Abstract
Myocardial hypertrophy and dysfunction occur in response to excessive catecholaminergic drive. Adverse cardiac remodelling is associated with activation of proinflammatory cytokines in the myocardium. To test the hypothesis that exercise training can prevent myocardial dysfunction and production of proinflammatory cytokines induced by β-adrenergic hyperactivity, male Wistar rats were assigned to one of the following four groups: sedentary non-treated (Con); sedentary isoprenaline treated (Iso); exercised non-treated (Ex); and exercised plus isoprenaline (Iso+Ex). Echocardiography, haemodynamic measurements and isolated papillary muscle were used for functional evaluations. Real-time RT-PCR and Western blot were used to quantify tumour necrosis factor α, interleukin-6, interleukin-10 and transforming growth factor β1 (TGF-β1) in the tissue. NF-κB expression in the nucleus was evaluated by immunohistochemical staining. The Iso rats showed a concentric hypertrophy of the left ventricle (LV). These animals exhibited marked increases in LV end-diastolic pressure and impaired myocardial performance in vitro, with a reduction in the developed tension and maximal rate of tension increase and decrease, as well as worsened recruitment of the Frank–Starling mechanism. Both gene and protein levels of tumour necrosis factor α and interleukin-6, as well as TGF-β1 mRNA, were increased. In addition, the NF-κB expression in the Iso group was significantly raised. In the Iso+Ex group, the exercise training had the following effects: (1) it prevented LV hypertrophy; (ii) it improved myocardial contractility; (3) it avoided the increase of proinflammatory cytokines and improved interleukin-10 levels; and (4) it attenuated the increase of TGF-β1 mRNA. Thus, exercise training in a model of β-adrenergic hyperactivity can avoid the adverse remodelling of the LV and inhibit inflammatory cytokines. Moreover, the cardioprotection is related to beneficial effects on myocardial performance.
Introduction
It is established that left ventricular hypertrophy is an independent and powerful predictor of heart failure and mortality (Levy et al. 1990; Lips et al. 2003; Reichek et al. 2009). There is a growing body of evidence showing that heart failure is often associated with an increase in intracardiac sympathetic nerve activity, which intensifies myocardial remodelling (Brum et al. 2002; Floras, 2009; Oliveira et al. 2009).
Considering the inconvenience of sustained β-adrenergic cardiac stimulation, much effort is currently being undertaken to find novel treatment modalities to prevent or even reverse the remodelling phenotype induced by β-adrenergic overload. Several potential pharmacological schemes for preventing myocardial remodelling of β-adrenergic-induced injury have been previously evaluated, as follows: β-adrenergic receptor antagonists (Brouri et al. 2004); angiotensin-converting enzyme inhibitors (Gallego et al. 2001); calcium channel blockers (Okuda et al. 2005); AT1 angiotensin II receptor blockers (Okuda et al. 2005); and antioxidants (Buttros et al. 2009). Additionally, information has been published regarding the cardioprotective effects of exercise training prior to β-adrenergic hyperactivity (Darrah & Engen, 1982; Brodowicz & Lamb, 1991; Frederico et al. 2009). These studies have reported that exercise training only attenuates myocardial remodelling caused by very large doses of isoprenaline.
We have recently confirmed that, in rats, repeated injections of isoprenaline stimulate rapid myocardial hypertrophy (Serra et al. 2008). In addition to myocardial hypertrophy, these rats develop cardiac necrosis, apoptosis, fibrosis, myocardial oedema and reduced capillary density. Furthermore, we demonstrated that exercise training completely prevents myocardial inflammation (Serra et al. 2008). Nevertheless, we did not analyse the myocardial function and possible mechanisms by which the exercise training induced cardioprotection in these animals. Therefore, the purpose of this study was to examine the role of exercise training in the myocardial dysfunction and local expression of inflammatory cytokines in rats submitted to β-adrenergic hyperactivity.
Methods
Animals and isoprenaline administration
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). The experiments were performed in agreement with the policy and UK regulations described in The Journal of Physiology (Drummond, 2009). The protocol was approved by the Institutional Research Ethics Committee of the Federal University of São Paulo, Brazil. Forty-one male Wistar rats, weighing 160–190 g and aged 6–7 weeks, were randomly assigned to one of the following four groups: (1) Con (n= 12), non-trained rats that received only vehicle (olive oil, 1 ml s.c.); (2) Iso (n= 13), non-trained rats that received isoprenaline injections (0.3 mg kg−1 day−1s.c.) diluted in 1 ml of olive oil; (3) Ex (n= 8), exercise-trained rats that received only vehicle; and (4) Iso+Ex (n= 8), exercise-trained rats that received isoprenaline injections (0.3 mg kg−1 day−1s.c.) diluted in vehicle.
Exercise protocol
Rats were subjected to a programme of running on a motor-driven treadmill (CL – 4002; Caloi, São Paulo, Brazil) for 13 weeks, as previously reported (Serra et al. 2008). Rats ran six times a week, and each session lasted up to 60 min. For each session, the treadmill speed was 18 m min−1 for 30 min and 22 m min−1 for the remaining 30 min. Isoprenaline or olive oil was administered on the last day of week 12 and during the 7 days of week 13, thus providing 8 days of treatment. Twenty-four hours after the last exercise session, the rats were anaesthetized (urethane, 1.2 g kg−1i.p.) and subjected to transthoracic echocardiography and catheterization of the left ventricle (LV).
Echocardiography
As previously described (Portes et al. 2009), transthoracic echocardiography was performed using an HP Sonos-5500 (Hewlett Packard, Andover, MA, USA) echocardiograph with a 12 MHz linear transducer. Rats were imaged in the left lateral decubitus position with three electrodes placed on paws for the electrocardiogram. Two-dimensional parasternal long- and short-axis views were recorded, as were two-dimensional targeted M-mode traces throughout the anterior and posterior left ventricular walls. The diastolic LV posterior wall thickness (LVPWd), systolic LV posterior wall thickness (LVPWs), LV end-diastolic (LVEDD) and LV end-systolic (LVESD) diameters, and LV fractional shortening (FS) were determined. Since the high heart rates caused fusion of the A and E waves, diastolic function was not evaluated by Doppler ultrasound.
Left ventricular haemodynamics
Immediately after echocardiography, the rats were intubated, ventilated (rodent ventilator, model 683, Harvard Apparatus, Holliston, MA, USA) and a 2-F gauge (length 140 cm) Millar catheter-tip micromanometer (model SPR-320, Millar Instruments, Houston, TX, USA) was inserted through the right carotid artery into the LV cavity. Measurements of LV parameters, including LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), and maximal positive (+dP/dt) and negative (−dP/dt) time derivatives of the developed pressure were studied using AcqKnowledge 3.5.7 software (Biopac Systems Inc., Santa Barbara, CA, USA).
