Abstract
Heart failure (HF) patients have a reduced cardiac reserve and increased work of breathing. Increased locomotor muscle blood flow demand may result in competition between respiratory and locomotor vascular beds. We hypothesized that HF patients would demonstrate improved locomotor blood flow with respiratory muscle unloading during activity. Ten patients (ejection fraction = 31 ± 3%) and 10 controls (CTL) underwent two cycling sessions (60% peak work). Session 1 (S1): 5 min of normal breathing (NB), 5 min respiratory muscle unloading with a ventilator, and 5 min of NB. Session 2 (S2): 5 min NB, 5 min of respiratory muscle loading with inspiratory resistance, and 5 min of NB. Measurements included: leg blood flow (LBF, thermodilution), cardiac output 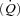 , and oesophageal pressure (Ppl, index of pleural pressure). S1: Ppl was reduced in both groups (HF: 73 ± 8%; CTL: 60 ± 13%, P < 0.01). HF:
, and oesophageal pressure (Ppl, index of pleural pressure). S1: Ppl was reduced in both groups (HF: 73 ± 8%; CTL: 60 ± 13%, P < 0.01). HF: 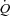 increased (9.6 ± 0.4 vs. 11.3 ± 0.8 l min−1, P < 0.05) and LBF increased (4.8 ± 0.8 vs. 7.3 ± 1.1 l min−1, P < 0.01); CTL: no changes in
increased (9.6 ± 0.4 vs. 11.3 ± 0.8 l min−1, P < 0.05) and LBF increased (4.8 ± 0.8 vs. 7.3 ± 1.1 l min−1, P < 0.01); CTL: no changes in 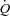 (14.7 ± 1.0 vs. 14.8 ± 1.6 l min−1) or LBF (10.9 ± 1.8 vs. 10.3 ± 1.7 l min−1). S2: Ppl increased in both groups (HF: 172 ± 16%, CTL: 220 ± 40%, P < 0.01). HF: no change was observed in
(14.7 ± 1.0 vs. 14.8 ± 1.6 l min−1) or LBF (10.9 ± 1.8 vs. 10.3 ± 1.7 l min−1). S2: Ppl increased in both groups (HF: 172 ± 16%, CTL: 220 ± 40%, P < 0.01). HF: no change was observed in 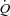 (10.0 ± 0.4 vs. 10.3 ± 0.8 l min−1) or LBF (5.0 ± 0.6 vs. 4.7 ± 0.5 l min−1); CTL:
(10.0 ± 0.4 vs. 10.3 ± 0.8 l min−1) or LBF (5.0 ± 0.6 vs. 4.7 ± 0.5 l min−1); CTL: 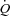 increased (15.4 ± 1.4 vs. 16.9 ± 1.5 l min−1, P < 0.01) and LBF remained unchanged (10.7 ± 1.5 vs. 10.3 ± 1.8 l min−1). These data suggest HF patients preferentially steal blood flow from locomotor muscles to accommodate the work of breathing during activity. Further, HF patients are unable to vasoconstrict locomotor vascular beds beyond NB when presented with a respiratory load.
increased (15.4 ± 1.4 vs. 16.9 ± 1.5 l min−1, P < 0.01) and LBF remained unchanged (10.7 ± 1.5 vs. 10.3 ± 1.8 l min−1). These data suggest HF patients preferentially steal blood flow from locomotor muscles to accommodate the work of breathing during activity. Further, HF patients are unable to vasoconstrict locomotor vascular beds beyond NB when presented with a respiratory load.
Introduction
Patients with heart failure (HF) are often limited in their activities by symptoms of dyspnoea and fatigue. Accordingly, exercise intolerance is a hallmark of symptomatic HF. Due to the pathophysiological sequelae of HF, initial studies attempted to link exercise capacity with measures of ventricular function (i.e. left ventricular ejection fraction (LVEF), left ventricular dimensions and cardiac index). These studies demonstrated little relationship between cardiac function and exercise tolerance in HF patients (Franciosa et al. 1979; Weber et al. 1984; Szlachcic et al. 1985; Pina et al. 1993).
While limited cardiac function is clearly an initiating process, HF becomes a systemic illness that impacts multiple organ systems. One system particularly influenced is the pulmonary system. The pulmonary system is intimately linked with the cardiovascular system anatomically and haemodynamically and plays a significant role in exercise intolerance through a number of mechanisms (Olson et al. 2006a,b;). One understudied mechanism is the work and cost associated with breathing. Independent of smoking history, patients with HF often develop mild ventilation/perfusion (VA/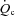 ) inhomogeneities, have increased ventilatory drive contributing to chronic mild hyperventilation in the setting of restrictive and obstructive lung changes, and develop alveolar–capillary diffusion abnormalities which alters gas exchange efficiency (Light & George, 1983; Wright et al. 1990; Dimopoulou et al. 1998; Johnson et al. 2000a; Johnson, 2000, 2001; Agostoni et al. 2002). These changes result in a high work and cost of breathing which is exacerbated during activities of daily living or moderate exercise intensities. This is particularly concerning when coupled with a severely blunted ability to augment cardiac output. A known compensatory mechanism is a high degree of vasoconstriction throughout the circulatory system in an attempt to adequately redistribute blood flow to working locomotor muscles (Zelis et al. 1981; Vanhoutte, 1983).
) inhomogeneities, have increased ventilatory drive contributing to chronic mild hyperventilation in the setting of restrictive and obstructive lung changes, and develop alveolar–capillary diffusion abnormalities which alters gas exchange efficiency (Light & George, 1983; Wright et al. 1990; Dimopoulou et al. 1998; Johnson et al. 2000a; Johnson, 2000, 2001; Agostoni et al. 2002). These changes result in a high work and cost of breathing which is exacerbated during activities of daily living or moderate exercise intensities. This is particularly concerning when coupled with a severely blunted ability to augment cardiac output. A known compensatory mechanism is a high degree of vasoconstriction throughout the circulatory system in an attempt to adequately redistribute blood flow to working locomotor muscles (Zelis et al. 1981; Vanhoutte, 1983).
It has been suggested that the diaphragm will preferentially steal blood flow from working locomotor muscles during increased activity (Bradley & Leith, 1978; Musch, 1993). In healthy adults, the cost of breathing is <5% of the total oxygen consumption at low level exercise but approaches 15% during heavy exercise in young athletes or older fit subjects (Aaron et al. 1992; Dempsey & Johnson, 1992). Further, a reflex vasoconstriction of the locomotor muscles is evident when a substantial respiratory load is applied sufficient to elicit diaphragm fatigue (Sheel et al. 2002).
Therefore, the aim of this study was to determine the relationship between the work of breathing and leg blood flow during moderate intensity exercise in HF patients. We hypothesized that the normal work of breathing during exercise results in a blood flow redistribution away from the locomotor skeletal muscles to the respiratory muscles and that reducing the respiratory muscle work would improve locomotor blood flow. To test this we measured leg blood flow using the thermodilution technique in HF patients with chronic systolic dysfunction under conditions of respiratory muscle unloading and loading during moderate exercise and compared this to matched healthy adults.
Methods
Participant characteristics
Ten HF patients from the Mayo Clinic Heart Failure Service and Cardiovascular Health Clinic and 10 healthy matched control participants (CTL) were recruited (Table 1). Patient inclusion criteria included: history of ischaemic or idiopathic dilated cardiomyopathy, duration of HF symptoms >1 year, stable symptoms >3 months, left ventricular ejection fraction ≤35%, body mass index (BMI) <35 kg m−2, and non-smokers with a smoking history <15 pack-years ((packs smoked per day) × (years as a smoker)) and no clinical diagnosis of chronic obstructive lung disease. Patients were treated with standard optimized medications at the time of the study. Attempts were made to match CTL participants for age and sex. These participants were not on medications, had normal cardiac function with no evidence of exercise-induced ischaemia, BMI <35 kg m−2, were non-smokers, and were without history of hypertension, lung disease, or coronary artery disease.
Table 1.
