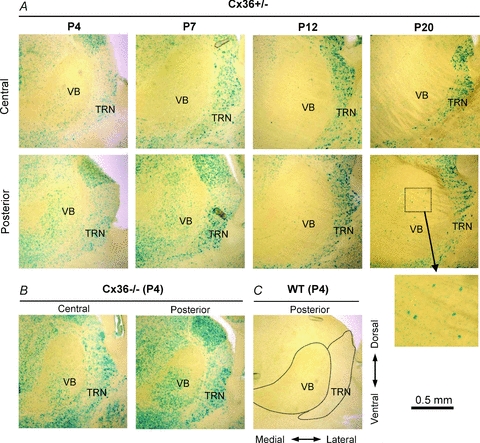
A, images of β-galactosidase staining in thalamic slices from Cx36 heterozygote mice (Cx36+/−) at different postnatal ages. Bottom row images are from the posterior part of VBN, and top row are from the central part of VBN (i.e. more anterior). The inset in the lower-right region shows the boxed VBN region of the posterior P20 section at higher magnification. B, β-galactosidase signal in thalamic slices from a P4 Cx36 KO mouse (homozygote, Cx36−/−). C, β-galactosidase signal in a thalamic slice from a P4 WT mouse (Cx36+/+). Dotted lines outline approximate boundaries of VBN and TRN.
At the later ages examined (i.e. P12 and P20), Cx36 β-gal signals became very weak in VBN but remained strong in TRN, consistent with the continued presence of electrical coupling among TRN neurons (Landisman et al. 2002; Parker et al. 2009). Nevertheless, a few isolated cells with intense β-gal signal intensity were still observed even in the P20 VBN (Fig. 5A). The β-gal data imply that there is substantial Cx36 expression in the young VBN (P4, P7) and that expression decreases as animals mature, leading to the near absence of Cx36 in the VBN by P20.
Chemical synaptic interactions between VBN relay neurons
Our recordings indicated that electrical synaptic communication among VBN neurons is functionally down-regulated during the second postnatal week. We also examined the appearance of chemical synaptic interactions over this period.
It is generally believed that thalamic relay cells do not make excitatory chemical synapses with other relay cells (Steriade et al. 1997). However, we did observe rare excitatory connections between VBN cell pairs. Such excitation was observed in two VBN pairs (from two WT mice; one P4 and the other P15; Fig. 6), from a sample of >300 potential synaptic connections in rats and mice. The time course, relatively short and invariant latency, short-term depression and a reversal potential close to 0 mV in both pairs all suggested that these connections were generated by monosynaptic excitatory synapses, presumably mediated by glutamate (Fig. 6C). To our knowledge, these are the first direct measurements of chemical synaptic connections between thalamic relay neurons (cf. Cox et al. 2003), although a small number of studies provided indirect evidence (Montero, 1991; Soltesz & Crunelli, 1992).
Disynaptic inhibition was a more frequently observed form of chemical synaptic communication between VBN cells. Excitatory neurons in thalamic relay nuclei synapse with inhibitory neurons of the thalamic reticular nucleus (TRN) via feedback connections organized in a generally topographic pattern, with substantial divergence and convergence (Desilets-Roy et al. 2002; Gentet & Ulrich, 2003; Guillery & Harting, 2003; Pinault, 2004; Lam & Sherman, 2005). Given this circuitry (Fig. 7D), it might be predicted that spikes from single VBN neurons could produce disynaptic IPSPs in neighbouring VBN cells. In fact, we observed such inhibition from relatively mature slices of both rat and mouse (Fig. 7A–C). In many recordings, recurrent disynaptic IPSPs were also evident, i.e. spikes triggered in cell 1 evoked IPSPs in cell 1 (Fig. 7A and C; arrows) as well as in cell 2 (cf. (Lo & Sherman, 1994; Kim & McCormick, 1998).
In rat VBN, no disynaptic inhibition was observed during the first 6 postnatal days (n= 38 pairs recorded, so 76 connections tested), a few connections were observed at P7 (n= 3/14 connections), and the majority were from P10 or older (n= 9/82 connections; Fig. 7E). Thus, at ages when electrical synaptic communication began to decrease (Fig. 2), disynaptic chemical communication emerged. Our recordings from mice are consistent with this developmental pattern. No disynaptic inhibitory connections were observed in mouse VBN cells during the first postnatal week (0/64 connections in P4–5 WT; and 0/26 in P6–7 WT), while such connections were seen regularly by the end of the second week (8/44 in P12–14 of WT, 2/36 in P12–14 of KO). Although we observed disynaptic IPSPs more frequently in WT compared to KO cells, the results were not significantly different (P= 0.089, WT vs. KO, chi square).
