Abstract
Myocardial ischaemia activates cardiac sympathetic afferents leading to chest pain and reflex cardiovascular responses. Previous studies have shown that a brief period of myocardial ischaemia increases endothelin in cardiac venous plasma draining ischaemic myocardium and that exogenous endothelin excites cutaneous group III and IV sensory nerve fibres. The present study tested the hypothesis that endogenous endothelin stimulates cardiac afferents during ischaemia through direct activation of endothelin A receptors (ETARs). Nerve activity of single unit cardiac sympathetic afferents was recorded from the left sympathetic chain or rami communicates (T2–T5) in anaesthetized cats. Single fields of 38 afferents (CV = 0.25–3.86 m s−1) were identified in the left or right ventricle with a stimulating electrode. Five minutes of myocardial ischaemia stimulated all 38 cardiac afferents (8 Aδ, 30 C-fibres) and the responses of these 38 afferents to chemical stimuli were further studied in the following protocols. In the first protocol, injection of endothelin 1 (ET-1, 1, 2 and 4 μg) into the left atrium (LA) stimulated seven ischaemically sensitive cardiac afferents in a dose-dependent manner. Second, BQ-123, a selective ETAR antagonist, abolished the responses of nine afferents to 2 μg of ET-1 injected into the left atrium and attenuated the ischaemia-related increase in activity of eight other afferents by 51%. In contrast, blockade of ETB receptors caused inconsistent responses to exogenous ET-1 as well as to ischaemia. Furthermore, in the absence of ETAR blockade, cardiac afferents responded consistently to repeated administration of ET-1 (n= 7) and to recurrent myocardial ischaemia (n= 7). Finally, using an immunocytochemical staining approach, we observed that ETA receptors were expressed in cardiac sensory neurons in thoracic dorsal root ganglia. Taken together, these data indicate that endogenous endothelin contributes to activation of cardiac afferents during myocardial ischaemia through direct stimulation of ETA receptors likely to be located in the cardiac sensory nervous system.
Introduction
During myocardial ischaemia cardiac sympathetic (spinal) afferent nerve endings are exposed to multiple ischaemic metabolites that activate or sensitize these afferents, leading to angina pectoris and excitatory cardiac-cardiovascular reflex responses (Malliani et al. 1981; Meller & Gebhart, 1992; Fu et al. 2008a; Fu & Longhurst, 2009). We and others have demonstrated that a number of ischaemic metabolites, including thromboxane A2, 5-hydroxytryptamine (5-HT), histamine, lactic acid (protons), reactive oxygen species and bradykinin (BK), but not adenosine, stimulate cardiac spinal afferents during ischaemia and reperfusion in an interactive and multifactorial fashion (Uchida & Murao, 1974; Baker et al. 1980; Pan & Longhurst, 1995; Fu & Longhurst, 2002, 2005; Fu et al. 2008b; Fu & Longhurst, 2009, 2010). However, despite almost complete inhibition of action induced by any of the receptor agonists, the specific antagonist never fully blocks afferent activity during ischaemia, indicating that other mediators are likely to contribute to excitation of these cardiac afferents during ischaemia.
Several lines of evidence suggest that endothelin-1 (ET-1), originally identified as a potent vasoconstrictor, may play an important role in stimulation of cardiac spinal afferents during ischaemia. First, investigators have observed increased plasma endothelin in patients with myocardial ischaemia, unstable angina and myocardial infarction (Wieczorek et al. 1994; Vojacek et al. 1999). Second, clinical and experimental studies have demonstrated that exogenous ET-1 elicits nociception when it is injected into several species of mammals at different peripheral locations, including verbally reported pain following injection into the human forearm (Katugampola et al. 2000; Hans et al. 2007), overt nociceptive behaviour when injected into the knee joint and hind paw of rats and dogs (Ferreira et al. 1989; De-Melo et al. 1998b) or after it has been applied epineurally onto or injected intraneurally into the rat sciatic nerve (Davar et al. 1998). Third, in vivo neurophysiological data have indicated that subcutaneous administration of ET-1 excites cutaneous group III and IV sensory nerve fibres, and that this effect is completely prevented by blockade of endothelin A receptors (ETARs) with BQ-123, a highly selective ETAR antagonist (Gokin et al. 2001). Finally, ET-1 mRNA and ET-1 immunoreactivity are expressed in sympathetic and somatic sensory neurons located in dorsal root ganglia (Hemsen & Lundberg, 1991; Milner et al. 2000). Despite the absence of any studies on the sensory role of endothelin in ischaemia or its action on visceral afferents, the above studies led us to believe that the potential exists for endogenous ET-1 to contribute to activation of cardiac afferent nerve endings duringmyocardial ischaemia.
In mammals, ET-1 produces its biological effects through activation of two receptor subtypes, the ETA and ETB receptors (ETBR), respectively. Both receptors are distributed widely throughout the body, but each receptor has a unique distribution. ETAR mRNA is expressed in vascular smooth muscle in a variety of tissues, whereas ETBR mRNA is abundant in a variety of cell types, predominately in vascular endothelial and glial cells in the brain (Hori et al. 1992). Recently, investigators observed that ETARs arepresent primarily in small-diameter neurons of the rat dorsal root ganglion (DRG) whereas ETBRs are expressed in DRG satellite cells (Pomonis et al. 2001; Peters et al. 2003). However, there is no evidence supporting the existence of ETARs in cardiac spinal afferent neurons.
The aim of the present study, therefore, was to determine the role of endogenously produced ET-1 in stimulation of cardiac spinal afferents during myocardial ischaemia. We employed neurophysiological and immunocytochemical approaches to test our overall hypothesis that endogenous endothelin stimulates cardiac sympathetic afferents during ischaemia through an ETAR, but not through an ETBR mechanism. Part of this study has been presented as a preliminary report (Fu & Longhurst, 2007).
Methods
Surgical preparation
Surgical and experimental protocols used in this study were approved by the Animal Use and Care Committee at the University of California at Irvine. The studies conformed to the American Physiological Society's Guiding Principles in the Care and Use of Animals. A total 49 adult cats of either sex (2.76 ± 0.35 kg, mean ±s.e.m.) were anaesthetized by intramuscular injection of ketamine (20–30 mg kg−1, Phoenix Scientific, Inc., St Joseph, MO, USA), followed by intravenous injection of α-chloralose (40–50 mg kg−1) through the femoral vein. Additional injections of α-chloralose (5–10 mg kg−1, i.v.) were given as necessary to maintain an adequate depth of anaesthesia that was assessed by observing the absence of a conjunctival reflex. The trachea of each animal was intubated and respiration was maintained artificially (Harvard pump, model 661, Ealing, South Natick, MA, USA). Cats were ventilated by air supplemented with 100% O2 through the respirator. The femoral vein and artery were cannulated for administration of drugs and fluid, and the measurement of blood pressure, respectively. A pressure transducer (Statham P 23 ID, Gould) was connected to the femoral arterial catheter for measuring arterial blood pressure. Arterial blood gases were assessed frequently with a blood gas analyser (Radiometer ABL-5, Copenhagen, Denmark) and maintained within physiological limits (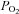 > 100 mmHg,
> 100 mmHg, 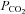 = 28–35 mmHg, pH 7.35–7.45) by adjusting the respirator rate or tidal volume, or by intravenously administering 2–3 ml of 1 m of NaHCO3 (8.4%, w/v). Another PE 90 catheter was introduced into the left atrium through the left atrial appendage for intra-cardiac injection of solutions. Body temperature was monitored with a rectal thermistor and was maintained at 36–38°C with a circulating water heating pad and a heat lamp. Animals were killed at the end of each experiment by administration of a solution of saturated potassium chloride into the femoral vein under deep anaesthesia, ensured by administering an additional dose of α-chloralose (50 mg kg−1).
= 28–35 mmHg, pH 7.35–7.45) by adjusting the respirator rate or tidal volume, or by intravenously administering 2–3 ml of 1 m of NaHCO3 (8.4%, w/v). Another PE 90 catheter was introduced into the left atrium through the left atrial appendage for intra-cardiac injection of solutions. Body temperature was monitored with a rectal thermistor and was maintained at 36–38°C with a circulating water heating pad and a heat lamp. Animals were killed at the end of each experiment by administration of a solution of saturated potassium chloride into the femoral vein under deep anaesthesia, ensured by administering an additional dose of α-chloralose (50 mg kg−1).