Participant characteristics
| Healthy control | Heart failure | P value | |
|---|---|---|---|
| Age (years) | 43.2 ± 2.9 | 54.2 ± 5.1 | 0.14 |
| Sex (M/F) | 6/4 | 6/4 | 1.00 |
| Height (cm) | 174.4 ± 2.8 | 172.9 ± 2.7 | 0.69 |
| Weight (kg) | 79.1 ± 3.0 | 87.9 ± 6.1 | 0.21 |
| BMI (kg m−2) | 26.1 ± 1.2 | 29.4 ± 2.0 | 0.17 |
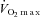 (ml kg−1 min−1) (ml kg−1 min−1) |
34.9 ± 3.0 | 17.1 ± 1.5 | <0.001 |
| LVEF (%) | 30.6 ± 2.6 | ||
| NYHA Class | I = 3 II = 7 | ||
| Aetiology (ischaemic/idiopathic) | 5/5 | ||
| Medications | |||
| ACE inhibitors | 5 (50) | ||
| AII receptor blockers | 4 (40) | ||
| ß-Blockers | 9 (90) | ||
| Digitalis | 3 (30) | ||
| Aspirin | 7 (70) | ||
| Diuretics | 6 (60) | ||
BMI, body mass index; 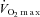 , maximal oxygen consumption; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ACE, angiotensin converting enzyme; AII, angiotensin II. Data are presented as mean ±s.e.m. or number of participants (percentage of population).
, maximal oxygen consumption; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ACE, angiotensin converting enzyme; AII, angiotensin II. Data are presented as mean ±s.e.m. or number of participants (percentage of population).
All participants gave written informed consent after being provided with a description of the study requirements. The protocol was approved by the Mayo Clinic Institutional Review Board; all procedures conformed to the Declaration of Helsinki.
Protocol
All participants underwent 2 days of exercise testing procedures in an environmentally controlled laboratory separated by at least 48 h. Day 1 consisted of a maximal exercise test on an electronically braked cycle ergometer (Corival, Lode Medical Technology, The Netherlands). Day 2 consisted of two submaximal exercise sessions at 60% of peak work at a cadence of 65 r.p.m. For both days, participants were asked to avoid strenuous physical activity for 24 h and refrain from eating or consuming caffeine for 3 h prior to arrival. All ventilatory, gas exchange, heart rate and oxygen saturation data were measured continuously during all exercise sessions. On day 1, participants were verbally encouraged to continue the exercise protocol to maximal exertion, identified as a rating of perceived exertion (RPE) ≥17 on the Borg 6–20 scale or a respiratory exchange ratio (RER) of ≥1.10.
On day 2, all participants completed two steady-state exercise sessions consisting of the following. Session 1: 3 min of resting data collection, 5 min of steady-state submaximal cycle ergometry while breathing normally under room air conditions, 5 min of respiratory muscle unloading with the assistance of a mechanical ventilator during inspiration, and 5 min of breathing normally under room air conditions. Session 2, 3 min of resting data collection, 5 min of breathing normally under room air conditions, 5 min of respiratory muscle loading via inspiratory resistance, and 5 min of breathing normally under room air conditions. The two exercise sessions were separated by 15–20 min of rest.
Measurement of gas exchange, ventilation and cardiac output
Oxygen consumption 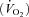 , carbon dioxide production
, carbon dioxide production 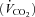 , minute ventilation
, minute ventilation 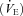 , tidal volume (VT), respiratory rate (RR), inspiratory time (TI), and total respiratory cycle time (TTOT) were measured continuously via a metabolic measurement system through a mouth piece and pneumotachograph while wearing a nose clip (CPX/D, Medical Graphic, St Paul, MN, USA). Manual volume calibration was performed with a 3 litre syringe and gas calibration was performed with manufacturer-recommended gases of known concentration. All calibration procedures were conducted immediately prior to each testing protocol.
, tidal volume (VT), respiratory rate (RR), inspiratory time (TI), and total respiratory cycle time (TTOT) were measured continuously via a metabolic measurement system through a mouth piece and pneumotachograph while wearing a nose clip (CPX/D, Medical Graphic, St Paul, MN, USA). Manual volume calibration was performed with a 3 litre syringe and gas calibration was performed with manufacturer-recommended gases of known concentration. All calibration procedures were conducted immediately prior to each testing protocol.
Cardiac output 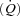 was measured using an open-circuit acetylene wash-in technique previously validated against direct Fick measures (Johnson et al. 2000b). Briefly, the pneumotachograph was connected to a non-rebreathing Y valve with the inspiratory port connected to a pneumatic switching valve which allowed for rapid switching from room air to the
was measured using an open-circuit acetylene wash-in technique previously validated against direct Fick measures (Johnson et al. 2000b). Briefly, the pneumotachograph was connected to a non-rebreathing Y valve with the inspiratory port connected to a pneumatic switching valve which allowed for rapid switching from room air to the 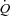 gas mixture (a bag reservoir containing 0.6% C2H2, 21% O2, 9% He, balance N2). Gases were sampled using a mass spectrometer (MGA 1100, Marquette Electronics, Milwaukee, WI, USA) integrated with custom analysis software for the assessment of
gas mixture (a bag reservoir containing 0.6% C2H2, 21% O2, 9% He, balance N2). Gases were sampled using a mass spectrometer (MGA 1100, Marquette Electronics, Milwaukee, WI, USA) integrated with custom analysis software for the assessment of 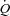 . Stroke volume (SV) was calculated by dividing the
. Stroke volume (SV) was calculated by dividing the 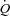 by heart rate (HR).
by heart rate (HR).
Respiratory muscle unloading and loading
A non-invasive ventilatory support system (BiPAP Vision, Respironics, Pittsburg, PA, USA) was used to provide inspiratory support and reduce the work of the respiratory muscles during exercise. Briefly, the ventilator circuit tubing was connected to the inspiratory port of the non-rebreathing Y valve. Each participant was provided with two practice sessions using the ventilator for inspiratory support during exercise. Each participant was verbally coached to relax and allow the ventilator to support their breathing with typical unloading of 10–15 cmH2O of pressure support. Inspiratory loading was accomplished by placing a fixed diameter orifice on the inspiratory port of the non-rebreathing Y valve. The diameter of the orifice was determined during the same practice sessions and chosen to double the negative swing in peak inspiratory pressure.
Measurement of pulmonary pressures
Oesophageal pressure, an index of pleural pressure (Ppl), was measured using a small 10 cm latex balloon attached to PE200 tubing and positioned in the lower one-third of the oesophagus through the nares (approximately 45 cm distal to the nares). The PE200 tubing was connected to a Validyne transducer (MP45, Northridge, CA, USA). Airflow was determined simultaneously with the pressure measurements, and volume was obtained by the digital integration of the flow signal, corrected for drift (Yeh et al. 1982). Signals were digitized and sent to a personal computer via an analog-to-digital converter board and analysed using a software program developed in our laboratory (Beck et al. 1999). Briefly, at rest and during exercise, the last 30 s of each phase of the session was recorded and used for analysis. These data were analysed for invalid breaths (e.g. cough, throat clear, etc.) and both the pressure–time and pressure–tidal volume (work of breathing, Wb) integrals were calculated using the following equations:
Oesophageal pressure–time integral during inspiration
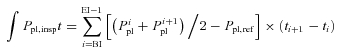 |
Oesophageal pressure–tidal volume integral during inspiration (Wb)
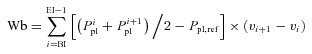 |
The sums of the integrals were taken per breath, the valid breaths were averaged, and these averaged sums were multiplied by the respiratory rate per minute. The oesophageal pressure–tidal volume loops were obtained by taking the tidal volume range for each participant and this was then divided into 50 equal parts to obtain a 50 point loop. After normalization, each of the 50 point loops were averaged (within each group and condition, respectively) to obtain the average oesophageal pressure–volume loop for that group and condition (Fig. 1).
Figure 1. Influence of inspiratory pressure assistance and inspiratory loading on the pleural pressure volume response during steady-state exercise.