In a subset of the disynaptically connected cell pairs, IPSPs could be evoked by single presynaptic spikes (13/22 connections), while the others (9/22) required trains of spikes (>30 Hz) to elicit inhibition. Spike trains at medium frequencies (30–60 Hz) generally evoked IPSPs that depressed in amplitude, but nevertheless resulted in substantial summation (Fig. 7B). Bursts of spikes at ≥150 Hz (produced by abrupt release from hyperpolarization) were nearly always more effective at evoking IPSPs than either single spikes or medium frequency trains. IPSPs were larger and/or more reliable when evoked by bursts (Fig. 7A–C). For example, we directly compared the ability of single spikes vs. bursts to induce IPSPs in 16 confirmed disynaptic connections (8 from rat, 7 from mouse). In these pairs, bursts evoked IPSPs on 96.0 ± 3.2% of trials tested, whereas single spikes elicited IPSPs on just 51.0 ± 11.1% of trials (P < 0.001, paired t test). The effectiveness of bursts was probably due to strong temporal summation at the high intra-burst spike frequencies (150–400 Hz), including summation of EPSPs in the TRN cells and IPSPs in VBN cells. Given that bursting activity only emerged during the second postnatal week (above), it would appear that the potency of disynaptic inhibition increases during this period. Thus, as was the case with the prevalence of disynaptic inhibition, this increased potency occurs approximately when electrical synaptic function declines.
Discussion
Our study shows that relay neurons in the VBN of the thalamus are electrically coupled during early postnatal development, and that this coupling is functionally down-regulated as animals mature and disynaptic inhibitory connections emerge. This is the first direct demonstration of electrical synapses using paired intracellular recording in thalamic relay neurons. The absence of measurable electrical synapses in mature relay cells suggests that gap junctions serve transient roles in the developing VBN circuit.
Strength of VBN electrical synapses and their possible roles
The average coupling coefficients and junctional conductances in immature VBN cells were relatively low compared to those of mature neocortical interneurons and thalamic reticular neurons (Gibson et al. 1999, 2005; Landisman et al. 2002; Parker et al. 2009). This is consistent with the relatively weak β-gal expression in the VBN of Cx36 mice (Fig. 5). However, we did observe some VBN pairs with reasonably strong coupling coefficients (>0.08) in P4–5 mice. This strength of coupling is probably great enough to produce some synchronization of action potentials in the coupled neurons (Long et al. 2004; Mancilla et al. 2007). In addition toelectrophysiological synchronization, gap junctions between young VBN neurons could provide a means of exchanging nutrients, second messengers, or other molecules involved in developmental signalling (Kandler & Katz, 1998).
Mechanism of down-regulation of electrical synapses
Our study showed that electrical coupling declined after birth and became undetectable after about P12 in rat and mouse VBN. A variety of mechanisms could account for the down-regulation of coupling. One important factor seems to be the large decrease in input resistance of VBN neurons during the initial two postnatal weeks (Table 1). Low input resistances in older VBN neurons would reduce coupling coefficients even if junctional conductance remained constant. Therefore, some of the weaker junctional conductances might have become undetectable (i.e. below noise levels) as the input resistances of VBN neurons decreased with age. However, the decrease of input resistance does not seem to explain all of the reduction in electrical coupling. Many cell pairs with junctional conductances higher than ∼0.06 nS were observed in P6–P9 rats (Fig. 2D) and P4–5 mice, and connections that strong would have generated detectable coupling coefficients (>0.01) even in neurons with input resistances as low as the P14 VBN neurons. However, the percentage of coupled pairs with those junctional conductances (> ∼0.06 nS) was much lower in P10–14 rats than in P6–9 rats (6 out of 30 pairs in P6–9, 2 out of 40 pairs in P10–14). It seems likely that declining input resistance as well as diminishing junctional conductances contributed to reductions of VBN coupling across development. This contrasts with the thalamic reticular nucleus, where junctional conductances increase and offset declining cellular input resistances, producing relatively stable coupling coefficients across development (Parker et al. 2009).