Cardiac spinal afferent recording
Single-unit activity of cardiac afferents was recorded as described previously (Fu & Longhurst, 2002, 2005). In brief, a midline sternotomy was performed and the first to seventh left ribs and the left lung were removed. The left paravertebral sympathetic chain was isolated, then draped over a Plexiglas platform and covered with warm mineral oil. Small nerve filaments were dissected gently from the chain and rami communicates between T2 and T5 under an operating microscope (Zeiss, Germany) and the rostral ends were placed across one pole of the recording electrode. The other pole of the recording electrode was grounded with a cotton thread to the animal. The recording electrode was attached to a high impedance probe (model HIP511, Grass Instruments, Quincy, MA, USA). Action potentials of afferents were amplified (×50,000) and bandpass filtered (100–3000 Hz) through an AC amplifier (model P511 Preamplifier, Grass) and processed through an audioamplifier (AM8B, Audiomonitor, Grass) and an oscilloscope (model 2201, Tektronix, Beavertown, OR, USA). Nerve activity and blood pressure signals were recorded on a Pentium computer using data acquisition and analysis software (Spike2), which sampled these signals at 10,000 Hz through an analog-to-digital converter (CED micro 1401 mkII, Cambridge Electronic Design, Cambridge, UK) for on- and offline quantitative analysis. Discharge frequency was quantified by using a software window discriminator; a histogram was generated for each afferent. Accurate counting of the impulse activity of each afferent was verified by comparing the constructed histogram with the original neurogram.
The precise location of the afferent nerve ending was identified by placing a stimulating electrode directly on the surface of the myocardium to evoke the afferent's action potential as described previously (Fu et al. 2008b). In brief, while recording afferent fibre activity the epicardium was mapped gradually from the apex to the base of the heart using a bipolar stimulating electrode to search for the location of nerve endings electrically (6–12 V, 0.5 ms, and 1 Hz). The location of the afferent nerve ending was confirmed by mechanical stimulation of the heart by gently probing the heart with a cotton swab and constricting the thoracic aorta as well as chemical stimulation with epicardial application of BK (1–3 μg) on the ventricles. The conduction velocity of each afferent fibre was calculated by dividing conduction distance by conduction time. The conduction time was determined by measuring the time interval from electrical stimulation to the evoked afferent's action potential. Conduction distance was estimated by measuring the length of a wet thread between the receptive field and the recording electrode (Fu & Longhurst, 2002, 2005). Unmyelinated C- and finely myelinated Aδ- fibre afferents were classified as those with conduction velocities (CV) of <2.5 and 2.5–30 m s−1, respectively. In the present study, each afferent had a single receptive field that could be located precisely in one of the ventricles. Myocardial ischaemia was induced by complete occlusion of the appropriate coronary artery supplying the regional receptive field of the cardiac afferent nerve with a thread placed around the vessel. Ischaemia was confirmed by observing a regional change in the colour of the myocardium, which has been closely correlated to the production of lactic acid as indicated by a reduction in tissue pH (Pan et al. 1999). Afferents were considered to be ischaemically sensitive if their discharge activity during 3–5 min ofmyocardial ischaemia increased at least 50% above baseline (Fu & Longhurst, 2002, 2005). To determine whether an afferent was chemosensitive, bradykinin (3 μg) was injected into the left atrium and the afferent response was recorded. Mechanosensitive afferents were identified by evaluating responses to aortic constriction, which raised systolic blood pressure to ∼170–190 mmHg for 15 s. Aortic constriction was induced by partially occluding aorta with an occlusion cuff that had been placed around the descending thoracic aorta at the level of T6. Afferents were classified mechanosensitive if their activity increased at least 50% above baseline during constriction.
Surgical preparation for cardiac sensory neurons (CSNs) labelling
Pre-anaesthesia of cats was induced withsubcutaneous ketamine and midazolam at a dose of 5–10/0.1–0.2 mg kg−1. Cats were intubated with a cuffed endotracheal tube and connected to an anaesthesia machine (model: Narkomed II, North American Drager, Telford, PA, USA). Anaesthesia was maintained withisoflurane (1–2%) in 100% oxygen mixed with room air through inhalation. Similar to the afferent recording studies, during the surgical procedures for labelling cardiac sensory nerves, body temperature, monitored with a rectal probe, was maintained at 37°C with a circulating heating pad and a heat lamp. Blood oxygenation and heart rate were monitored using a pulse oximeter (model: 8500AV, Nonin Medical, Plymouth, MN, USA). The heart was exposed through a left lateral thoracotomy at the fifth intercostal space. One hundred μl of a suspension of 17 mg ml−1 of 1,1′-dioctadecyl-3,3′,3′-tetramethyl indocarbocyanine perchlorate (DiI; Molecular Probes) in a solution of saline was injected into the pericardial space to access the ventricular wall of the heart. Care was taken to eliminate any leaks of DiI around the injection site by tightening the small puncture hole with a silk suture. We observed no leakage over a 5 min period after injection. The ribs then were approximated, the thoracic cavity evacuated and the incision closed in layers. Post-operatively the cats were isolated and treated for pain and infection. Pain was controlled by prophylactic administration of buprenorphine (0.01 mg kg−1, i.m.) every 8–12 h for the first 24–48 h period while infection was prevented by administration of penicillin G procaine (20,000 IU kg−1, i.m.) every 8–12 h for 4 days.
Experimental protocols
Dose-responses of ischaemically sensitive cardiac spinal afferents to ET-1
This protocol examined the response of ischaemically sensitive cardiac spinal afferents (n= 7 afferents) to graded doses of ET-1 (1, 2 and 4 μg). After identifying the location of the receptive field of an afferent fibre in the ventricle, its response to 3–5 min of myocardial ischaemia was evaluated. If the afferent responded to ischaemia, ET-1 or phosphate buffer solution (PBS, pH 7.35, vehicle) was injected into left atrium (LA) and afferent activity was recorded. Dose–response curves were generated with three doses of ET-1 (1, 2 and 4 μg, American Peptide Co., Inc., Sunnyvale, CA, USA) applied at least 20 min apart to avoid tachyphylaxis. ET-1 and the vehicle were applied randomly. To prepare a stock solution, ET-1 (1 mg) was dissolved in 1 ml of PBS to achieve an initial concentration of 1 mg ml−1 that was stored in a −70°C freezer. A working solution of 40 μg ml−1 was made by first removing 40 μl from the ET-1 stock solution and adding 960 μl of PBS to obtain the final concentration. Solutions of 10 and 20 μg ml−1 of ET-1 were made by further diluting the 10 μg ml−1 solution with PBS. PBS served as the vehicle.
Effect of ETA and ETB receptor blockade on responses of ET-1
In this protocol, we examined the influence of blockade of ETA receptors with BQ-123 on afferent responses to ET-1. After locating the receptive field of an afferent on the heart, the response to brief myocardial ischaemia was measured. If the afferent responded to ischaemia, we then recorded the response to LA injection of ET-1 (2 μg). This dose of ET-1 was chosen based on the ET-1 dose–response data. Repeated LA injections of ET-1 were conducted 15 min after intravenous administration of BQ-123 (0.1 mg kg−1, American Peptide Co.) and 30 min after the initial stimulation with ET-1. BQ-123 was dissolved in 1 ml of PBS and diluted as needed with PBS to a concentration of 1 mg ml−1. Previous studies have demonstrated that this dose or even a lower dose of BQ-123 selectively and completely inhibited ET-1 induced activation in cutaneous sensory nerve fibres by blocking ETA receptors (Gokin et al. 2001). Furthermore, BQ-123 is highly selective for ETA receptors (Masaki et al. 1994). We administered BK (3 μg) into the LA to establish responsiveness of the afferent after treatment with BQ-123. Six ischaemically sensitive afferents were studied in this group.
We also examined the influence of blockade of ETB receptors with BQ-788 on afferent responses to ET-1 in another group (n= 4) of afferents in a preliminary study. After identifying an ischaemically sensitive unit, each animal in this group was treated identically, except that BQ-788 (0.1 mg kg−1, American Peptide Co.) was used in place of BQ-123. BQ-788 (1 mg) was dissolved in 0.1 ml of 100% DMSO and diluted with 0.9 ml of PBS to a concentration of 1 mg ml−1. A similar dose of BQ-788 selectively and completely abolished ETBR-mediated pulmonary vasoconstriction in lambs (Black et al. 2003).
To determine reproducibility of afferent responses to ET-1, six additional afferents in six animals were studied as time controls. After identifying an ischaemically sensitive unit, each animal in this group was treated identically, except that vehicle (PBS, 2–4 ml, i.v.) was used in place of BQ-123.