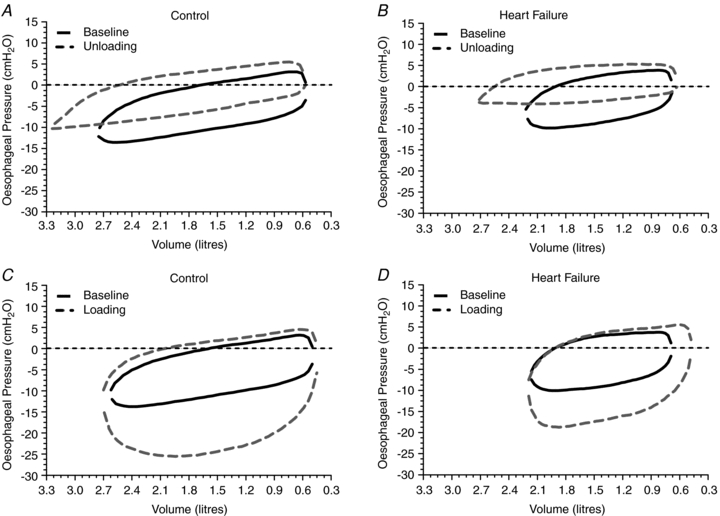
A, inspiratory assistance in CTL participants. B, inspiratory assistance in HF patients. C, inspiratory loading in CTL participants. D, inspiratory loading in HF patients. Baseline measurements represent an average of the normal breathing under room air conditions before and after the experimental condition.
Measurement of leg blood flow and arterial pressure
Leg blood flow (LBF) was measured using the constant infusion thermodilution technique described previously (Andersen & Saltin, 1985; Proctor et al. 1998). Briefly, an 18-gauge, 4.0-French, high-flow catheter was introduced percutaneously into the left femoral vein immediately distal to the inguinal ligament and advanced ∼8 cm toward the heart (Royal Flush Plus Angiographic Catheter, Cook Medical Inc., Bloomington, IN, USA) for venous blood sampling and infusion of iced saline. A second 18-gauge catheter was introduced at the same insertion point and advanced ∼20 cm toward the heart. A thin (0.64 mm diameter) Teflon-coated thermocouple (IT-18, Physi-temp Instruments, Clifton, NJ, USA) was introduced through the second catheter which was removed, leaving the thermistor in place. The infusion catheter and thermistor were secured and placement was not altered between sessions.
Iced saline (∼3–5°C, measured via thermocouple at the catheter inlet) was infused for 15–20 s until femoral vein blood temperature was reduced and stabilized at the lower temperature. The saline infusion rate was adjusted with a roller pump controller to achieve an approximate 1°C drop in femoral vein temperature. The volume of infusate was determined by the slope of the change in the weight of the saline reservoir over time measured via displacement transducer (FT10C, Grass Instruments, Quincy, MA, USA). All data signals were exported to a personal computer outfitted with a digital oscilloscope (PowerLab 16/30 and Chart 5, ADInstruments Inc., Colorado Springs, CO, USA) facilitating real-time observation of each measurement to ensure stabilization of temperature changes. Leg blood flow was calculated using the thermal-balance principle and doubled to provide two-leg blood flow values (l min−1) (Andersen & Saltin, 1985).
A 20-gauge Teflon catheter (FA-04020, Arrow International Inc., Reading, PA, USA) was introduced into the left radial artery for blood sampling and measurement of arterial pressure. Real-time recordings from the radial artery pressure transducer (PX-MK099, Edwards Lifesciences, Irvine, CA, USA) were exported to the same digital oscilloscope described previously. Mean arterial pressure (MAP) represents the true mean of the arterial waveform and leg vascular resistance was calculated as MAP divided by leg blood flow.
Measurement of blood gases and leg oxygen consumption calculation
Duplicate samples of arterial and venous blood were drawn anaerobically over 10–15 s for measurement of the partial pressure of oxygen and carbon dioxide (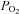 and
and 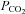 , respectively), haemoglobin (Hgb), and arterial and venous saturation of oxygen (
, respectively), haemoglobin (Hgb), and arterial and venous saturation of oxygen (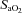 and
and 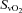 , respectively) (IL-1620, Instrumentation Laboratories, Lexington, MA, USA). Arterial and mixed venous oxygen content (
, respectively) (IL-1620, Instrumentation Laboratories, Lexington, MA, USA). Arterial and mixed venous oxygen content (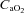 and
and 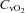 , respectively) were calculated as:
, respectively) were calculated as:
Leg 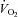 was calculated as LBF multiplied by the difference in arterio-venous oxygen content.
was calculated as LBF multiplied by the difference in arterio-venous oxygen content.
Statistical analysis
Statistical analysis and graphic presentation were accomplished using SPSS (v12.0, Chicago, IL, USA) and GraphPad Prism (v4.0, San Diego, CA, USA). To account for potential cardiovascular drift, percentage change was calculated using the average of normal breathing in room air before and after each experimental condition (Tables 3 and 4). Differences between specific means were evaluated using Student's t tests. Sex was analysed using Fisher's exact test. Analysis of variance (ANOVA) with repeated measures was used to compare means within groups over time. Bonferroni post hoc analyses were applied when the main effect was significant. Statistical significance was set at an α level of 0.05 for all analyses. Data are presented as mean ± standard error of the mean (s.e.m.) unless otherwise indicated.
Table 3.
Physiological impact of acute inspiratory pressure unloading
| Healthy control %Δ | Heart failure %Δ | P value | |
|---|---|---|---|
| Pulmonary pressures | |||
| Wb | −56.7 ± 12.6 | −55.5 ± 12.0 | 0.95 |
| Ppl mean insp | −38.9 ± 7.0 | −46.8 ± 6.5 | 0.15 |
| ∫Ppl,inspt | −59.9 ± 13.0 | −73.2 ± 7.5 | 0.41 |
| ∫Ppl,totalt | −130.2 ± 36.2 | −190 ± 27.8 | 0.24 |
| Haemodynamics | |||
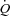 |
−3.0 ± 6.1 | 17.1 ± 5.2 | 0.02 |
| SV | −5.6 ± 6.4 | 13.0 ± 5.0 | 0.03 |
| HR | 2.8 ± 0.8 | 3.7 ± 3.0 | 0.78 |
| SBP | 1.4 ± 2.1 | −3.6 ± 1.7 | 0.10 |
| DBP | 0.5 ± 1.6 | −0.8 ± 1.6 | 0.58 |
| MAP | 1.0 ± 1.9 | −2.0 ± 1.6 | 0.26 |
| LBF | −2.4 ± 2.1 | 44.1 ± 5.9 | <0.0001 |
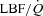 |
7.0 ± 7.7 | 25.4 ± 8.1 | 0.04 |
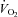 |
1.1 ± 2.6 | 46.2 ± 5.7 | <0.0001 |
| LVR | 3.7 ± 2.1 | −32.7 ± 2.0 | <0.0001 |
| Blood gases | |||
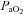 |
−0.3 ± 2.5 | 7.6 ± 1.9 | 0.03 |
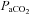 |
−0.4 ± 2.8 | −3.1 ± 2.0 | 0.45 |
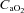 |
1.2 ± 0.8 | −1.4 ± 1.1 | 0.08 |
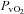 |
−3.6 ± 1.4 | −1.5 ± 2.0 | 0.42 |
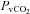 |
−0.7 ± 1.8 | −1.8 ± 2.1 | 0.69 |
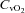 |
−5.0 ± 1.9 | −0.8 ± 3.1 | 0.28 |
| a–v O2 diff | 3.6 ± 1.1 | −0.8 ± 1.3 | 0.03 |
| Ventilation | |||
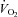 |
−1.9 ± 6.1 | 18.3 ± 5.7 | 0.04 |
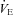 |
23.0 ± 6.5 | 32.3 ± 7.5 | 0.36 |
| VT | 25.3 ± 7.0 | 29.0 ± 4.3 | 0.66 |
| RR | −0.3 ± 5.8 | 2.9 ± 4.9 | 0.68 |
| TI | 10.0 ± 8.2 | −9.7 ± 8.6 | 0.98 |
| TTOT | −2.1 ± 8.0 | −1.4 ± 6.0 | 0.74 |
Wb, work of breathing; Ppl, pleural pressure; insp, inspiratory; 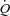 , cardiac output; SV, stroke volume; HR, heart rate; SBP, systolic blood pressure (direct arterial); DBP, diastolic blood pressure (direct arterial); MAP, mean arterial pressure (direct arterial); LBF, leg blood flow;
, cardiac output; SV, stroke volume; HR, heart rate; SBP, systolic blood pressure (direct arterial); DBP, diastolic blood pressure (direct arterial); MAP, mean arterial pressure (direct arterial); LBF, leg blood flow; 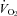 , oxygen consumption; LVR, leg vascular resistance;
, oxygen consumption; LVR, leg vascular resistance; 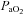 , partial pressure of arterial oxygen;
, partial pressure of arterial oxygen; 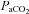 , partial pressure of arterial carbon dioxide;
, partial pressure of arterial carbon dioxide; 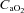 , arterial oxygen content;
, arterial oxygen content; 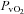 , partial pressure of venous oxygen;
, partial pressure of venous oxygen; 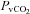 , partial pressure of venous carbon dioxide;
, partial pressure of venous carbon dioxide; 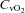 , venous content of oxygen; a–v O2 diff, arterio-venous oxygen difference;
, venous content of oxygen; a–v O2 diff, arterio-venous oxygen difference; 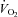 , volume of oxygen consumption;
, volume of oxygen consumption; 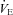 , ventilation; VT, tidal volume; RR, respiratory rate; TI, inspiratory time; TTOT, total respiratory cycle time. Data are presented as mean ±s.e.m.