Myocardial mechanics
After haemodynamic study, the animals received a urethane overdose (4.8 g kg−1i.p.), the hearts were quickly removed and the posterior papillary muscles were placed in a tissue bath containing modified Krebs–Henseleit solution (mm: 130 NaCl, 5.0 KCl, 1.2 MgCl2, 1.5 CaCl2, 11 glucose, 20 U insulin and 20 Hepes) bubbled with 100% O2 and maintained at 29°C, pH 7.4. Myocardial mechanics were evaluated as previously described (Portes et al. 2009). Preparations were stimulated 12 times min−1 with 5 ms square-wave pulses through parallel platinum electrodes at voltages that were approximately 10% greater than the minimal stimulus required to produce a maximal mechanical response.
After a 60 min equilibration period, during which preparations were permitted to contract isotonically in light loading conditions (0.4 g), papillary muscles were loaded to contract isometrically for 15 min and stretched to the apices of their length–tension curves (Lmax). The parameters were recorded through the use of AcqKnowledge 3.5.7 software (Biopac Systems Inc.) for later determination of peak developed tension (DT), maximal rate of tension increase (+dT/dt) and decrease (−dT/dt), and resting tension (RT). The developed and resting length–tension curves were derived from data obtained at lengths corresponding to 92, 94, 96, 98 and 100% of the Lmax. At the end of the experiment, the muscle length at Lmax was measured, and the muscular portion between the two clips was blotted dry and weighed. The cross-sectional area (CSA) was estimated from the muscle weight and length by assuming a cylindrical shape and a specific gravity of 1.0. All force-related data were normalized for the CSA.
Quantitative real-time RT-PCR analysis
Total RNA in LV samples was extracted with TRIzol (Gibco BRL, Gaithersburg, MD, USA). The RNA was subjected to DNase digestion (Invitrogen, Carlsbad, CA, USA). Reverse transcripition (RT) reaction was performed using random primers with 200 units of Moloney murine leukaemia virus reverse transcriptase (Invitrogen). Real-time PCR was done with a Mastercycler ep Realplex (Eppendorf, Hamburg, Germany) using a SYBRGreen core reaction kit (Applied Biosystems, Foster City, CA, USA). The primers used were as follows: rat tumour necrosis factor-α (TNF-α) 195–305 (GenBank™ accession number X66539), forward primer 5′-AAATGGGCTCCCTCTATCAGTTC-3′ and reverse primer 5′-TCTGCTTGGTGGTTTGCTACGAC-3′; rat interleukin-6 (IL-6; GenBank accession number E02522), forward primer 5′-TCCTACCCCAACTTCCAATGCTC-3′ and reverse primer 5′-TTGGATGGTCTTGGTCCTTAGCC-3′; rat interleukin-10 (IL-10; GenBank accession number NM012854), forward primer 5′-AAAGCAAGGCAGTGGAGCAG-3′ and reverse primer 5′-TCAAACTCATTCATGGCCTTGT-3′; rat transforming growth factor β1 (TGF-β1; GenBank accession number 021578), forward primer 5′-TGGCGTTACCTTGGTAACC-3′ and reverse primer 5′-GGTGTTGAG CCCTTTCCAG-3′; and rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GenBank accession number NM017008), forward primer 5′-TGCACCACCAACTGCTTAGC-3′ and reverse primer 5′-GCCCCACGGCCATCA-3′. One microlitre of RT reaction was used for real-time PCR. The ΔΔCt values were normalized with the values obtained for amplification of GAPDH.
Western blot analysis
The frozen left ventricle was homogenized in cell lysis buffer (100 mm Tris, 50 mm NaCl, 10 mm EDTA and 1% Triton X-100) and a proteinase inhibitor cocktail (Sigma Chemical Corp., St Louis, MO, USA). Samples containing 30 μg of the homogenate were subjected to SDS-PAGE in 10% polyacrylamide gels. Separated proteins were transferred onto Hydrophobic Polyvinylidene (PVDF) membranes (Hybond-P, Amersham Biosciences; Piscataway, NJ, USA), and the transfer efficiency was monitored with 0.5% Ponceau S staining of the blot membrane. The membrane was soaked in a blocking buffer (5% non-fat dry milk, 10 mm Tris–HCl, pH 7.6, 150 mm NaCl and 0.1% Tween 20) for 2 h at room temperature and then incubated overnight at 4°C with rabbit antirat TNF-α (1:500 dilution; IBL-America, Minneapolis, MN, USA), goat antirat IL-6 (1:500 dilution; Abcam, Cambridge, MA, USA) and goat antirat IL-10 (1:200 dilution; R&D Systems, Minneapolis, MN, USA). After overnight incubation, membranes were washed three times and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution; Zymed, San Franscisco, CA, USA). Detection was performed with enhanced chemiluminescence reagents (Amersham Biosciences). Although identical amounts of protein were loaded into each well, the GAPDH expression levels were used as a loading control and to normalize the results.
Immunohistochemistry assay
Paraffin sections (5 μm thick) were deparaffinized and fixed in 10% buffered formaldehyde, and endogenous peroxidase activity was inactivated with 3% H2O2 for 15 min. Rabbit antirat antibodies against TNF-α (1:800 dilution) and NF-κB (1:2000 dilution) were used as primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The primary antibodies or normal blocking serum (Dako Corporation, Inc., Carpinteria, CA, USA) was added and incubated overnight at 4°C. Biotin-conjugated rabbit antirat IgG (Dako, Ely, UK), diluted to 1:400, was used as the secondary antibody and incubated for 30 min at 37°C. An avidin–biotin–peroxidase complex (Vector Laboratories, Burlingame, CA, USA) was consecutively added and incubated for 20 min, and the reaction was developed with diaminobenzidine (Sigma Chemical Corp.). Omission of primary antibody served as a negative control. The slides were counterstained with antimouse α-smooth muscle actin (Sigma Chemical Corp.), diluted to 1:600. Finally, sections were covered with a glass coverslip (Merck Ltd, Lutterworth, Germany) and observed under an optical microscope. Immunohistochemistry staining intensity was evaluated in a blinded manner and scored on a percentage positive area for TNF-α (identified by brown staining), and the cells per area demonstrating nuclear localization for NF-κB were automatically detected in 10 different fields at ×40 magnification and averaged.
Data analysis
All grouped data were expressed as the means ±s.e.m., and one-way ANOVA was used for the comparisons using GraphPad Prism software (version 4.0, GraphPad Software, Inc, La Jolla, CA, USA). Subsequent analyses were performed using the Newman–Keuls method. The developed length–tension relationship was evaluated by linear regression analysis. The values of developed tension at each resting length were compared by one-way ANOVA. The resting length–tension curves were fitted to a monoexponential relationship, as follows: y=β0 eβ1x+ɛi, where β0,β1 and ɛi are constants of the curve. These non-linear relationships were compared between groups by the values of the constant stiffness, β1.