Effect of ETA and ETB receptor blockade on responses of cardiac afferents to ischaemia
This protocol consisted of evaluation of the responses of three groups of afferents to myocardial ischaemia before and after blockade of ETA or ETB receptors. Seven afferents were studied in the first group. After locating the receptive field of an afferent on the heart, discharge activity was measured during 5 min of regional myocardial ischaemia. If the afferent responded to ischaemia, a second period of ischaemia was repeated 30–40 min later in the presence of BQ-123 (0.1 mg kg−1, i.v.). We administered BK (3 μg) into the LA to establish responsiveness of the afferent 20 min after the second period of ischaemia.
In the second group of afferents (n= 4), we evaluated the influence of blockade of ETB receptors with BQ-788 on afferent responses to ischaemia. Repeated brief periods (5 min) of myocardial ischaemia were conducted as described above, except that BQ-788 (0.1 mg kg−1) was used in place of BQ-123.
To differentiate between variations in afferent responses to drug- and time-related effects, seven additional ischaemically sensitive afferents were examined to determine the repeatability of the afferent response to ischaemia in the third group of seven animals. In this group, after identification, each afferent fibre was treated in an identical manner, except that the vehicle (PBS) was used in place of BQ-123.
Histochemistry and Immunohistochemistry to identify labelled CSNs
Dorsal root ganglia (DRG) tissue preparations. During the 10 week postoperative period, DiI was transported retrogradely to the cell bodies of the CSNs. Then, cats were re-anaesthetized with ketamine (40–50 mg kg−1, i.m.) followed by α-chloralose (100 mg kg−1, i.v.) administered to induce deep anaesthesia as judged by the lack of a withdrawal response to toe pinch and corneal reflexes. Subsequently, animals were perfused transcardially with 0.9% saline and cold 4% paraformaldehyde in phosphate buffer (PB, pH 7.2). The T1-3 DRGs were harvested bilaterally since cardiac sympathetic sensory spinal input occurs at these levels (Kuo et al. 1984). The DRGs were stored in 4% paraformaldehyde for 2 h and then transferred to 30% sucrose for 48 h to prevent ice crystallization.
Transverse 30 μm DRG sections were cut with a cryostat microtome (CM1850, Leica, Nussloch, Germany) and were mounted serially on superfrost/plus slides (Fisher Scientific, Pittsburgh, PA, USA). The sections were used to detect DiI staining and to conduct immunohistochemical labelling for the ETA receptor, a seven-transmembrane domain G-protein coupled receptor (Masaki et al. 1994).
Fluorescent immunohistochemical labelling for ETA receptors
After rinsing three times (10 min each) with phosphate buffered saline containing 0.3% Triton X-100 (PBST, pH 7.4), DRG sections were treated with 1% normal donkey serum (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) for 1 h to block non-specific binding. The DRG sections were incubated with a rabbit anti-ETA receptor polyclonal antibody (200 μg ml−1, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in PBST at 4°C for 48 h. This antibody was raised specifically against ETA receptor C-terminal amino acids 323–343 of humans and has been used to identify ETA receptors in other species (Pomonis et al. 2001). The sections subsequently were incubated with fluorescein-conjugated donkey anti-rabbit antibodies (1:100; Jackson Immunoresearch Laboratories) in PBST for 24 h at 4°C. After rinsing the sections in phosphate buffered solution (pH 7.4) for 30 min (10 min, 3 times), they were air dried. The slides were coverslipped using mounting medium (Vector Laboratories, Burlingame, CA, USA). Immunohistochemical control studies were performed by omission of the primary or secondary antibodies. No labelling was detected under these conditions.
DRG sections were examined with a standard fluorescence microscope (Nikon, E400, Melville, NY, USA). Two epi-fluorescence filters (B-2A or G-2A), equipped in a fluorescence microscope, were used to identify single stains appearing as green (fluorescein) or red (DiI) in DRG sections. Selected sections were evaluated with a laser scanning confocal microscope (Zeiss LSM 510, Meta system, Thornwood, NY, USA) to confirm co-localization of two labels in the same cell (Fu et al. 2008b). This apparatus is equipped with argon and He–Ne lasers to allow operation of multiple channels. Lasers of 488 and 543 nm wavelengths were used to excite fluorescein (green) and DiI (red), respectively. Digital images of the immunoreactive structures were captured and analysed with software (Zeiss LSM) provided with the confocal microscope. Images in two colours in the same plane were merged to reveal the relationship between two immunoreactive elements. We identified cells that contained one-third or more of the cytoplasm stained bright red as DiI positive. We categorized a cell as ETA receptor positive if it contained labelling with bright green fluorescence. Co-localization of two labels was identified by the appearance of orange, reflecting a mixture of red and green.
Data analysis
Discharge activity of cardiac spinal afferents was expressed in imp s−1 and was averaged during the 3–5 min of pre-ischaemia and the 5 min of ischaemia. We measured the responses of cardiac afferent nerve endings to ET-1, BQ-123 and BQ-788 by averaging discharge rates of the afferents during the entire period of response, defined as the time during which sustained activity exceeded baseline activity by 20%. Five-minute sampling periods were used to measure afferent activity during myocardial ischaemia. During drug injection sampling periods varied between 1.5 and 8 min, depending on the responses of the afferent to the drug. Baseline activity was determined over the 3–5 min period immediately preceding ischaemia.
Data are expressed as means ±s.e.m. The effects of repeated injection of ET-1, BQ-123, and recurrent ischaemia on the responses of the afferents were compared using a one-way repeated-measures analysis of variance (ANOVA) followed by Tukey's post hoc test. If the data were not normally distributed, as determined by the Kolmogorov–Smirnov test, they were compared with Friedman's repeated-measures analysis of variance on ranks and Dunnett's post hoc test. We compared the effect of ET-1, on the afferent discharge activity using Student's paired t test. Alternatively, if data were not normally distributed, we used the Wilcoxon signed rank test to compare the paired data. All statistical calculations were performed with SigmaStat software (Systat Software Inc., San Jose, CA, USA). Values were considered to be significantly different when P < 0.05.
Results
Profile of cardiac afferents
The present study evaluated the activities of 38 ischaemically sensitive cardiac afferents. Approximately 25% of these afferents were mechanosensitive, while all were chemosensitive. Endings of most (97%) afferents were located in the anterior (n= 16) and posterior (n= 21) wall of the left ventricle (Fig. 1). One afferent was located on the posterior wall of the right ventricle. The afferent endings studied in this project appeared to be located near the epicardial surface since it was possible to activate them by gentle stroking or epicardial chemical stimulation of the receptive field. The conduction velocity for these afferents ranged from 0.25 to 3.86 m s−1. The majority of these afferents (30 fibres, 79%) were classified as C fibres (CV = 0.64 ± 0.06 m s−1). The remaining units (8 afferents) were classified as Aδ fibres (CV = 3.03 ± 0.16 m s−1). The responsiveness of the fibres to chemical stimulation or ischaemia was not related to the fibres’ conduction velocities.
Figure 1. Location of the receptive fields of ischaemically sensitive cardiac afferents on epicardial surface of left ventricle.
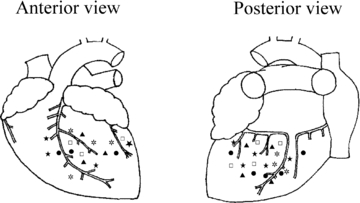
Receptive fields of cardiac afferents included in the study: ▴, ET-1 (n= 7); ⋆, repeated ET-1 (n= 7); ⋆, ET-1 + BQ-123 (n= 9); □, repeated ischaemia (n= 7); •, ischaemia + BQ-123 (n= 8).
Effect of ET-1 on activity of ischaemically sensitive afferents
The activity of seven ischaemically sensitive cardiac afferents (one Aδ, CV = 2.79 m s−1, six C-fibres, CV = 0.63 ± 0.12 m s−1) was increased from 0.43 ± 0.11 to 2.86 ± 0.55 imp s−1 by myocardial ischaemia. In the preliminary study we observed that LA injection of 0.5 μg of ET-1 stimulated only two of the four afferents. However, higher doses of ET-1 (1–4 μg, LA) stimulated all seven fibres, significantly increasing their discharge activity in a dose-dependent manner (Fig. 2), with responses ranging from 0.43 ± 0.06 to 1.23 ± 0.23, 1.82 ± 0.43 and 3.15 ± 0.54 imp s−1 following injection of 1, 2 and 4 μg, respectively. LA injection of ET-1 (4 μg) also increased mean blood pressure from 87 ± 5 to 113 ± 6 mmHg. We observed that two afferents in this group responded to cardiac distention induced by aortic constriction when blood pressure was raised to 170–190 mmHg (threshold pressure). Thus, we classified these afferents as bi-model in their responsiveness (i.e. mechano- and chemo-sensitive afferents). However, none of the afferents responded to cardiac distention occurring when mean BP was raised to 113 ± 6 mmHg, the level which was elevated by ET-1. In contrast, injection of the vehicle did not stimulate any of seven fibres tested (0.47 ± 0.08 to 0.49 ± 0.07 imp s−1). The locations of each of the seven afferent nerve endings that responded to the ET-1 are shown in Fig. 1.