, ventilation; VT, tidal volume; RR, respiratory rate; TI, inspiratory time; TTOT, total respiratory cycle time. Data are presented as mean ±s.e.m.
Table 4.
Physiological impact of acute inspiratory pressure loading
| Healthy control %Δ | Heart failure %Δ | P value | |
|---|---|---|---|
| Pulmonary pressures | |||
| Wb | 209.9 ± 43.2 | 208.6 ± 23.4 | 0.13 |
| Ppl mean insp | 98.1 ± 14.0 | 89.7 ± 7.8 | 0.62 |
| ∫Ppl,inspt | 219.7 ± 39.5 | 183.0 ± 12.7 | 0.44 |
| ∫Ppl,totalt | 304.5 ± 74.9 | 333.0 ± 72.4 | 0.79 |
| Haemodynamics | |||
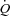 |
9.8 ± 2.3 | 2.1 ± 5.3 | 0.20 |
| SV | 7.9 ± 2.4 | −2.1 ± 6.0 | 0.14 |
| HR | 1.8 ± 0.5 | 4.6 ± 2.2 | 0.23 |
| SBP | 4.3 ± 1.3 | 4.0 ± 1.1 | 0.88 |
| DBP | −0.3 ± 3.3 | 0.2 ± 2.1 | 0.91 |
| MAP | 4.6 ± 1.2 | 2.5 ± 1.2 | 0.25 |
| LBF | 0.3 ± 6.0 | −5.4 ± 6.0 | 0.26 |
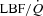 |
−7.9 ± 5.5 | −4.3 ± 7.8 | 0.36 |
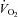 |
−0.8 ± 5.7 | −6.2 ± 5.7 | 0.24 |
| LVR | 6.7 ± 9.1 | 17.6 ± 11.3 | 0.24 |
| Blood gases | |||
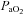 |
−7.9 ± 1.3 | −3.2 ± 1.8 | 0.05 |
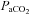 |
7.1 ± 1.6 | 1.9 ± 1.8 | 0.06 |
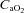 |
−0.7 ± 0.6 | −2.0 ± 0.6 | 0.35 |
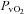 |
1.6 ± 1.0 | −6.9 ± 2.2 | 0.004 |
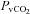 |
3.0 ± 0.9 | 4.4 ± 1.8 | 0.50 |
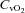 |
1.3 ± 2.5 | −8.8 ± 3.2 | 0.02 |
| a–v O2 diff | −1.0 ± 0.8 | 0.1 ± 1.5 | 0.56 |
| Ventilation | |||
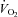 |
8.4 ± 2.7 | 3.7 ± 6.3 | 0.52 |
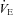 |
−9.1 ± 4.2 | −2.8 ± 5.9 | 0.40 |
| VT | 3.8 ± 7.7 | 15.1 ± 8.1 | 0.33 |
| RR | −9.3 ± 6.7 | −12.9 ± 4.3 | 0.65 |
| TI | 37.0 ± 12.2 | 36.8 ± 11.6 | 0.99 |
| TTOT | 7.6 ± 6.8 | 15.1 ± 7.7 | 0.47 |
Symbols and abbreviations as defined in Table 3. Data are presented as mean ±s.e.m.
Results
Participant characteristics
Participant characteristics and patient medications are reported in Table 1. Despite efforts to match groups on age, the control group was approximately 9 years younger; however, this difference was not statistically significant. Also, there were no differences between the groups for sex, height, weight, or BMI. The HF patients demonstrated lower 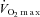 compared to the CTL group (P < 0.001).
compared to the CTL group (P < 0.001).
Pulmonary pressures, haemodynamics and ventilation at rest
Table 2 gives the pulmonary mechanical, haemodynamic and ventilatory measures at rest. There were no differences between the groups for resting measures of mean Ppl during inspiration or the time integral for inspiratory or total Ppl. The HF patients demonstrated lower 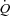 compared to the CTL participants due to a reduced SV. There were no differences between the groups for arterial blood pressure. Despite a trend towards an increased RR, the HF patients had similar
compared to the CTL participants due to a reduced SV. There were no differences between the groups for arterial blood pressure. Despite a trend towards an increased RR, the HF patients had similar 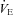 .
.
Table 2.
Resting physiological measurements
| Healthy control | Heart failure | P value | |
|---|---|---|---|
| Pulmonary pressures | |||
| Ppl mean insp (cmH2O) | −6.1 ± 0.8 | −6.1 ± 0.9 | 0.96 |
| ∫Ppl,inspt (cmH2O s min−1) | 83.3 ± 11.9 | 82.7 ± 9.6 | 0.97 |
| ∫Ppl,totalt (cmH2O s min−1) | 90.2 ± 12.8 | 78.9 ± 13.8 | 0.57 |
| Haemodynamics | |||
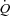 (l min−1) (l min−1) |
6.6 ± 0.5 | 5.0 ± 0.4 | 0.02 |
| SV (ml beat−1) | 98.8 ± 8.1 | 70.1 ± 7.2 | 0.02 |
| HR (beats min−1) | 67.3 ± 2.6 | 72.7 ± 4.0 | 0.27 |
| SBP (mmHg) | 157.9 ± 4.1 | 151.6 ± 6.8 | 0.46 |
| DBP (mmHg) | 88.2 ± 4.3 | 78.9 ± 4.5 | 0.17 |
| MAP (mmHg) | 111.3 ± 4.0 | 103.1 ± 4.5 | 0.20 |
| Blood gases | |||
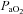 (mmHg) (mmHg) |
101.1 ± 1.8 | 97.3 ± 4.9 | 0.48 |
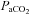 (mmHg) (ml (100 ml)−1) (mmHg) (ml (100 ml)−1) |
37.5 ± 1.3 | 37.2 ± 1.0 | 0.87 |
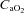 (ml (100 ml)−1) (ml (100 ml)−1) |
18.7 ± 0.5 | 18.3 ± 0.7 | 0.68 |
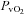 (mmHg) (mmHg) |
30.1 ± 2.6 | 27.2 ± 1.7 | 0.35 |
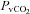 (mmHg) (mmHg) |
48.1 ± 1.9 | 47.8 ± 1.1 | 0.89 |
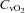 (ml (100 ml)−1) (ml (100 ml)−1) |
9.6 ± 1.0 | 8.3 ± 0.8 | 0.30 |
| a–v O2 diff (ml (100 ml)−1) | 9.0 ± 1.1 | 9.7 ± 1.1 | 0.66 |
| Ventilation | |||
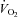 (ml min−1) (ml min−1) |
456.3 ± 89.7 | 396.6 ± 45.0 | 0.56 |
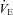 (l min−1) (l min−1) |
13.6 ± 2.5 | 13.4 ± 1.4 | 0.94 |
| VT (ml) | 944.4 ± 108.9 | 830.9 ± 89.4 | 0.43 |
| RR (breaths min−1) | 14.0 ± 0.9 | 16.3 ± 0.9 | 0.08 |
| TI (s) | 2.2 ± 0.2 | 1.9 ± 0.2 | 0.27 |
| TTOT (s) | 4.5 ± 0.3 | 3.7 ± 0.2 | 0.40 |
Ppl, pleural pressure; insp, inspiratory; 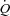 , cardiac output; SV, stroke volume; HR, heart rate; SBP, systolic blood pressure (direct arterial); DBP, diastolic blood pressure (direct arterial); MAP, mean arterial pressure (direct arterial);
, cardiac output; SV, stroke volume; HR, heart rate; SBP, systolic blood pressure (direct arterial); DBP, diastolic blood pressure (direct arterial); MAP, mean arterial pressure (direct arterial); 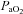 , partial pressure of arterial oxygen;
, partial pressure of arterial oxygen; 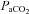 , partial pressure of arterial carbon dioxide;
, partial pressure of arterial carbon dioxide; 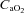 , arterial oxygen content;
, arterial oxygen content; 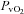 , partial pressure of venous oxygen;
, partial pressure of venous oxygen; 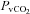 , partial pressure of venous carbon dioxide;
, partial pressure of venous carbon dioxide; 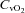 , venous content of oxygen; a–v O2 diff, arterio-venous oxygen difference;
, venous content of oxygen; a–v O2 diff, arterio-venous oxygen difference; 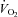 , volume of oxygen consumption;
, volume of oxygen consumption; 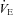 , ventilation; VT, tidal volume; RR respiratory rate; TI, inspiratory time; TTOT, total respiratory cycle time. Data are presented as mean ±s.e.m.