Results
Exercise training inhibits isoprenaline-induced myocardial hypertrophy
The body weight was similar among the groups (Table 1). The administration of isoprenaline over an 8 day period resulted in a significant increase in cardiac and LV mass between Iso and the other groups. Exercise training did not affect the myocardial mass and, more importantly, completely blunted the hypertrophic response to β-adrenergic stimulation (Fig. 1A and B). The exercise training and the isoprenaline, alone or incombination, had no impact on the right ventricle mass (Fig. 1C).
Table 1.
Influence of isoprenaline and exercise training on LV morphology and function
| Experimental group |
|||||
|---|---|---|---|---|---|
| Variable | Con (n= 10) | Iso (n= 13) | Ex (n= 8) | Iso+Ex (n= 8) | P value |
| Body weight (g) | 367 ± 13 | 359 ± 7 | 347 ± 21 | 349 ± 16 | 0.70 |
| CSA (mm2) | 0.77 ± 0.04 | 1.30 ± 0.02* | 0.67 ± 0.02 | 0.65 ± 0.02 | <0.001 |
| Echocardiography | |||||
| LVPWd (mm) | 1.44 ± 0.10 | 1.92 ± 0.13* | 1.46 ± 0.06 | 1.54 ± 0.09 | <0.05 |
| LVPWs (mm) | 2.50 ± 0.12 | 3.34 ± 0.15* | 2.46 ± 0.11 | 2.56 ± 0.07 | <0.001 |
| LVEDD (mm) | 7.66 ± 0.21 | 7.11 ± 0.17 | 7.66 ± 0.34 | 7.97 ± 0.18 | 0.06 |
| LVESD (mm) | 4.52 ± 0.16 | 3.12 ± 0.20* | 4.63 ± 0.32 | 5.03 ± 0.15 | <0.001 |
| FS (%) | 41 ± 1 | 56 ± 2* | 40 ± 2 | 37 ± 1 | <0.001 |
| Haemodynamics | |||||
| HR (beats min−1) | 401 ± 9 | 483 ± 20* | 415 ± 10 | 386 ± 15 | <0.01 |
| LVSP (mmHg) | 127 ± 2 | 116 ± 2* | 124 ± 3 | 131 ± 3 | <0.05 |
| LVEDP (mmHg) | 7.9 ± 0.5 | 15.2 ± 0.9* | 6.8 ± 0.8 | 7.3 ± 0.9 | <0.001 |
| +dP/dt (mmHg s−1) | 8023 ± 175 | 10090 ± 354* | 8122 ± 163 | 8313 ± 129 | <0.001 |
| −dP/dt (mmHg s−1) | 6342 ± 296 | 6946 ± 331 | 6569 ± 215 | 6516 ± 156 | 0.43 |
| MAP (mmHg) | 101 ± 3 | 84 ± 3* | 103 ± 3 | 106 ± 3 | <0.01 |
Abbreviations: CSA, cross-section area of papillary muscles; LVPWd, diastolic LV posterior wall thickness; LVPWs, systolic LV posterior wall thickness; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; FS, LV fractional shortening; HR, heart rate; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; +dP/dt, maximal positive time derivative of developed pressure; −dP/dt, maximal negative derivative of developed pressure; and MAP, mean arterial pressure.
Significant differences by ANOVA vs. other groups.
Figure 1. Effects of exercise training on the myocardial hypertrophy induced by isoprenaline.
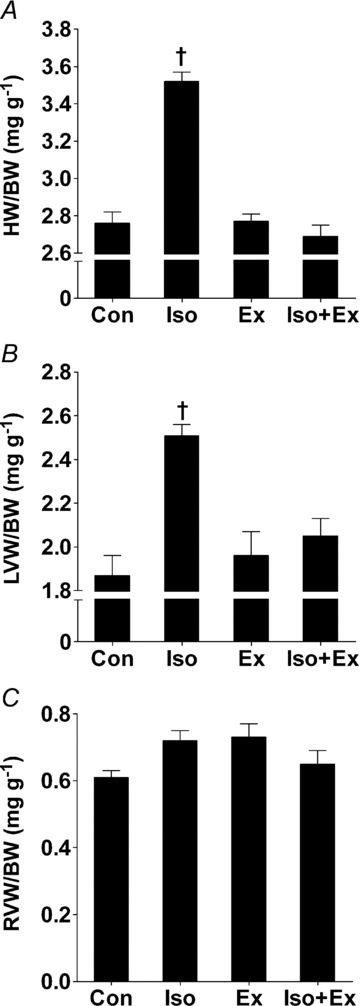
A, heart weight/body weight (HW/BW) ratio for non-trained (Con, n= 12; Iso, n= 13) and exercise-trained rats (Ex, n= 8; Iso+Ex, n= 8) determined after 13 weeks of follow-up. B, left ventricular weight/body weight (LVW/BW) ratio for all groups. C, right ventricular weight/body weight (RVW/BW) ratio. Significant differences in ANOVA, †P < 0.001 vs. other groups.
Exercise training effects on LV structure and function after β-adrenergic overload
On transthoracic echocardiography (Table 1), isoprenaline induced changes similar to concentrichypertrophy, namely, significant thickening of LVPWd and significant reduction of LVEDD. Moreover, the β-adrenergic overload resulted in a remarkable increase in FS (+36%) in treated, non-trained rats compared with the other groups. On LV haemodynamics measurements (Table 1), the heart rate and +dP/dt were significantly higher in the Iso group compared with the Con group. The Iso group also showed a reduction in LVSP and mean arterial pressure. In addition, there was marked left ventricular diastolic dysfunction, as assessed by the exacerbated increase in LVEDP. Exercise training did not affect LV structure and function but, interestingly,prevented the alterations provoked by isoprenaline.
Exercise training enhanced the myocardial mechanics even in isoprenaline-treated animals
There were striking differences in papillary muscle morphology between the non-trained group treated with isoprenaline and the other groups. Indeed, CSA was significantly greater (Table 1) in the Iso group compared with the Con group. Exercise training by itself did not alter the papillary muscle CSA, and notably it was effective in maintaining normal values in isoprenline-injected rats.