Figure 2. Impulse activity of seven cardiac sympathetic afferents to graded doses of ET-1 (1–4 μg) injected into left atrium.
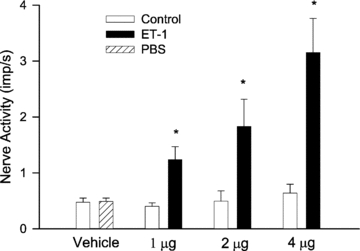
Columns and error bars represent means ±s.e.m.*P < 0.05 compared with control.
Effect of blockade of ETA receptors on cardiac afferent responses to ET-1
The responses of two groups of ischaemically sensitive cardiac afferents to ET-1 before and after treatment with BQ-123 (0.1 mg kg−1, i.v.) are displayed in Fig. 3. In the first group, ET-1 (2 μg) significantly increased the discharge activity of nine afferents (three Aδ, CV = 2.71, 2.87 and 3.5 m s−1, six C-fibres, CV = 0.69 ± 0.13 m s−1) from 0.34 ± 0.07 to 1.51 ± 0.23 imp s−1 (Fig. 3A). After blockade of ETA receptors with BQ-123, however, the responses of these afferents to ET-1 were abolished. BQ-123 itself did not change the basal discharge rate of these afferents. In addition, after blockade with BQ-123, these afferents still responded to application of BK (0.68 ± 0.24 to 3.06 ± 0.48 imp s−1, P < 0.05). In the second group, seven additional afferents (one Aδ, CV = 2.69 m s−1, six C-fibres, CV = 0.58 ± 0.12 m s−1) responded consistently to 2 μg of ET-1 following administration of the vehicle (PBS, Fig. 3B). Figure 3C illustrates the summated 2 s nerve activity of all nine cardiac afferents in response to ET-1 applied before and after treatment with BQ-123. Similar to the changes in averaged afferent nerve activity, the summated nerve response to ET-1 was abolished after blockade of ETA receptors with BQ-123. An example of the response of an ischaemically sensitive cardiac C-fibre afferent (CV = 0.32 m s−1) innervating the posterior of wall of the left ventricle to ET-1 before and after treatment with ETA receptor antagonist, BQ-123, is shown in Fig. 4. The impulse activity of this afferent was increased from 0.49 to 2.89 imp s−1 during ischaemia. Blockade of ETA receptors with BQ-123 eliminated the response of this afferent to ET-1 compared with the initial ET-1 response (Fig. 4). The locations of the 16 afferent nerve endings studied in this protocol are provided in Fig. 1.
Figure 3.
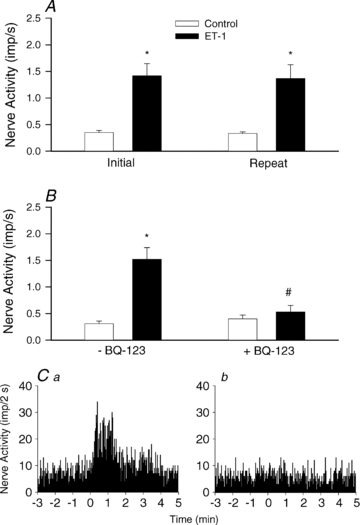
Responses of peak activity of cardiac sympathetic afferents to repeated LA injection of ET-1 (2 μg) with vehicle (PBS, 1.5 ml, i.v., n= 7, A) or treatment with BQ-123 (0.1 mg kg−1, i.v., n= 9, B). C, neurohistograms of summated 2 s discharge activity from all nine cardiac afferents in response to ET-1 stimulation before (a) and after (b) treatment with BQ-123. Columns and error bars represent means ±s.e.m.*P < 0.05 compared with control. †P < 0.05 post-BQ-123 vs. pre-BQ-123.
Figure 4. Neurohistogram showing response of a cardiac sympathetic C-fibre (CV = 0.32 m s−1) innervating the posterior wall of the left ventricle to ET-1 (2 μg, LA) before and after treatment with BQ-123 (0.1 mg kg−1, i.v.).
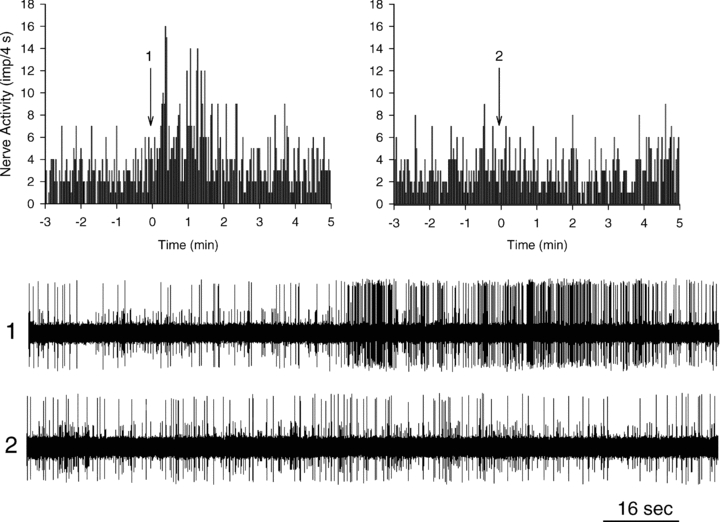
A, administration of ET-1 increased baseline activity of this afferent from 0.68 to 1.81 imp s−1. B, BQ-123 abolished the increase (0.74 to 0.73 imp s−1) in discharge activity of this afferent during repeated injection off ET-1. Panels 1 and 2 are representative tracings of the discharge activity of the afferent at times indicated by the arrows above histograms.
In preliminary studies, we examined the effect of blockade of ETBR on the responses of four different ischaemically sensitive afferents (one Aδ, CV = 3.29 m s−1, three C-fibres, CV = 0.85 ± 0.58 m s−1) to ET-1. Initial administration of ET-1 excited all four afferents and increased their activity from 0.53 ± 0.09 to 1.11 ± 0.13 imp s−1. Following blockade of ETBRs with BQ-788, the responses of two afferents to repeated ET-1 were higher than initial responses (increased by 32%), whereas the responses of two other afferents to ET-1 were lower (attenuated by 13%). These inconsistent responses led us to dismiss further study of this group.
Effect of blockade of ETA receptors on activity of cardiac afferents during myocardial ischaemia
Representative tracings of a cardiac Aδ afferent (CV = 3.1 m s−1) that responded to myocardial ischaemia in the absence and presence of BQ-123 are shown in Fig. 5. Ischaemia increased the discharge activity of this afferent from 0.39 to 2.42 imp s−1 (Fig. 5A). Antagonism of ETA receptors with BQ-123 (0.1 mg kg−1, i.v.) attenuated the ischaemia-induced increase by 48% when averaged over a 5 min period (2.42 to 1.62 imp s−1) (Fig. 5B).
Figure 5. Neurohistogram showing response of a cardiac sympathetic Aδ-fibre (CV = 3.1 m s−1) innervating the posterior wall of the left ventricle to 5 min of myocardial ischaemia before and after treatment with BQ-123 (0.1 mg kg−1, i.v.).