, ventilation; VT, tidal volume; RR respiratory rate; TI, inspiratory time; TTOT, total respiratory cycle time. Data are presented as mean ±s.e.m.
Influence of acute inspiratory pressure unloading during exercise
Pulmonary pressures
Figure 1A and B highlights the influence of acute inspiratory pressure unloading on the pleural pressure–volume response during exercise in both the HF and CTL groups. With unloading, there was a similar increase (less negative) in mean inspiratory Ppl (P < 0.01, both groups) and a similar reduction in the inspiratory Ppl–time integral (P < 0.01, both groups). Further, there was a similar reduction in the total Ppl–time integral (P < 0.01, both groups) (Table 3; see Fig. 2 for absolute changes).
Figure 2. Influence of inspiratory pressure assistance and inspiratory loading on pulmonary pressures during steady-state exercise.
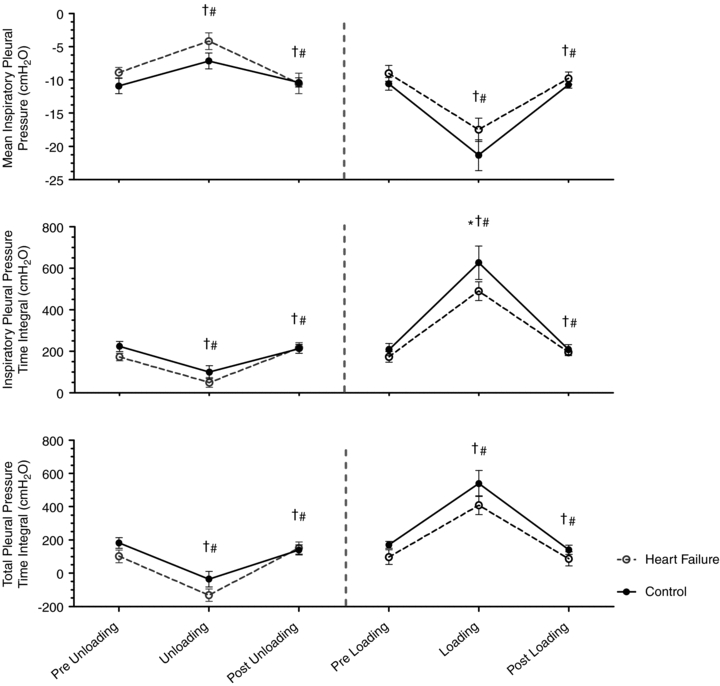
Figures represent absolute values over time for all exercise conditions. * indicates a significant difference between the HF and CTL groups (P < 0.05), # indicates a significant difference from the preceding exercise condition within the CTL group (P < 0.05), and † indicates a significant difference from the preceding exercise condition within the HF group (P < 0.05).
Haemodynamics
With unloading, HF patients had an increase in SV and a rise in 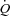 (P= 0.03 and P < 0.01, respectively) (Table 3, see Fig. 3 for absolute changes). There was also an increase in both LBF and
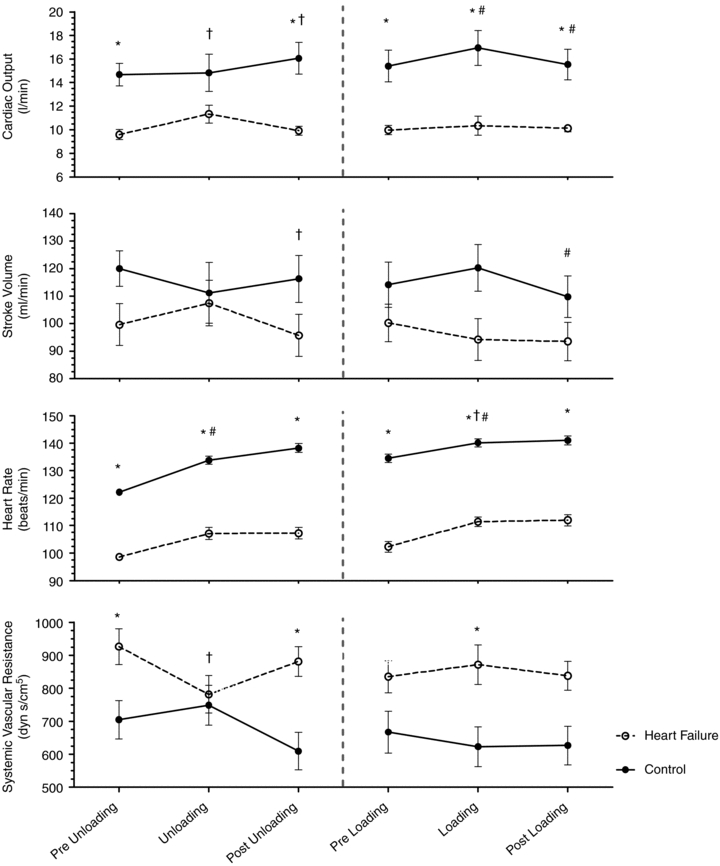
(P= 0.03 and P < 0.01, respectively) (Table 3, see Fig. 3 for absolute changes). There was also an increase in both LBF and 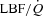 (P < 0.01 and P= 0.02, respectively) with an associated increase in Leg
(P < 0.01 and P= 0.02, respectively) with an associated increase in Leg 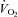 and reduction in leg vascular resistance (LVR) (P < 0.01 for both) (Table 3, see Fig. 4 for absolute changes). In contrast, the CTL group had a slight but non-significant drop in SV with a slight but non-significant reduction in
and reduction in leg vascular resistance (LVR) (P < 0.01 for both) (Table 3, see Fig. 4 for absolute changes). In contrast, the CTL group had a slight but non-significant drop in SV with a slight but non-significant reduction in 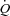 (Table 3; see Fig. 3 for absolute changes). Moreover, there were no changes in LBF,
(Table 3; see Fig. 3 for absolute changes). Moreover, there were no changes in LBF, 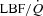 , Leg
, Leg 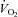 , or LVR in the CTL group (Table 3, see Fig. 4 for absolute changes).
, or LVR in the CTL group (Table 3, see Fig. 4 for absolute changes).
Figure 3. Influence of inspiratory pressure assistance and inspiratory loading on cardiovascular haemodynamics during steady-state exercise.
Figures represent absolute values over time for all exercise conditions. * indicates a significant difference between the HF and CTL groups (P < 0.05), # indicates a significant difference from the preceding exercise condition within the CTL group (P < 0.05), and † indicates a significant difference from the preceding exercise condition within the HF group (P < 0.05).
Figure 4. Influence of inspiratory pressure assistance and inspiratory loading on leg blood flow and haemodynamics during steady-state exercise.
Figures represent absolute values over time for all exercise conditions. * indicates a significant difference between the HF and CTL groups (P < 0.05), # indicates a significant difference from the preceding exercise condition within the CTL group (P < 0.05), and † indicates a significant difference from the preceding exercise condition within the HF group (P < 0.05).