There were remarkable differences in myocardial performance between the four groups. We confirmed findings of previous studies (Vassallo et al. 1988; Stein et al. 1996) in which the sustained administration ofisoprenaline resulted in muscle that developed less force than their respective controls; the effect is depicted as a decrease in DT, as well as a smaller +dT/dt (Fig. 2A and B). Furthermore, −dT/dt was significantly diminished in β-adrenergically stimulated non-trained rats compared with non-trained rats that received only vehicle (Fig. 2C). The myocardial contractile performance was significantly enhanced after the exercise training programme. Indeed, papillary muscles from the Ex group exhibited a significant increase in DT and +dT/dt (Fig. 2A and B). Moreover, exercise training did more than simply to preserve the myocardial performance after β-adrenergic hyperactivity; the contractile properties were markedly improved in the Iso+Ex group as assessed by DT and +dT/dt. In addition, −dT/dt of exercise-trained rats was maintained, even when rats were treated with isoprenaline. The RT was not altered by isoprenaline or exercise training (Fig. 2D).
Figure 2. Exercise training increases the myocardial performance, even after β-adrenergic hyperactivity.
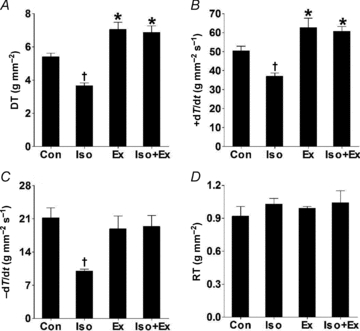
Data were obtained at muscle lengths corresponding to 100% of Lmax from non-trained (Con, n= 8; Iso, n= 13) and exercise-trained rats (Ex, n= 8; Iso+Ex, n= 6), as described in the Methods. A, peak developed tension (DT). B, maximal positive time derivative of developed tension (+dT/dt). C, maximal negative time derivative of developed tension (−dT/dt). D, resting tension (RT). Significant differences in ANOVA, *P < 0.05 vs. Con group, †P < 0.05 vs. other groups.
Additionally, as shown in Fig. 3A, the DT was plotted as a function of muscle length. There was a linear relationship between DT and muscle length for all experiments, as evidenced by the coefficient of determination (r2), which was typically >0.97. The active length–tension relationship from the Iso group was shifted downwards, with a reduced slope of linear regression in relationship to the other groups. Notably, the slope of the linear projection of tension was preserved in exercise-trained rats after chronic β-adrenergic stimulation. In this way, it is possible to define that for the same variation of muscle length, the recruitment of the Frank–Starling mechanism ispreserved in trained myocardium after β-adrenergic hyperactivity. Furthermore, the length–tension curves from the exercise-trained groups (Ex and Iso+Ex) were shifted upwards from that of the Con and Iso groups, indicating that, even though the Frank–Starling mechanism was unchanged, for the same stretch level, greater muscle force is generated in exercised rats (Fig. 3A). The resting length–tension curves were similar between the groups as evaluated by mean values of β1, reflecting thatisoprenaline as well as exercise training did not affectmyocardial stiffness (Fig. 3B).
Figure 3. Left ventricular papillary muscle developed and resting length–tension curves obtained from Con (○), Iso (▴), Ex (•) and Iso+Ex groups (▵) as described in the Methods.
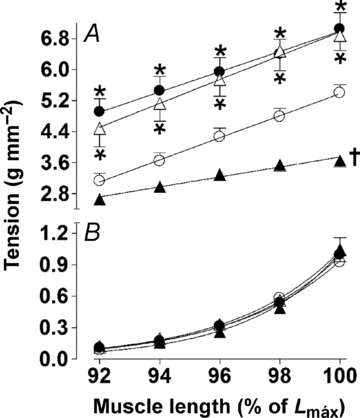
A, straight lines were fitted to the developed length–tension relationships using linear regression analysis. The resulting mean slopes, corresponding to developed tension, were compared between groups. ANOVA and post hoc Newman–Keuls test were used for multiple comparisons. *P < 0.01 when the developed tension was compared with Con and Iso groups for each stretching, †P < 0.001 when slope was compared with other groups. B, the resting length–tension curves for the four groups were fitted to monoexponential non-linear relationships. The resulting means β1 corresponded to resting tension were compared between groups.
Effects of exercise training on gene expression of myocardial cytokines induced by isoprenaline
It is well known that adrenergic nervous system activation acts as a powerful stimulus to synthesis of myocardial inflammatory cytokines (Murray et al. 2000). Thus, β-adrenergic hyperactivity significantly increased TNF-α and IL-6 mRNA in the Iso group compared with the Con group (Fig. 4A). More importantly, exercise training did not affect local cytokines and inhibited the increases in TNF-α and IL-6 induced by isoprenaline. Moreover, the exercise training significantly increased the IL-10 mRNA expression in isoprenaline-affected myocardium. Remarkably, in the trained and isoprenaline-treated rats, the ratios of TNF-α/IL-10 and IL-6/IL-10 were maintained (Fig. 4B).
Figure 4. Gene expression by real-time RT-PCR in myocardium as described in the Methods.
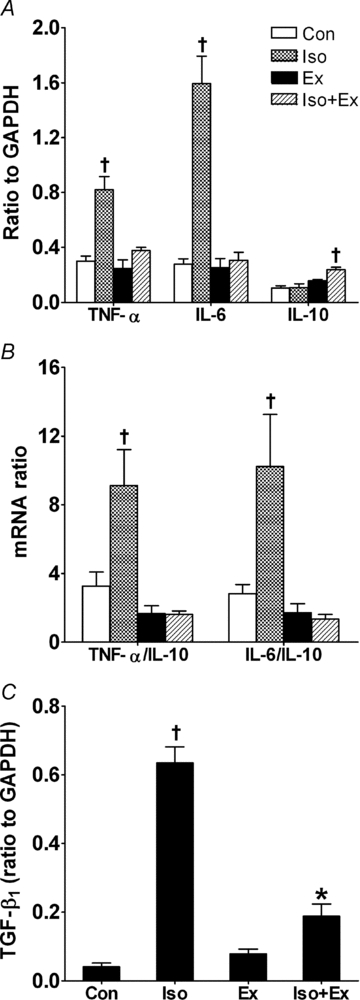
A, gene expression of proinflammatory (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10). B, ratio of TNF-α/IL-10 and IL-6/IL-10. C, gene expression of TGF-β1. All values were normalized for levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data are from five rats in each group. ANOVA and post hoc Newman–Keuls test were used for multiple comparisons. *P < 0.05 vs. Con and Ex groups. †P < 0.05 vs. other groups.
Consistent with other observations in that chronic β-adrenergic activation is thought to promote hypertrophy via local stimulation of TGF-β1 (Osadchii, 2007), isoprenaline treatment increased the TGF-β1 mRNA level by ∼15.6-fold in the Iso group compared with the Con group (Fig. 4C). As shown by quantitative RT-PCR analysis, the exercise training did not result in significant modification of expression of TGF-β1 mRNA, yet the physical conditioning resulted in attenuation of the increase in TGF-β1 mRNA in rats treated withisoprenaline.