Our β-gal staining from Cx36 heterozygote mice showed that Cx36 reporter was expressed in VBN neurons at the youngest age tested (P4) and decreased to almost undetectable levels by P20, consistent with previous in situ hybridization studies of Cx36 mRNA (Belluardo et al. 2000). This implies that Cx36 itself and the gap junction channels it comprises are down-regulated in the VBN during postnatal development (Arumugam et al. 2005). Other potential mechanisms include reductions in gap junction channel conductance due to various forms of modulation (Pereda et al. 2004; Landisman & Connors, 2005).
Role of Cx36 and Cx45 in gap junction coupling in thalamus
Cx36 and Cx45 are reported to be expressed in thalamic relay nuclei during early postnatal development (Belluardo et al. 2000; Maxeiner et al. 2003; Sohl et al. 2005). Partly because of the low expression of the primary neuronal connexin, Cx36, in mature thalamic relay nuclei (Condorelli et al. 2000), the electrical synapses in these nuclei had received only limited study (Hughes et al. 2002, 2004). However, as shown by our data and previous in situ hybridization studies (Belluardo et al. 2000), Cx36 is expressed in VBN neurons in early postnatal development and even appears to be required for most VBN electrical coupling. Interestingly, the level of Cx36 β-gal reporter expression was not uniform within VBN. This suggests that Cx36-based electrical synapses might be regionally variable within VBN. Variable Cx36 expression could account for the wide variations in coupling strength we observed among relay cell pairs.
Cx45 is interesting because of its strong, persistent and relatively selective expression in neurons of thalamic relay nuclei (Maxeiner et al. 2003; Sohl et al. 2005). We did observe some distinct Cx36-independent electrical coupling between VBN neurons, and it is possible that this coupling depends on Cx45-dependent gap junctions. However, the persistence through adulthood of Cx45 expression in VBN does not correspond with our electrophysiological observations, since we did not find electrical coupling in VBN neurons older than P13. Nevertheless we cannot exclude the possibility that Cx45 mediates very low conductance junctions, undetectable by our methods, which could contribute to biochemical coupling between VBN neurons.
Thalamic relay cell development and electrical synapses
Electrical synapses exist in many types of neurons in the adult mammalian brain, where they appear to be important for synchronizing activity (Connors & Long, 2004). There is a long-standing hypothesis that early developmental expression of gap junctions is somehow important for the maturation of neural circuits (Westerfield & Frank, 1982; Kandler & Katz, 1995; Roerig & Feller, 2000), including the formation of neural circuits involving chemical synapses. For example, reducing electrical coupling between motor neurons accelerated neuromuscular synapse elimination in mice (Personius et al. 2007). Recent work on the olfactory bulb showed that Cx36-dependent electrical synapses between mitral cells were necessary for proper development of a glutamatergic synaptic circuit (Maher et al. 2009).
In the vertebrate central nervous system there issubstantial evidence for early gap junctional coupling among pyramidal neurons of the cerebral cortex, corticalsubplate neurons, and spinal cord motor neurons (Fulton et al. 1980; Westerfield & Frank, 1982; Connors et al. 1983; Lo Turco & Kriegstein, 1991; Peinado et al. 1993; Kandler & Katz, 1995; Chang & Balice-Gordon, 2000; Mentis et al. 2002; Montoro & Yuste, 2004; Dupont et al. 2006). In those regions there is extensive dye-coupling between neurons during early circuit formation periods, and neurons generally uncouple as animals mature. Our electrophysiological evidence for VBN electrical synapses, together with the patterns of Cx36 reporter expression we observed, suggest a similar course of transient coupling and expression early in the develop of thalamic relay cells.
It is possible that gap junctions in thalamic relay nuclei play a role in the formation or maturation of thalamic and thalamocortical circuits during development. Electrical coupling among thalamic relay cells, as well as among the inhibitory TRN cells (Parker et al. 2009), seems toprecede the maturation of functional chemical synapses that interconnect neurons of the relay nuclei and TRN later in development (Warren & Jones, 1997; Pangratz-Fuehrer et al. 2007; Evrard & Ropert, 2009). Most thalamic neurons are formed before birth in rat and mouse, and thalamocortical axon terminals have reached the cortical subplate before P0 (Lopez-Bendito & Molnar, 2003). However neurons, axons, and synapses continue to develop rapidly during the postnatal period. Electrical coupling between thalamic relay cells may support synchronized activity of ascending thalamic axons, and this may promote some level of refinement of intrathalamic and thalamocortical axonal arborizations and synapses (Khazipov & Luhmann, 2006; Blankenship & Feller, 2009; Hanganu et al. 2009). However, this remains an open question.