Blood gases
The HF patients had an increase in  (98.9 ± 3.6 vs. 106.2 ± 3.1 mmHg, P < 0.01) with no change in
(98.9 ± 3.6 vs. 106.2 ± 3.1 mmHg, P < 0.01) with no change in 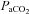 (35.8 ± 1.1 vs. 34.6 ± 0.9 mmHg, P= 0.18), or
(35.8 ± 1.1 vs. 34.6 ± 0.9 mmHg, P= 0.18), or 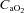 (19.2 ± 1.0 vs. 19.0 ± 1.1 ml (100 ml)−1, P= 0.30) during unloading. Also during unloading, the HF patients had no changes in
(19.2 ± 1.0 vs. 19.0 ± 1.1 ml (100 ml)−1, P= 0.30) during unloading. Also during unloading, the HF patients had no changes in 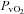 (21.3 ± 1.5 vs. 20.9 ± 1.4 mmHg, P= 0.42),
(21.3 ± 1.5 vs. 20.9 ± 1.4 mmHg, P= 0.42), 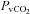 (60.6 ± 1.6 vs. 59.4 ± 1.6 mmHg, P= 0.38), or
(60.6 ± 1.6 vs. 59.4 ± 1.6 mmHg, P= 0.38), or 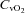 (4.9 ± 0.5 vs. 4.8 ± 0.5 ml (100 ml)−1, P= 0.71). These changes resulted in no change in the arterio-venous oxygen difference (a–v O2 diff) (12.3 ± 1.0 vs. 14.1 ± 1.0 ml (100 ml)−1, P= 0.60). In contrast, the CTL group had no change in
(4.9 ± 0.5 vs. 4.8 ± 0.5 ml (100 ml)−1, P= 0.71). These changes resulted in no change in the arterio-venous oxygen difference (a–v O2 diff) (12.3 ± 1.0 vs. 14.1 ± 1.0 ml (100 ml)−1, P= 0.60). In contrast, the CTL group had no change in 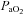 (95.6 ± 2.4 vs. 95.3 ± 3.3 mmHg, P= 0.45),
(95.6 ± 2.4 vs. 95.3 ± 3.3 mmHg, P= 0.45), 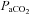 (38.6 ± 1.4 vs. 38.5 ± 1.9 mmHg, P= 0.46), or
(38.6 ± 1.4 vs. 38.5 ± 1.9 mmHg, P= 0.46), or 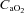 (19.3 ± 0.5 vs. 19.5 ± 0.5 mmHg, P= 0.07). However, during unloading the CTL group had reductions in
(19.3 ± 0.5 vs. 19.5 ± 0.5 mmHg, P= 0.07). However, during unloading the CTL group had reductions in 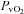 (22.5 ± 0.7 vs. 22.0 ± 0.5 mmHg, P= 0.02) and
(22.5 ± 0.7 vs. 22.0 ± 0.5 mmHg, P= 0.02) and 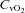 (5.3 ± 0.5 vs. 5.2 ± 0.5 ml (100 ml)−1, P= 0.02) with no change in
(5.3 ± 0.5 vs. 5.2 ± 0.5 ml (100 ml)−1, P= 0.02) with no change in 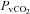 (64.2 ± 2.4 vs. 64.6 ± 2.7 mmHg, P= 0.38). In contrast to the HF group, these changes resulted in an increase in a–v O2 diff (14.0 ± 0.3 vs. 14.4 ± 0.4 ml (100 ml)−1, P < 0.01).
(64.2 ± 2.4 vs. 64.6 ± 2.7 mmHg, P= 0.38). In contrast to the HF group, these changes resulted in an increase in a–v O2 diff (14.0 ± 0.3 vs. 14.4 ± 0.4 ml (100 ml)−1, P < 0.01).
Ventilation
During unloading, the HF patients had an increase in 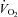 (1268.6 ± 179.9 vs. 1680.5 ± 170.7 ml min−1, P < 0.01). As a result of an increase in VT, HF patients also demonstrated an increase in
(1268.6 ± 179.9 vs. 1680.5 ± 170.7 ml min−1, P < 0.01). As a result of an increase in VT, HF patients also demonstrated an increase in 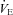 with inspiratory pressure unloading (P < 0.01 for both) with no change in RR. Furthermore, there were no changes in TI or TTOT in the HF patients (Table 3). Similarly, the CTL group also demonstrated an increase in
with inspiratory pressure unloading (P < 0.01 for both) with no change in RR. Furthermore, there were no changes in TI or TTOT in the HF patients (Table 3). Similarly, the CTL group also demonstrated an increase in 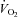 (2149.7 ± 180.6 vs. 2014.0 ± 222.5 ml min−1, P= 0.80). The CTL group also had an increase in
(2149.7 ± 180.6 vs. 2014.0 ± 222.5 ml min−1, P= 0.80). The CTL group also had an increase in 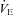 as a result of an increase in VT (P < 0.01 for both) with no change in RR. There was no significant change in TI or TTOT for the CTL group (Table 3).
as a result of an increase in VT (P < 0.01 for both) with no change in RR. There was no significant change in TI or TTOT for the CTL group (Table 3).
During unloading, there was a strong relationship between %Δ in mean inspiratory Ppl and the %Δ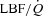 in the HF patients (Fig. 5) suggesting the increase in LBF was associated with a less negative inspiratory Ppl. In contrast, there was no clear relationship between the inspiratory Ppl and
in the HF patients (Fig. 5) suggesting the increase in LBF was associated with a less negative inspiratory Ppl. In contrast, there was no clear relationship between the inspiratory Ppl and 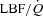 in the CTL group (r=−0.18, P= 0.62).
in the CTL group (r=−0.18, P= 0.62).
Figure 5. Relationship between the percentage change in mean inspiratory Ppl and the percentage change in LBF/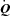 during the transition from baseline exercise to inspiratory assistance in the HF patients.
during the transition from baseline exercise to inspiratory assistance in the HF patients.
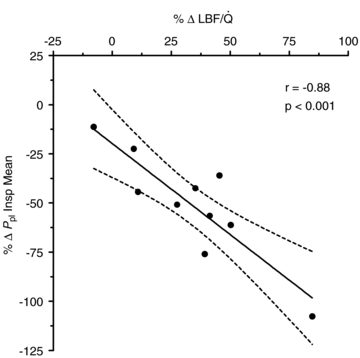
This relationship demonstrates the impact of a reduction in the work of breathing on LBF in HF patients.
Influence of acute inspiratory pressure loading during exercise
Pulmonary pressures
Figure 1C and D highlights the influence of acute inspiratory loading on the pleural pressure–volume response during exercise in the HF patients and CTL group. Inspiratory loading resulted in an increase in mean inspiratory Ppl (P < 0.01, both groups), as well as the increase in the time integrals for inspiratory Ppl (P < 0.01 for both groups) and total Ppl (P < 0.01, both groups) (Table 4; see Fig. 2 for absolute changes).
Haemodynamics
With loading, the HF patients had no change in 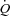 although there was a tendency for a fall in SV and a rise in HR (Table 4; see Fig. 3 for absolute changes). There were no changes in LBF,
although there was a tendency for a fall in SV and a rise in HR (Table 4; see Fig. 3 for absolute changes). There were no changes in LBF, 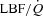 , Leg
, Leg 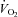 , or LVR (Table 4; see Fig. 4 for absolute changes). In contrast, the CTL group had an increase in
, or LVR (Table 4; see Fig. 4 for absolute changes). In contrast, the CTL group had an increase in 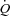 due to an increase in HR and SV (P < 0.01 for all) (Table 4; see Fig. 3 for absolute changes). Interestingly, there were no changes in LBF,
due to an increase in HR and SV (P < 0.01 for all) (Table 4; see Fig. 3 for absolute changes). Interestingly, there were no changes in LBF, 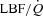 , Leg
, Leg 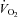 , or LVR (Table 4; see Fig. 4 for absolute changes).
, or LVR (Table 4; see Fig. 4 for absolute changes).