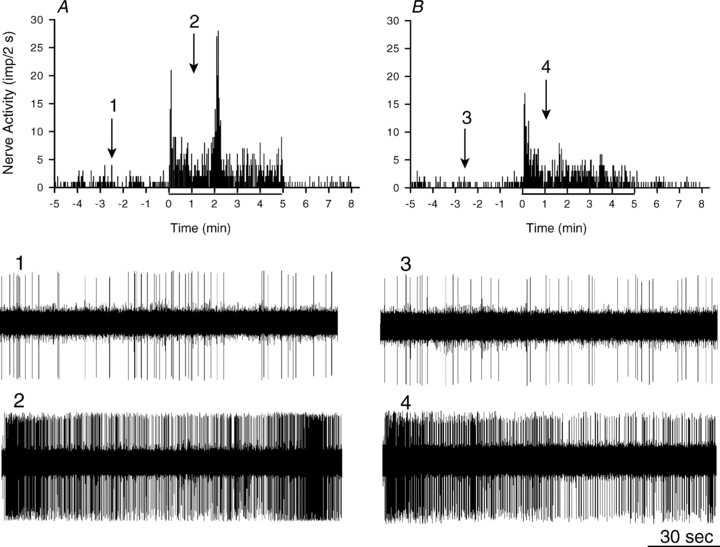
A, 5 min of myocardial ischaemia increased baseline activity of this afferent from 0.39 to 2.42 imp s−1. B, BQ-123 attenuated the increase (0.29 to 1.62 imp s−1) in discharge activity of this afferent during repeated ischaemia, particularly the second peak of the biphasic response. Panels 1–4 are representative tracings of the discharge activity of the afferent at times indicated by the arrows above histograms.
The responses of two groups of cardiac afferents to brief myocardial ischaemia are displayed in Fig. 6. In the first group, 5 min of ischaemia significantly increased the discharge activity of eight afferents (two Aδ, CV = 2.76 and 3.1 m s−1, six C-fibres, CV = 0.86 ± 0.22 m s−1) from 0.47 ± 0.15 to 3.19 ± 0.79 imp s−1 (Fig. 6A). After blockade of ETA receptors with BQ-123, however, the ischaemia-induced increase in afferent activity was significantly attenuated (3.19 ± 0.79 vs. 1.69 ± 0.49 imp s−1), compared to the initial period of ischaemia (Fig. 6A). In contrast, the responses to BK administration (3 μg, LA) were unaltered (0.72 ± 0.27 to 3.56 ± 0.38 imp s−1vs. 0.74 ± 0.32 to 3.33 ± 0.52 imp s−1, P > 0.05) by BQ-123. In another group, seven additional afferents (one Aδ, CV = 3.86 m s−1, six C-fibres, CV = 0.46 ± 0.06 m s−1) responded consistently to 5 min of repeated myocardial ischaemia in the presence of the vehicle (Fig. 6B). Figure 6C shows the summated 2 s discharge activity from all eight cardiac afferents during 5 min of ischaemia before and after blockade of ETA receptors with BQ-123. Similar to the changes in averaged afferent nerve activity, the summated nerve activity during the entire 5 min period of ischaemia was attenuated by approximately 51% after treatment with BQ-123. Locations of the 15 afferent nerve endings tested in these protocols during ischaemia are provided in Fig. 1.
Figure 6. Responses of cardiac afferents to ischaemia before and after blockade of ETA with receptors with BQ-123.
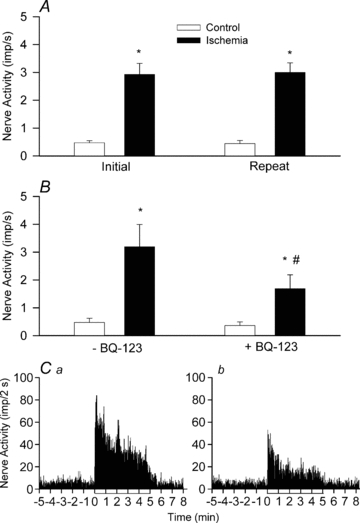
A, bar graph summarizing changes in activity of seven cardiac sympathetic afferents before (open bar) and during (filled bar) 5 min of myocardial ischaemia. B, responses of another eight cardiac sympathetic afferents to myocardial ischaemia before and after treatment with BQ-123. C, neurohistograms of summated 2 s discharge activity from all eight afferents during ischaemia before (a) and after (b) treatment with BQ-123. *P < 0.05 compared with control. †P < 0.05 post-BQ-123 vs. pre-BQ-123.
In preliminary studies, we observed that four other ischaemically sensitive afferents (one Aδ, CV = 3.52 m s−1, three C-fibres, CV = 0.74 ± 0.47 m s−1) that displayed an increase in discharge activity from 0.55 ± 0.12 to 2.45 ± 0.19 imp s−1 yielded inconsistent responses to blockade of the ETBR with BQ-788. Thus, two of the afferents demonstrated higher activity than initial ischaemia (increased by 46%), whereas the two others responded with lower activity (attenuated by 19%) during the second period of ischaemia following blockade. As a result we did not further evaluate the response to ETB receptor blockade.
DiI labelling and immunohistochemical staining of ETA receptors in DRG
DiI labelled perikarya were observed bilaterally in all of the DRGs between T1 and T3, but there was no labelling in the L1 ganglia 10 weeks after instillation of DiI into the pericardial space of two cats. We observed no DiI labelled neurons in the DRGs between T1 and T3 in a cat not treated with DiI. Neurons labelled with ETA receptors were observed in DRG sections of the DiI treated and the untreated cats. Importantly, DiI labelled DRG neurons were found to coexist with ETA receptors in ganglia between T1 and T3 in the DiI treated animals. Most DiI labelled neurons (∼95%) contained ETA receptors, while approximately half of the ETA labelled neurons (53%) were co-labelled with DiI (Fig. 7).
Figure 7. Confocal microscopic images showing coexistence of ETA receptors and DiI in dorsal root ganglion (DRG) neurons at spinal T2 level in a cat.
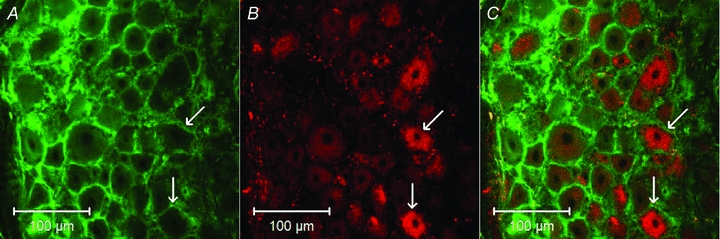
A and B demonstrate DRG neurons stained with ETA receptors and DiI, respectively. C is a merged image from A and B. Arrows indicate two neurons containing ETA receptors, DiI, or both of them, respectively. Scale bars in A–C represent 100 μm.
Discussion
There were three novel findings in this study. First, we found that injection of ET-1 into the left atrium stimulated ischaemically sensitive cardiac spinal afferents in a dose-dependent manner. Second, administration of the ETA receptor antagonist BQ-123 abolished the cardiac spinal afferent responses to ET-1 and significantly attenuated the afferents’ responses to regional myocardial ischaemia. Conversely, the responses to ETBR blockade were more variable. Third, we observed that ETA receptors were expressed in the cell membrane of cardiac sensory neurons located in the DRG of cats, assessed by double-labelling with the retrogradely transported DiI and ETA receptors. Taken together, these data confirm our original hypothesis that endogenous ET-1 contributes to activation of cardiac spinal afferents during myocardial ischaemia through direct stimulation of ETA receptors, likely to be located on spinal sensory endings in the ventricles. Conversely, ETB receptors do not appear to play a role in the ischaemia-mediated activation of cardiac sympathetic sensory afferents.
The 21-amino acid peptide endothelin (ET) was discovered 20 years ago (Yanagisawa et al. 1988). Since then, three ET isoforms have been identified (ET-1, ET-2, and ET-3), although ET-1 is the predominant and most potent isoform acting on the cardiovascular and other systems (Yasuda et al. 1990; Maguire & Davenport, 1995; Brehm et al. 1998). ET-1 is synthesized and released by various cells, including endothelial cells, epithelia, cardiomyocytes, leukocytes and macrophages (Hans et al. 2009). The concentration of plasma endothelin-1 is dramatically increased in patients during clinical myocardial ischaemic conditions, including both unstable angina and myocardial infarction, as well as provoked myocardial ischaemia in experimental animals (Qiu et al. 1993; Tonnessen et al. 1993; Wieczorek et al. 1994; Vojacek et al. 1999). For instance, investigators have observed that the plasma ET-1 concentration of patients rises sharply from 0.62 ± 0.56 to 4.95 ± 0.78 pg ml−1 after the onset of myocardial infarction (Stewart et al. 1991) and in unstable angina (Qiu et al. 1993). A brief period of myocardial ischaemia also increases endothelin in cardiac venous plasma draining ischaemic myocardium of swine (Tonnessen et al. 1993). In addition, bothhypoxia and thrombin, which are increased by ischaemia, induce the release of endothelin from the endothelial cells (Schini et al. 1989). In aggregate, these data indicate that myocardial ischaemia increases the concentration of endothelin in coronary circulation and myocardial tissue. Thus, it has been suggested that chest pain in patients with variant angina that is triggered by coronary spasm provoked by increased endothelin related to direct afferent stimulation, in addition to the ET-induced spasm (Toyo-oka et al. 1991).