Effects of exercise training on protein expression of myocardial cytokines induced by isoprenaline
The Western blot results are shown in Fig. 5A. Consistent with their mRNA levels, TNF-α and IL-6 proteins were significantly increased in the Iso group, and exercise training inhibited the TNF-α and IL-6 increase induced by isoprenaline. The level of IL-10 protein was significantly increased only in the Ex group. Furthermore, as in the analyses of gene expression, the TNF-α/IL-10 and IL-6/IL-10 ratios in the Ex and Iso+Ex groups were similar to that of the Con group, but were significantly higher in the Iso group (Fig. 5B).
Figure 5. The protein expression by Western blot in myocardium as described in the Methods.
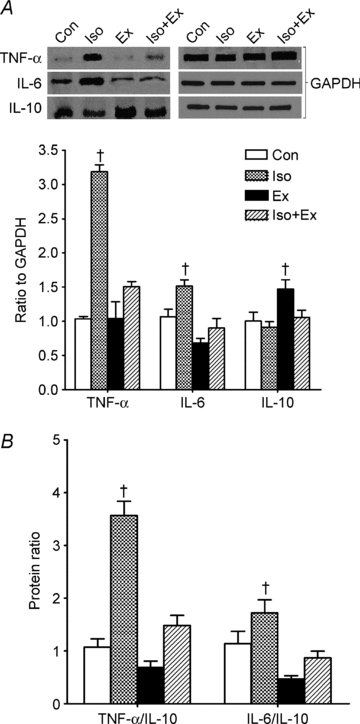
A, protein expression of TNF-α, IL-6 and IL-10. The upper panel is a representative Western blot. B, ratio of TNF-α/IL-10 and IL-6/IL-10. All values were normalized for levels of GAPDH. Specifics blots of the GAPDH for each cytokine protein are shown in A. Data are from five rats in each group. ANOVA and post hoc Newman–Keuls test were used for multiple comparisons. †P < 0.05 vs. other groups.
Localization of proinflammatory (TNF-α) cytokine in myocardium
The imunostaining revealed intense, diffuse immunoreactivity for TNF-α in myocardium of the Iso group (Fig. 6B). Positive immunostaining was noted in areas of inflammatory cell infiltration and in the myocardial tissue distinct from these areas. Importantly, positive immunoreactivity for TNF-α was rarely detected in Con (Fig. 6A) and Ex groups (Fig. 6C) or in the Iso+Ex group (Fig. 6D). The cardioprotector effect was confirmed by the TNF-α immunoreactivity scores (Fig. 6E).
Figure 6. Representative photomicrographs showing localization of TNF-α and NF-κB in myocardium.
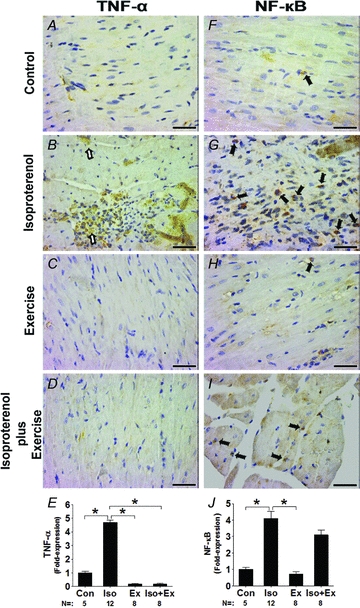
Brown stain indicates positive immunoreactivity. Intense immunoreactivity was detected in sedentary isoprenaline-treated animals (B). It is noteworthy that TNF-α was readily positive and localized to areas of inflammatory cell infiltration and uninjured myocardial tissue (open arrows). However, in exercise-trained animals there was inhibition of the intense proinflammatory staining after isoprenaline overload. Positive immunoreactivity to TNF-α was rarely detected in myocardium of Con (A), Ex (C) and Iso+Ex groups (D). In the right-hand panels, the filled arrows indicate positive nuclear immunoreactivity for NF-κB in Con (F), Iso (G), Ex (H) and Iso+Ex groups (I). E and J, statistical analysis for TNF-α and NF-κB, respectively. Numbers of rats used in each group and fold-expression relative to the Con group are shown. *P < 0.05 in ANOVA. Magnification ×40 (Scale bar, 250 μm).
Effects of isoprenaline and exercise training on nuclear NF-κB expression
Since activation of NF-κB may participate in the development of myocardial hypertrophy induced by isoprenaline (Chandrasekar et al. 2004; Freund et al. 2005), nuclear NF-κB expression was evaluated in the rats treated with isoprenaline. As shown in Fig. 6G, β-adrenergic hyperactivity significantly increased nuclear NF-κB expression by ∼4.1-fold in the Iso group compared with the Con group. Exercise training did not increase NF-κB expression (Fig. 6H). However, exercise training preceding isoprenaline administration partly blunted the expression of NF-κB increase (Fig. 6I), although not significantly.
Discussion
Recently, we demonstrated the cardioprotective effects of exercise training against LV lesions induced by prolonged stimulation with isoprenaline (Serra et al. 2008), yet the myocardial function and possible mechanisms for this occurrence were not established. The present study provides novel information in this regard. We found that the myocardial dysfunction induced by β-adrenergic hyperactivity was prevented by exercise training. This maintenance of myocardial function was accompanied by inhibition of myocardial gene and protein overexpression of TNF-α and IL-6 as well as an improvement in the anti-inflammatory IL-10 expression. Furthermore, the exercise training attenuated the increase of TGF-β1 mRNA and nuclear NF-κB expression, a well-known growth factor and a transcriptional regulator involved in β-adrenergic signalling, respectively (Takemoto et al. 1999; Osadchii, 2007). These molecular changes were paralleled by inhibition of myocardial hypertrophy.
As previously stated, sedentary rats treated withisoprenaline show a substantial increase in myocardial mass, usually characterized by a combination of muscle fibre enlargement, proliferation of non-myocyte cells and myocardial oedema (Serra et al. 2008). Moreover, on echocardiography, the aforementioned rats show chamber dimensions indicating that the isoprenaline-induced LV hypertrophy is of the concentric type, which corroborates previous findings in our laboratory (Murad & Tucci, 2000; Serra et al. 2008). We identified possible signalling triggers involved in the myocardial remodelling induced by β-adrenergic hyperactivity. Our results confirm that isoprenaline is a powerful stimulant to local myocardial expression of TNF-α and IL-6 (Murray et al. 2000). Furthermore, TNF-α immunoreactivity was not confined to regions of inflammatory cell infiltration, but was present even in preserved tissues, suggesting direct expression of cytokines by non-inflammatory cells (Murray et al. 2000). Considering that the interplay between β-adrenergic hyperactivity and overexpression of TNF-α and IL-6 may have implications with respect to adverse cardiac remodelling, one might expect that both cytokines could be involved in hypertrophic responses of cardiac myocytes in isoprenaline-treated rats. Indeed, proinflammatory cytokines have been shown to cause myocyte apoptosis, extracellular matrix alterations, contractile depression and concentric hypertrophy that progresses to dilated cardiomyopathy over time (Sivasubramanian et al. 2001; Janczewski et al. 2003; van Empel & De Windt, 2004; Hori & Nishida, 2009).