Blood gases
The HF group demonstrated trends for reduced 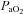 (96.5 ± 3.3 vs. 93.2 ± 3.2 mmHg, P= 0.06) and
(96.5 ± 3.3 vs. 93.2 ± 3.2 mmHg, P= 0.06) and 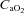 (34.8 ± 1.1 vs. 35.4 ± 1.2 ml (100 ml)−1, P= 0.07) with no change in
(34.8 ± 1.1 vs. 35.4 ± 1.2 ml (100 ml)−1, P= 0.07) with no change in 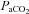 (34.8 ± 1.1 vs. 35.4 ± 1.2 mmHg, P= 0.19) during loading. Similarly, the HF patients had reductions in
(34.8 ± 1.1 vs. 35.4 ± 1.2 mmHg, P= 0.19) during loading. Similarly, the HF patients had reductions in 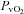 (21.2 ± 1.4 vs. 19.8 ± 1.5 mmHg, P= 0.01) and
(21.2 ± 1.4 vs. 19.8 ± 1.5 mmHg, P= 0.01) and 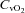 (4.7 ± 0.4 vs. 4.3 ± 0.5 ml (100 ml)−1, P= 0.02) with an increase in
(4.7 ± 0.4 vs. 4.3 ± 0.5 ml (100 ml)−1, P= 0.02) with an increase in 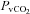 (55.4 ± 1.7 vs. 57 ± 1.6 mmHg, P= 0.02). These changes, however, resulted in no change in the a–v O2 diff (14.1 ± 0.9 vs. 14.2 ± 1.0 ml (100 ml)−1, P= 0.33). The CTL group, during loading, had reduced
(55.4 ± 1.7 vs. 57 ± 1.6 mmHg, P= 0.02). These changes, however, resulted in no change in the a–v O2 diff (14.1 ± 0.9 vs. 14.2 ± 1.0 ml (100 ml)−1, P= 0.33). The CTL group, during loading, had reduced 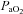 (92.7 ± 2.3 vs. 85.4 ± 2.6 mmHg, P < 0.01) with an increase in
(92.7 ± 2.3 vs. 85.4 ± 2.6 mmHg, P < 0.01) with an increase in 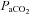 (36.9 ± 1.4 vs. 39.3 ± 1.0 mmHg, P < 0.01) and no change in
(36.9 ± 1.4 vs. 39.3 ± 1.0 mmHg, P < 0.01) and no change in 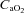 (18.8 ± 0.5 vs. 18.7 ± 0.5 ml (100 ml)−1, P= 0.24). In addition, the CTL participants had no change in
(18.8 ± 0.5 vs. 18.7 ± 0.5 ml (100 ml)−1, P= 0.24). In addition, the CTL participants had no change in 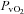 (20.3 ± 0.9 vs. 21.0 ± 0.8 mmHg, P= 0.19) or
(20.3 ± 0.9 vs. 21.0 ± 0.8 mmHg, P= 0.19) or 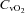 (4.7 ± 0.5 vs. 4.9 ± 0.5 ml (100 ml)−1, P= 0.78) with an increase in
(4.7 ± 0.5 vs. 4.9 ± 0.5 ml (100 ml)−1, P= 0.78) with an increase in 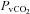 (58.8 ± 1.6 vs. 60.6 ± 1.7 mmHg, P= 0.01). These changes resulted in no change in a–v O2 diff (14.1 ± 0.4 vs. 13.8 ± 0.3 ml (100 ml)−1, P= 0.24).
(58.8 ± 1.6 vs. 60.6 ± 1.7 mmHg, P= 0.01). These changes resulted in no change in a–v O2 diff (14.1 ± 0.4 vs. 13.8 ± 0.3 ml (100 ml)−1, P= 0.24).
Ventilation
During loading, the HF patients had no change in 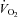 (1292.3 ± 177.4 vs. 1505.2 ± 166.0 ml min−1, P= 0.52). However, they had a reduction in RR (P= 0.02), an increase in VT and no change in
(1292.3 ± 177.4 vs. 1505.2 ± 166.0 ml min−1, P= 0.52). However, they had a reduction in RR (P= 0.02), an increase in VT and no change in 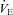 . There was an increase in TI (P= 0.02) although there was no change in TTOT in the HF patient group (Table 4). In contrast, the CTL group had no change in
. There was an increase in TI (P= 0.02) although there was no change in TTOT in the HF patient group (Table 4). In contrast, the CTL group had no change in 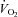 (2206.7 ± 235.2 vs. 1962.6 ± 264.8 ml min−1, P= 0.56), RR or VT and
(2206.7 ± 235.2 vs. 1962.6 ± 264.8 ml min−1, P= 0.56), RR or VT and 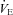 . Similar to the HF patients, the CTL group had an increase in TI (P= 0.02) with no change in TTOT (Table 4).
. Similar to the HF patients, the CTL group had an increase in TI (P= 0.02) with no change in TTOT (Table 4).
During loading, in contrast to the unloaded condition, there was no relationship between %Δ in inspiratory Ppl and the %Δ in 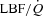 in the HF patients (r= 0.23, P= 0.52). This lack of correlation was similar in the CTL group (r= 0.24, P= 0.50).
in the HF patients (r= 0.23, P= 0.52). This lack of correlation was similar in the CTL group (r= 0.24, P= 0.50).
Discussion
Summary of findings
This study examined the influence of acute inspiratory pressure unloading and loading on blood flow distribution to the locomotor muscles during exercise in stable HF patients. Our data suggest that reducing the normal inspiratory muscle work via inspiratory pressure unloading results in a significant increase in active skeletal muscle blood flow. Further, despite an increase in the work of breathing during inspiratory pressure loading, neither the LBF nor total body 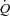 was altered suggesting that HF patients had reached a plateau in their ability to augment
was altered suggesting that HF patients had reached a plateau in their ability to augment 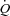 to meet respiratory muscle needs, and moreover, these patients were unable to vasoconstrict the vascular beds of the locomotor muscles in order to redistribute blood flow beyond that observed during normal breathing.
to meet respiratory muscle needs, and moreover, these patients were unable to vasoconstrict the vascular beds of the locomotor muscles in order to redistribute blood flow beyond that observed during normal breathing.
Influence of respiratory muscle work on cardiovascular haemodynamics and blood flow distribution in healthy humans
In healthy trained individuals, respiratory muscles preferentially recruit blood flow at the expense of locomotor muscles during maximal exercise. Harms and colleagues suggest that, during maximal exercise, respiratory muscle unloading by 63% of control results in a 4.3% increase in 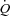 distribution to the legs whereas respiratory muscle loading of 28% results in a 7% reduction in
distribution to the legs whereas respiratory muscle loading of 28% results in a 7% reduction in 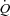 distribution to the legs. This implies that the work of breathing exhibited during heavy exercise results in a reflex vasoconstriction of the locomotor muscle vascular beds contributing to reduced leg blood flow and subsequently compromises locomotor muscle perfusion (Harms et al. 1997). Further, up to 14–16% of the total body
distribution to the legs. This implies that the work of breathing exhibited during heavy exercise results in a reflex vasoconstriction of the locomotor muscle vascular beds contributing to reduced leg blood flow and subsequently compromises locomotor muscle perfusion (Harms et al. 1997). Further, up to 14–16% of the total body 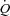 is directed to the respiratory muscles during maximal exercise in fit healthy adults and reduced locomotor muscle blood flow appears to be a direct result of the local reflex vasoconstriction (Harms et al. 1998). In addition, Vogiatzis recently demonstrated, in trained athletes, that intercostal muscle blood flow and vascular conductance are reduced during cycle exercise above 80% of maximal work compared to resting hyperpnoea at the same minute ventilation. These authors suggest that these findings indicate that the circulatory system is unable to meet the competing needs of both respiratory and locomotor muscles resulting in augmented oxygen extraction (Vogiatzis et al. 2009). As a whole, these studies suggest a necessary blood flow redistribution that occurs during heavy exercise in healthy humans to accommodate the shift in blood flow need.
is directed to the respiratory muscles during maximal exercise in fit healthy adults and reduced locomotor muscle blood flow appears to be a direct result of the local reflex vasoconstriction (Harms et al. 1998). In addition, Vogiatzis recently demonstrated, in trained athletes, that intercostal muscle blood flow and vascular conductance are reduced during cycle exercise above 80% of maximal work compared to resting hyperpnoea at the same minute ventilation. These authors suggest that these findings indicate that the circulatory system is unable to meet the competing needs of both respiratory and locomotor muscles resulting in augmented oxygen extraction (Vogiatzis et al. 2009). As a whole, these studies suggest a necessary blood flow redistribution that occurs during heavy exercise in healthy humans to accommodate the shift in blood flow need.
However, during submaximal work corresponding to 50 and 75% of maximal oxygen consumption, Wetter et al. reported that increasing or decreasing the work of breathing in healthy participants does not significantly alter leg vascular conductance, noradrenaline (norepinephrine) spillover, arterial pressure, or LBF (Wetter et al. 1999). These authors postulate that the work of breathing at these reduced ventilatory and total body work rates is not intense enough to elicit changes in cardiac function or sympathetically mediated vasoregulation and thus does not bring about change in vascular resistance or blood flow (Wetter et al. 1999). However, during sustained high-intensity submaximal exercise (90%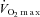 ) in healthy trained individuals, ventilatory unloading improves time to exhaustion by approximately 14% and reduces the rate of change in respiratory and limb discomfort perception throughout the duration of the exercise bout (Harms et al. 2000). Taken together, these results suggest that normal vasoregulation preferentially redistributes blood flow to the respiratory musculature during activities of high enough intensity to require substantially greater respiratory muscle energy production.