The influence of endothelin on the cardiovascular system as a potent stimulus of vascular smooth muscle has been studied extensively (Maguire & Davenport, 1995; Black et al. 2003; Bohm & Pernow, 2007). In contrast, the role of ET-1 with respect to its action on the visceral sensory nervous system remains poorly studied. Recently, studies have evaluated the effects of ET-1 on the somatic sensory nerves. In this regard, injection of ET-1 into the plantar footpad of rats causes behaviours consistent with overt pain (e.g. hindpaw flinching) at high concentrations (>30 μm, (Gokin et al. 2001; Khodorova et al. 2002) and sensitizes the paw (e.g. tactile allodynia) to lower concentrations (30 nm–10 um) (Piovezan et al. 1998; Balonov et al. 2006). Furthermore, somatic sensory nerve group III and IV fibres are excited by ET-1 injected into their receptive fields in a dose-dependent manner (Gokin et al. 2001). Application of ET-1 (100 nm) to the DRG neurons of rats activates a rapid inward current with a wide range of amplitudes at −60 mV (Plant et al. 2007). ET-1 also potentiates the capsaicin-activated current in DRG neurons of rats. Additionally, ET-1 may stimulate visceral sensory nerves since a single intraperitoneal (i.p.) injection of ET-1 into mice induces constriction of abdominal musculature, a nociceptive behavioural response that occurs within 15 min (Raffa et al. 1996). These studies suggest that ET-1 has the potential to act on visceral sensory nerves, although until the current study there has been no direct proof.
ET-1 exerts its actions in mammalian species by binding to two G protein linked transmembrane receptors, ETAR and ETBR (Hans et al. 2009). In the nervous system, activation of ETAR generally excites peripheral sensory nerves, whereas stimulation of ETBR induces a variable response (see below). In this respect, injection of ET-1 into the knee joint of naive rats causes nociceptive responses, an effect that can be halved by intra-articular injection of the ETAR antagonist BQ-123. Conversely, this response is not affected by the ETBR antagonist BQ-788 (De-Melo et al. 1998a; Bohm & Pernow, 2007), suggesting that these ETB receptors are not involved in somatic nociception. BQ-123 also blocks ET-1 induced activation of group III and IV fibres when co-injected with ET-1 into the hindpaw of rats (Gokin et al. 2001). In the acute and chronic inflammatory animal models, intraplantar injection of BQ-123 but not BQ-788 inhibits thermal hyperalgesia in the injected hindpaw (Baamonde et al. 2004). Furthermore, depleting expression of ETAR in sensory nerves, but not in non-neural peripheral tissues of mice, abrogates the nociceptive hypersensitivity caused by ET-1 (Stosser et al. 2010). It is important to note that no previous study has evaluated the influence of endogenous ET-1 during ischaemia. The present study clearly shows that ET-1, acting through stimulation of the ETA receptor, contributes to activation of spinal sensory cardiac afferents during myocardial ischaemia.
Several studies suggest that both endothelin receptor subtypes are present in the central and peripheral nervous system. For instance, immunocytochemical studies have documented the presence of ET-1 and endothelin receptors on neurons in most regions of the human and animal brain involved with regulation of cardiovascular function, including the hypothalamus, midbrain, pons, medulla and spinal cord (Hemsen & Lundberg, 1991; Peters et al. 2003). Peters and colleagues (2003) reported that ETARs are expressed in a subpopulation of primary afferent nerve fibres in laminae I–II of the dorsal horn in the spinal cord, whereas ETBRs are expressed primarily in radial glial cells, a small population of grey and white matter astrocytes. More importantly, although there are no studies in cats, in rat and rabbit DRG ETARs arepresent in the plasma membrane of primary afferent small-, medium-, and large-diameter sensory neurons (Pomonis et al. 2001; Plant et al. 2007). In contrast, ETBRs are expressed primarily in DRG satellite cells. In fact, ETBR immunoreactivity is almost completely absent from DRG neuronal cell bodies. In addition, in rat and rabbit sciatic nerve, there are a large number of ET receptor binding sites distributed along the nerve with dense areas of binding forming intermittent bands through the length of the nerve (Pomonis et al. 2001). Specifically, ETARs are localized on primary afferent fibres, whereas ETBRs are present on ensheathing Schwann cells. Our data indicate that ET-1 excites cardiac sympathetic afferent endings through direct activation of ETARs located on the cardiac afferent endings, assuming that our immunocytochemical data showing the existence of ETAR in cardiac afferent cell bodies located in thoracic DRG can be extrapolated to the axonal nerve endings in the heart. This assumption is likely to be true since previous evidence has shown that vanilloid receptors are located on both DRG and cardiac spinal sensory afferent endings (Zahner et al. 2003).
The role of ETBRs in regulation of peripheral sensory nerve activity is less clear. Several studies have shown that activation of ETBRs induces a nociception and algesia. For example, intraperitoneal injection of ETBR agonist into mice rapidly elicited a nociceptive behavioural response (Raffa et al. 1996). Phenylbenzoquinone (PBQ) commonly is used to induce algesia in experimental animals. Compared with wild-type (+/+) mice, PBQ-induced algesia is reduced by 80% in heterzogous (+/−) ETBR knockout mice and absent in homozygous (−/−) mice (Griswold et al. 1999). Also, blockade of ETBRs with A192621, a selective ETBR antagonist, inhibits PBQ-induced algesia by 74%. Moreover, blockade of ETBRs with BQ-788, a specific ETBR antagonist, inhibits the mechanical nociceptive response induced by intraplantar injection of ETs into the hindpaws of rats (da Cunha et al. 2004). In contrast, a number of other studies have documented that activation of ETBRs provides an anti-nociceptive action or analgesia in rodents (Piovezan et al. 2000; Khodorova et al. 2002). In this regard, blockade of ETBRs with BQ-788 enhances hindpaw flinching frequency (Khodorova et al. 2002; Houck et al. 2004). Application of IRL-1620, a selective ETBR agonist, suppresses somatic C-fibre afferent responses to ET-1. Additionally, in an acute pain model, administration of ET-1 enhanced injection of capsaicin into a mouse hindpaw produced licking behaviour response, but activation of ETBR with IRL-1620 or sarafotoxin failed to modify the nociceptive response to capsaicin (Piovezan et al. 2000). Of course, the issue with most of these studies is that they are based on pharmacology and pharmacologically administered agonists rather than endogenously produced mediators of algesia. In the present study we examined the effect of activation of ETBR on ET-1 mediated increase in cardiac spinal afferent activity as well as the role of this receptor system in mediating the afferent response to endogenously produced ET. We observed that blockade of ETBR with BQ-788 inconsistently altered the response of cardiac afferents to both ET-1 stimulation and to ischaemia. We conclude therefore that, unlike ETARs, ETBRs do not play an important role in excitation of cardiac spinal afferents during ischaemia that is mediated by endogenous endothelin.
There could be interactions between endothelin and reactive oxygen species in excitation of spinal sensory nerve endings innervating the heart during ischaemia and/or reperfusion. In this respect, oxidative stress occurs during ischaemia and reperfusion, as we have shownpreviously (O’Neill et al. 1996). In fact, reactive oxygen species like hydroxyl radicals excite cardiac spinal afferents during myocardial ischaemia and reperfusion (Huang et al. 1995). Thus, both reactive oxygen species and endothelin are produced and excite cardiac spinal afferents. However,previous studies have yielded conflicting results. For instance, on one hand reactive oxygen species can increase ET-1 generation from cultured endothelial and vascular smooth cells (Yura et al. 1999; Kahler et al. 2001; Ruef et al. 2001). Conversely, hydrogen peroxide decreases ET-1 release and down-regulates ET-1 mRNA levels (Saito et al. 2001). In addition, endothelin may interact with reactive oxygen species leading to deoxycorticosterone acetate-salt hypertension by increasing the production of vascular superoxide (Li et al. 2003a,b;). Therefore, although it is possible that endothelin may interact with reactive oxygen species in their action on cardiac afferents during ischaemia or reperfusion, they potentially could do so in a synergistic, additive or even a subtractive manner, as we have shown previously with other mediators (Fu & Longhurst, 2005, 2010). These possibilities require further investigation.