Several lines of evidence suggest that the cardiac hypertrophy is associated with an increased expression of TGF-β1 mRNA. Rosenkranz et al. (2002) reported that mice overexpressing TGF-β1 showed significant cardiac hypertrophy, accompanied by an increased expression of hypertrophy-associated genes. In fact, a positive correlation has been found between elevated myocardial TGF-β1 mRNA expression and increased heart-weight-to-body-weight ratio (Boluyt et al. 1995). In accordance with previous studies (Boluyt et al. 1995; Copaja Soto et al. 2008; Korkmaz et al. 2009), we also showed that myocardial levels of TGF-β1 mRNA were raised after isoprenaline infusion, which highlights the role of TGF-β1 in pathological hypertrophy associated with β-adrenergic hyperactivity.
The β-adrenergic hyperactivity increased the nuclear myocardial expression of NF-κB, a ubiquitous transcription factor, which is known for its role in cell growth, inflammation, apoptosis and embryonic development (Freund et al. 2005). The role of NF-κB in inducing a hypertrophic phenotype during β-adrenergic stimulation has been demonstrated by Freund et al. (2005), who showed that mice with cardiomyocyte-restricted expression of the NF-κB super-repressor IkappaBalphaDeltaN displayed an attenuated hypertrophic response to 7 days of isoprenaline treatment. Similarly, Chandrasekar et al. (2004) and Takemoto et al. (1999) showed that isoprenaline resulted in a significant increase in NF-κB-dependent DNA binding activity in the myocardium of mice and rats, respectively.
Of clinical relevance, we showed that exercise training inhibited LV remodelling in response to a pathological stimulus. The responsible mechanism(s) for this exercise-induced cardioprotection against β-adrenergic agonists has yet to be fully defined. To our best knowledge, only the study of Frederico et al. (2009) provides evidence for mechanisms contributing to cardioprotection, suggesting that exercise training provides cardioprotection via a reduction in reactive oxygen species in rats submitted to high doses of isoprenaline to induce myocardial infarction.
In the present study, we investigated the mechanisms responsible for exercise-induced cardioprotection. The myocardial TNF-α and IL-6 increase evoked by isoprenaline was completely abolished by exercise training, and this might have contributed to the cardiac antiremodelling effect of exercise training. The exercise training maintained TNF-α/IL-10 and IL-6/IL-10 ratios at normal levels. Consequently, exercise training may affect the LV remodelling after β-adrenergic overload by modulating the balance between proinflammatory and anti-inflammatory cytokines. Furthermore, the TGF-β1 and NF-κB signalling provoked by β-adrenergic hyperactivity was reduced in the exercised rats, which is in agreement with the current idea that exercise training may be an important modulator of growth factors and/or transcriptional regulators in pathological cardiac hypertrophy (Konhilas et al. 2006; Oliveira et al. 2009; Benito & Nattel, 2009).
The β-adrenergic hyperactivity of the non-trained rats was associated with concentric LV hypertrophy with increase of FS and +dP/dt, suggesting an illusory improvement of the LV contractile state. Nevertheless, we evaluated the myocardial contractile capacity in the papillary muscle preparation and, in this approach, our results are consistent with a depressed contractile state after β-adrenergic hyperactivity (Vassallo et al. 1988; Krenek et al. 2009). Moreover, the isoprenaline treatment provoked a downwards shift of length–active tension curves, suggesting that the Frank–Starling relationship is worsened (Matsubara et al. 2000). Considering that the mass/volume ratio increases in concentric hypertrophied hearts and taking the Laplace law into account, it is conceivable that +dP/dt should be higher in concentric hypertrophied LV in spite of force generation impairment. According to the simplest expression the law of Laplace, wall stress and intraventricular pressure are related by the equation: σ=PRi/2h, where σ is developed wall stress, P is pressure, Ri is chamber radius and h is wall thickness. Rearrangement of this equation shows that P= 2σh/Ri. Therefore, for the same wall force, intraventricular pressure generation will be facilitated if the h/Ri ratio increases, that is to say, +dP/dt should increase. Since in our experiments a concentric hypertrophy occurred, it is possible to understand that FS and +dP/dt should be elevated even when myocardial contractility was depressed.
Perhaps the most likely candidate to be appointed as responsible for mediating isoprenaline-induced diastolic dysfunction is an imbalance between muscular tissue and collagenous compartments (Hanft et al. 2008; Brooks & Conrad, 2009; Nakajima-Takenaka et al. 2009). Moreover, an increase of ventricular diastolic stiffness is a common consequence of concentric hypertrophy (Vangheluwe et al. 2006) and, additionally, there are data demonstrating that β-adrenergic hyperactivity induces deleterious alterations in the expression and/or phosphorylation of regulatory cardiac proteins, e.g. phospholamban and sarcoplasmic reticulum Ca2+-adenosine triphosphatase, as well as a reduced adenosine 3′,5′-cyclic monophosphate accumulation (Stein et al. 1996; Nakajima-Takenaka et al. 2009).
Exercise training has been shown to be effective in improving the functional characteristics of hearts from hypertensive (Morris et al. 2007), myocardial-infarcted (Leosco et al. 2008; Portes et al. 2009) and aged rats (Barmeyer et al. 2009). However, this is the first report to document that exercise training can prevent functional abnormalities in chronically β-adrenergically stimulated rats. It is noteworthy that the β-adrenergic hyperactivity did not inhibit the beneficial effect of exercise training on myocardial contractile capacity.
Clinical implications
The present study indicates that exercise training commencing before β-adrenergic hyperactivity insult is beneficial, resulting in prevention of LV hypertrophy and improved myocardial performance, without adverse effects on chamber remodelling. Our data show that exercise training, which is recommended as part of the gold standard treatment for attenuation of ventricular remodelling and heart failure (Hunt et al. 2005), is very effective in promoting heart protection against β-adrenergic hyperactivity. Exercise training can offer protection by creating a positive balance between anti-inflammatory and proinflammatory cytokines as well as by modulation of TGF-β1 signalling in pathological cardiac hypertrophy. Nevertheless, future studies should be aimed at investigating whether combined β-adrenoreceptor blockade and exercise training yield added benefit. For clinical purposes, assuming that the isoprenaline dose used in our study is excessive in terms of usual physiological β-adrenergic stimulation, exercise training can be considered as very effective in cardioprotection against β-adrenergic hyperactivity.