A recent report indicates a substantial level of neurogenesis in the VBN during the postnatal period (Mooney & Miller, 2007). This suggests another possibility, namely that the newly formed cells may be the subpopulation of VBN neurons that are electrically coupled. Our β-gal staining showed a heterogeneous Cx36 expression pattern within VBN, and it would be interesting to see if neurogenesis is occurring preferentially in thesubregions of VBN with high levels of Cx36. If so, perhaps synchronized electrical activity or biochemical coupling between newborn VBN neurons (via their gap junctions) could play a role in synapse formation with brain stem afferents or cortical targets.
Acknowledgments
We thank Saundra Patrick for outstanding assistance with histology and genotyping, and David Paul and Michael Deans for providing Cx36 KO mice. This work was supported by NS050434 and NS025983 from NIH.
Glossary
Abbreviations
- β-gal
β-galactosidase
- CC
coupling coefficient
- Cx36
connexin36
- Cx45
connexin45
- Gj
gap junction conductance
- TRN
thalamic reticular nucleus
- VBN
ventrobasal nucleus
Author contributions
All experiments were performed in the Department of Neuroscience at Brown University. This study was conceived and designed by S.-C.L., S.J.C., and B.W.C. S.-C.L. and S.J.C. performed the experiments and collected and analysed the data. S.-C.L., S.J.C., and B.W.C. interpreted the data and drafted the manuscript. All authors approved the final version of this paper.
References
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signalling. Nat Neurosci. 2005;8:1720–1726. doi: 10.1038/nn1588. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. doi: 10.1016/s0006-8993(00)02300-3. [DOI] [PubMed] [Google Scholar]
- Bennett MV. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966;137:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2009;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blethyn KL, Hughes SW, Crunelli V. Evidence for electrical synapses between neurons of the nucleus reticularis thalami in the adult brain in vitro. Thalamus Relat Syst. 2008;4:13–20. doi: 10.1017/S1472928807000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Dermietzel R. Structure and function of gap junctions in the developing brain. Cell Tissue Res. 2006;326:239–248. doi: 10.1007/s00441-006-0287-0. [DOI] [PubMed] [Google Scholar]
- Chang Q, Balice-Gordon RJ. Gap junctional communication among developing and injured motor neurons. Brain Res Brain Res Rev. 2000;32:242–249. doi: 10.1016/s0165-0173(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudo G. Expression of Cx36 in mammalian neurons. Brain Res Brain Res Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- Connors BW, Benardo LS, Prince DA. Coupling between neurons of the developing rat neocortex. J Neurosci. 1983;3:773–782. doi: 10.1523/JNEUROSCI.03-04-00773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Contreras D. The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–585. doi: 10.2174/187152706779025526. [DOI] [PubMed] [Google Scholar]
- Cox CL, Reichova I, Sherman SM. Functional synaptic contacts by intranuclear axon collaterals of thalamic relay neurons. J Neurosci. 2003;23:7642–7646. doi: 10.1523/JNEUROSCI.23-20-07642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Degen J, Meier C, Van Der Giessen RS, Sohl G, Petrasch-Parwez E, Urschel S, Dermietzel R, Schilling K, De Zeeuw CI, Willecke K. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol. 2004;473:511–525. doi: 10.1002/cne.20085. [DOI] [PubMed] [Google Scholar]
- Desilets-Roy B, Varga C, Lavallee P, Deschenes M. Substrate for cross-talk inhibition between thalamic barreloids. J Neurosci. 2002;22:RC218. doi: 10.1523/JNEUROSCI.22-09-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Evrard A, Ropert N. Early development of the thalamic inhibitory feedback loop in the primary somatosensory system of the newborn mice. J Neurosci. 2009;29:9930–9940. doi: 10.1523/JNEUROSCI.1671-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton BP, Miledi R, Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc R Soc Lond B Biol Sci. 1980;208:115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Ulrich D. Strong, reliable and precise synaptic connections between thalamic relay cells and neurones of the nucleus reticularis in juvenile rats. J Physiol. 2003;546:801–811. doi: 10.1113/jphysiol.2002.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Okabe A, Lessmann V, Luhmann HJ. Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex. 2009;19:89–105. doi: 10.1093/cercor/bhn061. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Blethyn KL, Cope DW, Crunelli V. Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience. 2002;110:395–401. doi: 10.1016/s0306-4522(01)00577-2. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Lorincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, Juhasz G, Crunelli V. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Neuronal coupling and uncoupling in the developing nervous system. Curr Opin Neurobiol. 1995;5:98–105. doi: 10.1016/0959-4388(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Kim U, McCormick DA. The functional influence of burst and tonic firing mode on synaptic interactions in the thalamus. J Neurosci. 1998;18:9500–9516. doi: 10.1523/JNEUROSCI.18-22-09500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Mapping by laser photostimulation of connections between the thalamic reticular and ventral posterior lateral nuclei in the rat. J Neurophysiol. 2005;94:2472–2483. doi: 10.1152/jn.00206.2005. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Fine structural localization of connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J Comp Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Lo FS, Sherman SM. Feedback inhibition in the cat's lateral geniculate nucleus. Exp Brain Res. 1994;100:365–368. doi: 10.1007/BF00227207. [DOI] [PubMed] [Google Scholar]
- Lo Turco JJ, Kriegstein AR. Clusters of coupled neuroblasts in embryonic neocortex. Science. 1991;252:563–566. doi: 10.1126/science.1850552. [DOI] [PubMed] [Google Scholar]
- Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neurosci. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there. Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Maher BJ, McGinley MJ, Westbrook GL. Experience-dependent maturation of the glomerular microcircuit. Proc Natl Acad Sci U S A. 2009;106:16865–16870. doi: 10.1073/pnas.0808946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci. 2007;27:2058–2073. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K. Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience. 2003;119:689–700. doi: 10.1016/s0306-4522(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Diaz E, Moran LB, Navarrete R. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol. 2002;544:757–764. doi: 10.1113/jphysiol.2002.028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Montero VM. A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Exp Brain Res. 1991;86:257–270. doi: 10.1007/BF00228950. [DOI] [PubMed] [Google Scholar]
- Montoro RJ, Yuste R. Gap junctions in developing neocortex: a review. Brain Res Brain Res Rev. 2004;47:216–226. doi: 10.1016/j.brainresrev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Postnatal generation of neurons in the ventrobasal nucleus of the rat thalamus. J Neurosci. 2007;27:5023–5032. doi: 10.1523/JNEUROSCI.1194-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Giant spontaneous depolarizing potentials in the developing thalamic reticular nucleus. J Neurophysiol. 2007;97:2364–2372. doi: 10.1152/jn.00646.2006. [DOI] [PubMed] [Google Scholar]
- Parker PR, Cruikshank SJ, Connors BW. Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology. J Neurosci. 2009;29:9761–9770. doi: 10.1523/JNEUROSCI.4568-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Pereda AE, Rash JE, Nagy JI, Bennett MV. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res Brain Res Rev. 2004;47:227–244. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Perez Velazquez JL, Carlen PL. Development of firing patterns and electrical properties in neurons of the rat ventrobasal thalamus. Brain Res Dev Brain Res. 1996;91:164–170. doi: 10.1016/0165-3806(95)00171-9. [DOI] [PubMed] [Google Scholar]
- Personius KE, Chang Q, Mentis GZ, O’Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci U S A. 2007;104:11808–11813. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Crunelli V. A role for low-frequency, rhythmic synaptic potentials in the synchronization of cat thalamocortical cells. J Physiol. 1992;457:257–276. doi: 10.1113/jphysiol.1992.sp019377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus. Kidlington, Oxford, UK: Elsevier; 1997. Chapter 3: thalamic cell types and intrinsic synaptic organization; pp. 175–267. [Google Scholar]
- Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Warren RA, Jones EG. Maturation of neuronal form and function in a mouse thalamo-cortical circuit. J Neurosci. 1997;17:277–295. doi: 10.1523/JNEUROSCI.17-01-00277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Westerfield M, Frank E. Specificity of electrical coupling among neurons innervating forelimb muscles of the adult bullfrog. J Neurophysiol. 1982;48:904–913. doi: 10.1152/jn.1982.48.4.904. [DOI] [PubMed] [Google Scholar]