We considered the possibility that the neuronal actions of ET-1 on cardiac spinal afferents may be secondary to the local action of ET-1 on coronary arterial smooth muscle-induced vasoconstriction and ischaemia, since endothelin is foremost a molecule that induces potent vasoconstriction (Maguire & Davenport, 1995; Bohm & Pernow, 2007). Thus, ET-1 might stimulate vascular smooth muscle to contract to increase blood pressure and thereby stimulate mechanosensitive cardiac afferent endings. However, the majority of afferents studied were not mechanosensitive. In fact, administration of ET-1 increased mean blood pressure to 113 ± 6 mmHg. However, BP elevations to this level during aortic constriction did not alter afferent activity of the ischaemia- and mechano-sensitive group of afferents in the present study. Hence, we do not believe that endothelin acted through stimulation of mechanoreceptors. Secondly, endothelin could induce myocardial ischaemia and through the generation of chemical mediators, which, in turn, activate cardiac spinal afferents. In this regard, others (Igarashi et al. 1989) previously observed that intracoronary administration of 0.25 μg (e.g. 100 pmol) of endothelin reduces coronary blood flow of canine by only 6–12% 1–5 min after injection, which is insufficient to induce myocardial ischaemia. In the present study we injected 1–4 μg of ET-1 into the left atrium. By the time this dose of ET-1 reached the coronary vasculature only a small portion (5%) of the injected ET-1 would be delivered to the coronary circulation since 5% of cardiac output is distributed to the coronary circulation. Thus, only approximately 0.05–0.2 μg of ET-1 reached the coronary circulation, an amount that would be insufficient to induce myocardial ischaemia. In addition, we have shown that ETA receptors are located on the cell membrane of cardiac afferent neurons. As such, we believe that ET-1 most likely stimulates cardiac afferents directly through an ETAR mechanism on the nerve endings rather than acting through the generation of other ischaemic mediators, such as serotonin or bradykinin.
In summary, using a combined neurophysiological and immunocytochemical approach, the present study provides the first evidence demonstrating that endogenous ET-1 contributes to excitation of cardiac spinal afferents during myocardial ischaemia through direct activation of ETA receptors located on cardiac spinal sensory nerves. This information extends our understanding of the role of endogenous ET-1 in pathophysiological responses to myocardial ischaemia. These new findings may help physicians developing strategies to treat angina pectoris by incorporating blockade of ETA receptors with selective antagonists in the future. Such therapy could relieve cardiac nociception and the associated excitatory cardiac cardiovascular reflex responses, thereby lessening not only the pain but the demand for oxygen to help sparemyocardium.
Acknowledgments
We gratefully acknowledge the technical assistance of Mrs Rainier Cabatbat and Alvin Nguyen. We also thank undergraduate students Rex Sanathra and Sherwin Barvarz for help with experimental procedures. This study was supported by National Institutes of Health HL-66217, the Larry K. Dodge and Susan Samueli Endowed Chairs (J.L.).
Glossary
Abbreviations
- BK
bradykinin
- CSN
cardiac sensory neuron
- CV
conduction velocity
- DiI
1,1′-dioctadecyl-3,3′,3′-tetramethylindocarbocyanine perchlorate
- DRG
dorsal root ganglion
- ET-1
endothelin 1
- ETAR
endothelin A receptor
- ETBR
endothelin B receptor
- LA
left atrium
Author contributions
L-W.F. contributed to conception and design of the study, writing and revising the manuscript, and collection, analysis and interpretation of data. Z-L.G. contributed to partial design of the study, writing a part of the manuscript, and collection, analysis and interpretation of partial data. J.L. contributed to conception of the study, analysis and interpretation of data, and revising the manuscript. All authors have approved the final version of the manuscript. The experiments were performed at Medical Sciences I, school of Medicine, University of California, Irvine.
References
- Baamonde A, Lastra A, Villazon M, Bordallo J, Hidalgo A, Menendez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–251. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- Baker D, Coleridge H, Coleridge J, Nerdrum T. Search for a cardiac nociceptor: Stimulation by bradykinin of sympathetic afferent nerve endings in the heart of the cat. J Physiol. 1980;306:519–536. doi: 10.1113/jphysiol.1980.sp013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Exp Biol Med. 2006;231:1165–1170. [PubMed] [Google Scholar]
- Black SM, Mata-Greenwood E, Dettman RW, Ovadia B, Fitzgerald RK, Reinhartz O, Thelitz S, Steinhorn RH, Gerrets R, Hendricks-Munoz K, Ross GA, Bekker JM, Johengen MJ, Fineman JR. Emergence of smooth muscle cell endothelin B-mediated vasoconstriction in lambs with experimental congenital heart disease and increased pulmonary blood flow. Circulation. 2003;108:1646–1654. doi: 10.1161/01.CIR.0000087596.01416.2F. [DOI] [PubMed] [Google Scholar]
- Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Brehm BR, Buttcher E, Beyer ME, Hoffmeister HM. Comparison of curculating endothelin-1 and big endothelin-1 levels in unstable versus stable angina pectoris. J Cardiovasc Pharmacol. 1998;31:S90–S93. doi: 10.1097/00005344-199800001-00028. [DOI] [PubMed] [Google Scholar]
- da Cunha JM, Rae GA, Ferreira SH, Cunha FQ. Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase C. Eur J Pharmacol. 2004;501:87–94. doi: 10.1016/j.ejphar.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Davar G, Hans G, Fareed MU, Sinnott C, Strichartz GR. Behavioural signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9:2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- De-Melo JD, Tonussi CR, Orleans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998a;77:261–269. doi: 10.1016/S0304-3959(98)00098-0. [DOI] [PubMed] [Google Scholar]
- De-Melo JD, Tonussi CR, Orleans-Juste P, Rae GA. Effects of endothelin-1 on inflammatory incapacitation of the rat knee joint. J Cardiovasc Pharmacol. 1998b;31:S518–S520. doi: 10.1097/00005344-199800001-00149. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13:S220–S222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- Fu L-W, Longhurst JC. Role of activated platelets in excitation of cardiac afferents during myocardial ischemia in cats. Am J Physiol Heart Circ Physiol. 2002;282:H100–H109. doi: 10.1152/ajpheart.2002.282.1.H100. [DOI] [PubMed] [Google Scholar]
- Fu L-W, Longhurst JC. Interactions between histamine and bradykinin in stimulation of ischaemically sensitive cardiac afferents in felines. J Physiol. 2005;565:1007–1017. doi: 10.1113/jphysiol.2005.084004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L-W, Longhurst JC. Regulation of cardiac afferent excitability in ischemia. Handb Exp Pharmacol. 2009:185–225. doi: 10.1007/978-3-540-79090-7_6. [DOI] [PubMed] [Google Scholar]
- Fu L-W, Longhurst J. Bradykinin and thromboxane A2 reciprocally interact to synergistically stimulate cardiac spinal afferents during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:H235–244. doi: 10.1152/ajpheart.00782.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L-W, Phan A, Longhurst JC. Myocardial ischemia-mediated excitatory reflexes: a new function for thromboxane A2? Am J Physiol Heart Circ Physiol. 2008a;295:H2530–H2540. doi: 10.1152/ajpheart.00790.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LW, Guo ZL, Longhurst JC. Undiscovered role of endogenous TxA2 in activation of cardiac sympathetic afferents during ischemia. J Physiol. 2008b;586:3287–3300. doi: 10.1113/jphysiol.2007.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Longhurst J. Endothelin stimulates cardiac sympathetic afferents during myocardial ischemia. FASEB. 2007;21:909.6. [Google Scholar]
- Gokin AP, Fareed MU, Pan H-L, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behaviour and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–5366. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold DE, Douglas SA, Martin LD, Davis TG, Davis L, Ao Z, Luttmann MA, Pullen M, Nambi P, Hay DW, Ohlstein EH. Endothelin B receptor modulates inflammatory pain and cutaneous inflammation. Mol Pharmacol. 1999;56:807–812. [PubMed] [Google Scholar]
- Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioural study. Neurosci Lett. 2007;418:117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Hans G, Schmidt BL, Strichartz G. Nociceptive sensitization by endothelin-1. Brain Res Rev. 2009;60:36–42. doi: 10.1016/j.brainresrev.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Hemsen A, Lundberg JM. Presence of endothelin-1 and endothelin-3 in peripheral tissues and central nervous system of the pig. Regul Pept. 1991;36:71–83. doi: 10.1016/0167-0115(91)90196-n. [DOI] [PubMed] [Google Scholar]
- Hori S, Komatsu Y, Shigemoto R, Mizuno N, Nakanishi S. Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology. 1992;130:1885–1895. doi: 10.1210/endo.130.4.1312429. [DOI] [PubMed] [Google Scholar]
- Houck CS, Khodorova A, Reale AM, Strichartz GR, Davar G. Sensory fibres resistant to the actions of tetrodotoxin mediate nocifensive responses to local administration of endothelin-1 in rats. Pain. 2004;110:719–726. doi: 10.1016/j.pain.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Huang H-S, Pan H-L, Stahl G, Longhurst J. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am J Physiol Heart Circ Physiol. 1995;269:H888–H901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Aizawa Y, Tamura M, Ebe K, Yamaguchi T, Shibata A. Vasoconstrictor effect of endothelin on the canine coronary artery: is a novel endogenous peptide involved in regulating myocardial blood flow and coronary spasm? Am Heart J. 1989;118:674–678. doi: 10.1016/0002-8703(89)90578-4. [DOI] [PubMed] [Google Scholar]
- Kahler J, Ewert A, Weckmuller J, Stobbe S, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol. 2001;38:49–57. doi: 10.1097/00005344-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85:839–846. [PubMed] [Google Scholar]
- Khodorova A, Fareed MU, Gokin AP, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci. 2002;22:7788–7796. doi: 10.1523/JNEUROSCI.22-17-07788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DC, Oravitz JJ, DeGroat WC. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res. 1984;321:111–118. doi: 10.1016/0006-8993(84)90686-3. [DOI] [PubMed] [Google Scholar]
- Li L, Chu Y, Fink GD, Engelhardt JF, Heistad DD, Chen AF. Endothelin-1 stimulates arterial VCAM-1 expression via NADPH oxidase-derived superoxide in mineralocorticoid hypertension. Hypertension. 2003a;42:997–1003. doi: 10.1161/01.HYP.0000095980.43859.59. [DOI] [PubMed] [Google Scholar]
- Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelinA-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003b;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliani A, Lombardi F, Pagani M. Functions of afferents in cardiovascular sympathetic nerves. J Auton Nerv Syst. 1981;3:231–236. doi: 10.1016/0165-1838(81)90065-5. [DOI] [PubMed] [Google Scholar]
- Masaki T, Vane JR, VanHoutte PM. International Union of Pharmacology nomenclature of endothelin receptors. Pharmacol Rev. 1994;46:137–142. [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501–524. doi: 10.1016/0306-4522(92)90398-l. [DOI] [PubMed] [Google Scholar]
- Milner P, Loesch A, Burnstock G. Neural endothelin in hypertension: increased expression in ganglia and nerves to cerebral arteries of the spontaneously hypertensive rat. J Vasc Res. 2000;37:39–49. doi: 10.1159/000025712. [DOI] [PubMed] [Google Scholar]
- O’Neill CA, Fu L-W, Halliwell B, Longhurst JC. Hydroxyl radical production during myocardial ischemia and reperfusion in cats. Am J Physiol Heart Circ Physiol. 1996;271:H660–H667. doi: 10.1152/ajpheart.1996.271.2.H660. [DOI] [PubMed] [Google Scholar]
- Pan H-L, Longhurst JC, Eisenach JC, Chen S-R. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischemia. J Physiol. 1999;518:857–866. doi: 10.1111/j.1469-7793.1999.0857p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H-L, Longhurst J. Lack of a role of adenosine in activation of ischemically sensitive cardiac sympathetic afferents in cats. Am J Physiol Heart Circ Physiol. 1995;269:H106–H113. doi: 10.1152/ajpheart.1995.269.1.H106. [DOI] [PubMed] [Google Scholar]
- Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol. 2003;180:1–13. doi: 10.1016/s0014-4886(02)00023-7. [DOI] [PubMed] [Google Scholar]
- Piovezan AP, D’Orleans-Juste P, Souza GE, Rae GA. Endothelin-1-induced ETA receptor-mediated nociception, hyperalgesia and oedema in the mouse hind paw: modulation by simultaneous ETB receptor activation. Br J Pharmacol. 2000;129:961–968. doi: 10.1038/sj.bjp.0703154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovezan AP, D’Orleans-Juste P, Tonussi CR, Rae GA. Effects of endothelin-1 on capsaicin induced nociception in mice. Eur J Pharmacol. 1998;12:15–22. doi: 10.1016/s0014-2999(98)00281-7. [DOI] [PubMed] [Google Scholar]
- Plant TD, Zollner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain. 2007;3:35. doi: 10.1186/1744-8069-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21:999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Theroux P, Marcil M, Solymoss BC. Plasma endothelin-1 levels in stable and unstable angina. Cardiology. 1993;82:12–19. doi: 10.1159/000175848. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Schupsky JJ, Jacoby HI. Endothelin-induced nociception in mice: mediation by ETA and ETB receptors. J Pharmacol Exp Ther. 1996;276:647–651. [PubMed] [Google Scholar]
- Ruef J, Moser M, Kubler W, Bode C. Induction of endothelin-1 expression by oxidative stress in vascular smooth muscle cells. Cardiovasc Pathol. 2001;10:311–315. doi: 10.1016/s1054-8807(01)00095-3. [DOI] [PubMed] [Google Scholar]
- Saito T, Itoh H, Chun TH, Fukunaga Y, Yamashita J, Doi K, Tanaka T, Inoue M, Masatsugu K, Sawada N, Sakaguchi S, Arai H, Mukoyama M, Tojo K, Hosoya T, Nakao K. Coordinate regulation of endothelin and adrenomedullin secretion by oxidative stress in endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281:H1364–H1371. doi: 10.1152/ajpheart.2001.281.3.H1364. [DOI] [PubMed] [Google Scholar]
- Schini VB, Hendrickson H, Heublein DM, Burnett JC, Jr, VanHoutte PM. Thrombin enhances the release of endothelin from cultured porcine aortic endothelial cells. Eur J Pharmacol. 1989;165:333–334. doi: 10.1016/0014-2999(89)90733-4. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Kubac G, Costello KB, Cernacek P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J Am Coll Cardiol. 1991;18:38–43. doi: 10.1016/s0735-1097(10)80214-1. [DOI] [PubMed] [Google Scholar]
- Stosser S, Agarwal N, Tappe-Theodor A, Yanagisawa M, Kuner R. Dissecting the functional significance of endothelin A receptors in peripheral nociceptors in vivo via conditional gene deletion. Pain. 2010;148:206–214. doi: 10.1016/j.pain.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Tonnessen T, Naess PA, Kirkeboen KA, Offstad J, Ilebekk A, Christensen G. Release of endothelin from the porcine heart after short term coronary artery occlusion. Cardiovasc Res. 1993;27:1482–1485. doi: 10.1093/cvr/27.8.1482. [DOI] [PubMed] [Google Scholar]
- Toyo-oka T, Aizawa T, Suzuki N, Hirata Y, Miyauchi T, Shin WS, Yanagisawa M, Masaki T, Sugimoto T. Increased plasma level of endothelin-1 and coronary spasm induction in patients with vasospastic angina pectoris. Circulation. 1991;83:476–483. doi: 10.1161/01.cir.83.2.476. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Murao S. Excitation of afferent cardiac sympathetic nerve fibres during coronary occlusion. Am J Physiol. 1974;226:1094–1099. doi: 10.1152/ajplegacy.1974.226.5.1094. [DOI] [PubMed] [Google Scholar]
- Vojacek J, Kolar J, Lisy O, Hrabos V, Simek S, Jindra A, Jr, Jachymova M. Time course of endothelin-1 plasma level in patients with acute coronary syndromes. Cardiology. 1999;91:114–118. doi: 10.1159/000006890. [DOI] [PubMed] [Google Scholar]
- Wieczorek I, Haynes WG, Webb DJ, Ludlam CA, Fox KA. Raised plasma endothelin in unstable angina and non-Q wave myocardial infarction: relation to cardiovascular outcome. Br Heart J. 1994;72:436–441. doi: 10.1136/hrt.72.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent casoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Kohno M, Tahara A, Itagane H, Toda I, Akioka K, Teragaki M, Oku H, Takeuchi K, Takeda T. Circulating immunoreactive endothelin in ischemic heart disease. Am Heart J. 1990;119:801–806. doi: 10.1016/s0002-8703(05)80315-1. [DOI] [PubMed] [Google Scholar]
- Yura T, Fukunaga M, Khan R, Nassar GN, Badr KF, Montero A. Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int. 1999;56:471–478. doi: 10.1046/j.1523-1755.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li D-P, Chen S-R, Pan H-L. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]