Conclusion
In summary, in rats with β-adrenergic hyperactivity exercise training induced a cardiac antiremodelling effect associated with deactivation of proinflammatory cytokines, avoiding the development of myocardial hypertrophy and dysfunction. These findings support the notion that deactivation of inflammatory cytokine signalling pathways is an important mechanism underlying the cardiac antiremodelling effect of exercise training in situations of excessive catecholaminergic drive.
Acknowledgments
This study was supported by grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). There is no conflict of interest in this manuscript. We thank BioMed Proofreading for critical reviewing of English language.
Glossary
Abbreviations
- CSA
cross-sectional area
- +dP/dt
maximal positive time derivate of the developed pressure
- −dP/dt
maximal negative time derivate of the developed pressure
- DT
developed tension
- +dT/dt
maximal rate of tension increase
- −dT/dt
maximal rate of tension decrease
- FS
left vetricular fractional shortening
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HR
heart rate
- IL-6
interleukin-6
- IL-10
interleukin-10
- Lmax
maximal length
- LV
left ventricle
- LVEDD
left vetricular end-diastolic diameter
- LVEDP
left vetricular end-diastolic pressure
- LVESD
left vetricular end-systolic diameter
- LVPWd
diastolic left vetricular posterior wall thickness
- LVPWs
systolic left vetricular posterior wall thickness
- LVSP
left vetricular systolic pressure
- MAP
mean arterial pressure
- NF-κB
nuclear factor-κB
- RT
resting tension
- TGF-β1
transforming growth factor β1
- TNF-α
tumour necrosis factor α
Author contributions
A.J.S. contributed to design of the experiments, drafting the article and revising it critically for important intellectual content. M.H.H.S. contributed to collection and analysis of data. D.S.B., A.A.S., J.A.S., F.C.M. and V.G.B. contributed to collection, analysis and interpretation of data. E.L.A. and R.F.L. contributed to collection of data. M.L.H. and J.E.K. contributed to conception of the experiments. P.J.F.T. contributed to drafting the article and revising it critically for important intellectual content. All authors approved the final version to be published. The experiments were carried out in the Department of Physiology of the Federal University of São Paulo, the Department of Pathology of the University of São Paulo and the Laboratory of Genetics and Molecular Cardiology of the University of São Paulo.
References
- Barmeyer A, Müllerleile K, Mortensen K, Meinertz T. Diastolic dysfunction in exercise and its role for exercise capacity. Heart Fail Rev. 2009;14:125–134. doi: 10.1007/s10741-008-9105-y. [DOI] [PubMed] [Google Scholar]
- Benito B, Nattel S. Exercise training as a treatment for heart failure: potential mechanisms and clinical implications. J Physiol. 2009;587:5011–5013. doi: 10.1113/jphysiol.2009.181339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluyt MO, Long X, Eschenhagen T, Mende U, Schmitz W, Crow MT, Lakatta EG. Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am J Physiol Heart Circ Physiol. 1995;269:H638–H647. doi: 10.1152/ajpheart.1995.269.2.H638. [DOI] [PubMed] [Google Scholar]
- Brodowicz GR, Lamb DR. Exercise training, indomethacin, and isoproterenol induced myocardial necrosis in the rat. Basic Res Cardiol. 1991;86:40–48. doi: 10.1007/BF02193870. [DOI] [PubMed] [Google Scholar]
- Brooks WW, Conrad CH. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med. 2009;59:339–343. [PMC free article] [PubMed] [Google Scholar]
- Brouri F, Hanoun N, Mediani O, Saurini F, Hamon M, Vanhoutte PM, Lechat P. Blockade of β1- and desensitization of β2-adrenoreceptors reduce isoprenaline-induced cardiac fibrosis. Eur J Pharmacol. 2004;485:227–234. doi: 10.1016/j.ejphar.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Brum PC, Kosek J, Patterson A, Bernstein D, Kobilka B. Abnormal cardiac function associated with sympathetic nervous system hyperactivity in mice. Am J Physiol Heart Circ Physiol. 2002;283:H1838–H1845. doi: 10.1152/ajpheart.01063.2001. [DOI] [PubMed] [Google Scholar]
- Buttros JB, Bergamaschi CT, Ribeiro DA, Fracalossi AC, Campos RR. Cardioprotective actions of ascorbic acid during isoproterenol-induced acute myocardial infarction in rats. Pharmacology. 2009;84:29–37. doi: 10.1159/000222245. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. β-Adrenergic stimulation induces interleukin-18 expression via β2-AR, PI3K, Akt, IKK, and NF-κB. Biochem Biophys Res Commun. 2004;319:304–311. doi: 10.1016/j.bbrc.2004.04.185. [DOI] [PubMed] [Google Scholar]
- Copaja Soto M, Valenzuela R, Saldaña A, Paz Ocaranza M, Jalil JE, Vio C, Lijnen P, Ordenes GE, Vivar Sanchez R, Lavandero S, Díaz-Araya G. Early expression of monocyte chemoattractant protein-1 correlates with the onset of isoproterenol-induced cardiac fibrosis in rats with distinct angiotensin-converting enzyme polymorphism. J Renin Angiotensin Aldosterone Syst. 2008;9:154–162. doi: 10.1177/1470320308096408. [DOI] [PubMed] [Google Scholar]
- Darrah MI, Engen RL. Beneficial effects of exercise on l-isoproterenol induced myocardial infarction in male rats. Med Sci Sports Exerc. 1982;14:76–80. doi: 10.1249/00005768-198201000-00015. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;4:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–85. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Frederico MJ, Justo SL, Da Luz G, Da Silva S, Medeiros C, Barbosa VA, Silva LA, Boeck CR, De Pinho RA, De Souza CT. Exercise training provides cardioprotection via a reduction in reactive oxygen species in rats submitted to myocardial infarction induced by isoproterenol. Free Radic Res. 2009;43:957–964. doi: 10.1080/10715760903159154. [DOI] [PubMed] [Google Scholar]
- Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, El-Jamali A, Dietz R, Scheidereit C, Bergmann MW. Requirement of nuclear factor-κB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005;111:2319–2325. doi: 10.1161/01.CIR.0000164237.58200.5A. [DOI] [PubMed] [Google Scholar]
- Gallego M, Espiña L, Vegas L, Echevarria E, Iriarte MM, Casis O. Spironolactone and captopril attenuates isoproterenol-induced cardiac remodelling in rats. Pharmacol Res. 2001;44:311–315. doi: 10.1006/phrs.2001.0865. [DOI] [PubMed] [Google Scholar]
- Hanft LM, Korte FS, MacDonald KS. Cardiac function and modulation of sarcomeric function by length. Cardiovasc Res. 2008;77:627–636. doi: 10.1093/cvr/cvm099. [DOI] [PubMed] [Google Scholar]
- Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Janczewski AM, Kadokami T, Lemster B, Frye CS, Mactiernan CF, Feldman AM. Morphological and functional changes in cardiac myocytes isolated from mice overexpressing TNF-α. Am J Physiol Heart Circ Physiol. 2003;284:H960–H969. doi: 10.1152/ajpheart.0718.2001. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98:540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, Veres G, Páli S, Seidel B, Zöllner S, Karck M, Szabó G. Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation. 2009;120:677–686. doi: 10.1161/CIRCULATIONAHA.109.870774. [DOI] [PubMed] [Google Scholar]
- Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, Ochodnicky P, Klimas J, Kyselovic J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 2009;11:140–146. doi: 10.1093/eurjhf/hfn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leosco D, Rengo G, Laccarino G, Golino L, Marchese M, Fortunato F, Zincarelli C, Sanzari E, Ciccarelli M, Galasso G, Altobelli GG, Conti V, Matrone G, Cimini V, Ferrara N, Filippelli A, Koch WJ, Rengo F. Exercise promotes angiogenesis and improves β-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res. 2008;78:385–394. doi: 10.1093/cvr/cvm109. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WB. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Lips DJ, deWindt LJ, van Kraaij DJ, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur Heart J. 2003;24:883–896. doi: 10.1016/s0195-668x(02)00829-1. [DOI] [PubMed] [Google Scholar]
- Matsubara LS, Matsubara BB, Okoshi MP, Cicogna AC, Janicki JS. Alterations in myocardial collagen content affect rat papillary muscle function. Am J Physiol Heart Circ Physiol. 2000;279:H1534–H1539. doi: 10.1152/ajpheart.2000.279.4.H1534. [DOI] [PubMed] [Google Scholar]
- Morris RT, Fine DM, Lees SJ, Booth FW, Link CD, Ferrario CM, Stump CS, Sowers JR. Exercise training prevents development of cardiac contractile dysfunction in hypertensive TG (mREN-2)27 rats. J Am Soc Hypertens. 2007;1:393–399. doi: 10.1016/j.jash.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad N, Tucci PJF. Isoproterenol-induced hypertrophy may result in distinct left ventricular changes. Clin Exp Pharmacol Physiol. 2000;27:352–357. doi: 10.1046/j.1440-1681.2000.03254.x. [DOI] [PubMed] [Google Scholar]
- Murray DR, Prabhu SD, Chandrasekar B. Chronic β-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–2341. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- Nakajima-Takenaka C, Zhang GX, Obata K, Tohne K, Matsuyoshi H, Nagai Y, Nishiyama A, Takaki M. Left ventricular function of isoproterenol-induced hypertrophied rat hearts perfused with blood: mechanical work and energetics. Am J Physiol Heart Circ Physiol. 2009;297:H1736–H1743. doi: 10.1152/ajpheart.00672.2009. [DOI] [PubMed] [Google Scholar]
- Okuda N, Hayashi T, Mori T, Inamoto S, Okabe M, Mieno S, Horimoto H, Kitaura Y. Nifedipine enhances the cardioprotective effect of an angiotensin-II receptor blocker in an experimental animal model of heart failure. Hypertens Res. 2005;28:431–438. doi: 10.1291/hypres.28.431. [DOI] [PubMed] [Google Scholar]
- Oliveira RSF, Ferreira JCB, Gomes ERM, Paixão NA, Rolim NPL, Medeiros A, Guatimosim S, Brum PC. Cardiac anti-remodelling effect of aerobic training is associated with a reduction in the calcineurin/NFAT signalling pathway in heart failure mice. J Physiol. 2009;587:3899–3910. doi: 10.1113/jphysiol.2009.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osadchii OE. Cardiac hypertrophy induced by sustained β-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- Portes LA, Saraiva RM, dos Santos AA, Tucci PJF. Swimming training attenuates remodelling, contractile dysfunction and congestive heart failure in rats with moderate and large myocardial infarctions. Clin Exp Pharmacol Physiol. 2009;36:394–399. doi: 10.1111/j.1440-1681.2008.05070.x. [DOI] [PubMed] [Google Scholar]
- Reichek N, Devereux RB, Rocha RA, Hilkert R, Hall D, Purkayastha D, Pitt B. Magnetic resonance imaging left ventricular mass reduction with fixed-dose angiotensin-converting enzyme inhibitor-based regimens in patients with high-risk hypertension. Hypertension. 2009;54:731–737. doi: 10.1161/HYPERTENSIONAHA.109.130641. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schlüter K-D, Böhm M. Alterations of β-adrenergic signalling and cardiac hypertrophy in transgenic mice overexpressing TGF-β1. Am J Physiol Heart Circ Physiol. 2002;283:H1253–H1262. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- Serra AJ, Higuchi ML, Ihara SS, Antonio EL, Santos MHH, Bombig MTNM, Tucci PJF. Exercise training prevents β-adrenergic hyperactivity-induced myocardial hypertrophy and lesions. Eur J Heart Fail. 2008;10:534–539. doi: 10.1016/j.ejheart.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodelling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- Stein B, Bartel S, Kirchhefer U, Kokott S, Krause EG, Neumann J, Schmitz W, Scholz H. Relation between contractile function and regulatory cardiac proteins in hypertrophied hearts. Am J Physiol Heart Circ Physiol. 1996;270:H2021–H2028. doi: 10.1152/ajpheart.1996.270.6.H2021. [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Yoshiyama M, Takeuchi K, Omura T, Komatsu R, Izumi Y, Kim S, Yoshikawa J. Increased JNK, AP-1 and NF-κB DNA binding activities in isoproterenol-induced cardiac remodelling. J Mol Cell Cardiol. 1999;31:2017–2030. doi: 10.1006/jmcc.1999.1033. [DOI] [PubMed] [Google Scholar]
- van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63:487–499. doi: 10.1016/j.cardiores.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Tjwa M, Van Den Bergh A, Louche WE, Beullens M, Dode L, Carmeliet P, Kranias E, Herijgers P, Sipido KR, Raeymaekers L, Wuytack F. A Serca 2 pump with an increased Ca2+ affinity can lead to severe cardiac hypertrophy, stress intolerance and reduced life span. J Mol Cell Cardiol. 2006;41:308–317. doi: 10.1016/j.yjmcc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Vassallo DV, Vasquez EC, Cabral AM. Contractile performance of papillary muscles of renovascular hypertensive and isoproterenol-pretreated rats. Pharmacol Res Commun. 1988;20:61–72. doi: 10.1016/s0031-6989(88)80607-6. [DOI] [PubMed] [Google Scholar]