) in healthy trained individuals, ventilatory unloading improves time to exhaustion by approximately 14% and reduces the rate of change in respiratory and limb discomfort perception throughout the duration of the exercise bout (Harms et al. 2000). Taken together, these results suggest that normal vasoregulation preferentially redistributes blood flow to the respiratory musculature during activities of high enough intensity to require substantially greater respiratory muscle energy production.
Influence of respiratory muscle work on cardiovascular haemodynamics and blood flow distribution in patients with HF
Chronic HF is associated with a number of pulmonary-related disorders including restrictive and to a lesser extent obstructive changes, reduced lung diffusing capacity for carbon monoxide, reduced respiratory muscle strength and oxygenation, and an overall increased work of breathing (Light & George, 1983; Wright et al. 1990; Mancini et al. 1994, 1997; Dimopoulou et al. 1998; O’Donnell et al. 1999; Terakado et al. 1999; Johnson et al. 2000a; Agostoni et al. 2002). These alterations are exacerbated during exercise and are suggested contributors to dyspnoea and fatigue. Due to the complex interrelationship between blood flow, exercise intensity, cardiac function, and bioenergetics, it is possible that even low levels of physical activity in HF patients (e.g. activities of daily living) may result in redistribution of blood flow away from the locomotor muscles to the respiratory muscles in an effort to compensate for the exaggerated increase in respiratory muscle work. If so, the respiratory muscle blood flow ‘steal’ may be one mechanism which contributes to the enhanced perception of fatigue that is commonly encountered in this population.
Mancini and colleagues examined the influence of the work of breathing on maximal exercise performance in HF patients by conducting two randomized, blinded exercise tests using room air or heliox gas (21% oxygen and 79% helium). These authors suggest that reducing the flow-resistive work of breathing via heliox gas extends the time to exhaustion without altering oxygen uptake during the exercise test. Further, 87% of the patients reported that the exercise with heliox was subjectively easier (Mancini et al. 1997). O’Donnell et al. have demonstrated that inspiratory pressure support alone, compared to continuous positive airway pressure, results in greater submaximal steady-state exercise duration in conjunction with reduced leg discomfort (O’Donnell et al. 1999). These results suggest a clear role for elevated respiratory muscle work contributing to exercise intolerance in HF. More recently and similar to the findings of O’Donnell and colleagues, Broghi-Silva et al. have also shown that respiratory muscle unloading with proportional assist ventilation increased exercise tolerance and time to exhaustion during constant load exercise at 70–80% of peak work in HF patients. Moreover, these authors further demonstrated increases in muscle oxygenation and blood volume despite no change in arterial oxygen content (Broghi-Silva et al. 2008).
Miller and colleagues demonstrated in the canine model that removing the normal intrathoracic pressure generated during submaximal exercise (via inspiratory positive pressure support) results in a 5% reduction in SV in healthy dogs, whereas in dogs with tachycardia-induced HF, removing this intrathoracic pressure resulted in increased SV and 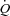 by ∼5% with an increase in hind limb blood flow out of proportion to the increase in
by ∼5% with an increase in hind limb blood flow out of proportion to the increase in 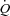 . In contrast, doubling the intrathoracic pressure excursion via inspiratory resistance did not significantly influence SV or
. In contrast, doubling the intrathoracic pressure excursion via inspiratory resistance did not significantly influence SV or 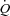 in healthy animals whereas it significantly reduced SV and
in healthy animals whereas it significantly reduced SV and 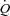 by approximately 10% and 4%, respectively, in the HF model. These authors suggest that the normally produced intrathoracic pressure generated during steady-state submaximal exercise in healthy animals is a necessary component of SV maintenance whereas this same intrathoracic pressure generated in animals with HF inhibits SV and limits
by approximately 10% and 4%, respectively, in the HF model. These authors suggest that the normally produced intrathoracic pressure generated during steady-state submaximal exercise in healthy animals is a necessary component of SV maintenance whereas this same intrathoracic pressure generated in animals with HF inhibits SV and limits 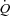 during submaximal exercise (Miller et al. 2007).
during submaximal exercise (Miller et al. 2007).
Our control group results are consistent with the findings of previous studies in healthy young adults during submaximal exercise and extend the findings to an older, sedentary but healthy population where we observed a small reduction in SV coupled with a tachycardic response that resulted in a sight reduction in 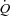 and no change in LBF. Despite this slight reduction in
and no change in LBF. Despite this slight reduction in 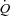 coupled with a slight increase in MAP, leg vascular resistance failed to increase significantly. This suggests that the exercise intensity utilized was not high enough to result in a respiratory muscle work load (and blood flow need) that would compete with locomotor muscles. In contrast, the HF patients demonstrated increased
coupled with a slight increase in MAP, leg vascular resistance failed to increase significantly. This suggests that the exercise intensity utilized was not high enough to result in a respiratory muscle work load (and blood flow need) that would compete with locomotor muscles. In contrast, the HF patients demonstrated increased 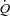 mediated by an increased SV. In the HF patients, the change in
mediated by an increased SV. In the HF patients, the change in 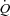 , when examined in the light of a slight reduction in MAP, resulted in a significant reduction in leg vascular resistance. Further, there was an increase in LBF out of proportion to the changes in
, when examined in the light of a slight reduction in MAP, resulted in a significant reduction in leg vascular resistance. Further, there was an increase in LBF out of proportion to the changes in 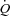 which resulted in improved Leg
which resulted in improved Leg 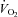 . These findings suggest a redistribution of blood flow away from the respiratory musculature to the locomotor muscles during the inspiratory muscle unloading in the HF patients.
. These findings suggest a redistribution of blood flow away from the respiratory musculature to the locomotor muscles during the inspiratory muscle unloading in the HF patients.
During the inspiratory muscle loading, CTL participants had a significant increase in 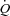 due to a combined increased in HR and SV with no change in LBF suggesting a specific distribution of
due to a combined increased in HR and SV with no change in LBF suggesting a specific distribution of 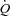 to the respiratory musculature with maintenance of existing blood flow to the locomotor muscles. The HF patients, however, had no change in
to the respiratory musculature with maintenance of existing blood flow to the locomotor muscles. The HF patients, however, had no change in 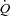 or LBF with loading. The lack of change in
or LBF with loading. The lack of change in 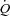 in the HF patients is probably due to reduced
in the HF patients is probably due to reduced 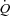 reserve. The lack of
reserve. The lack of 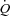 reserve coupled with a heightened baseline sympathetically mediated vascular tone and lack of additional vasoconstrictor reserve necessary for further redistribution of blood flow probably contributed to the lack of change in leg blood flow.
reserve coupled with a heightened baseline sympathetically mediated vascular tone and lack of additional vasoconstrictor reserve necessary for further redistribution of blood flow probably contributed to the lack of change in leg blood flow.
Limitations
Despite the reduced ejection fraction (≤35%) and significantly limited exercise capacity, most subjects enrolled in the present study were well compensated. Since pulmonary system changes are linked to disease severity, we would expect more robust respiratory muscle blood flow steal in sicker patients where the work and cost of breathing are further exaggerated and cardiac reserve is more significantly limited. In addition, our data apply more specifically to modest work intensities in an attempt to mimic intensities which may be encountered during activities of daily living and as such these findings may be exaggerated at high work loads.
Conclusions
The results of this study demonstrate that during whole body exercise, respiratory muscle unloading in HF patients results in an increase in LBF independent of changes in 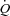 . However, respiratory muscle loading had no effect on
. However, respiratory muscle loading had no effect on 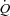 or LBF in HF patients, consistent with a lack of cardiac reserve to meet the needs of increased respiratory work. Further, the HF patients were unable to vasoconstrict locomotor vascular beds beyond that observed during normal breathing when presented with this respiratory load. These data suggest that during modest levels of activity, positive inspiratory pressure significantly increases blood flow to the locomotor muscles in patients with HF.
or LBF in HF patients, consistent with a lack of cardiac reserve to meet the needs of increased respiratory work. Further, the HF patients were unable to vasoconstrict locomotor vascular beds beyond that observed during normal breathing when presented with this respiratory load. These data suggest that during modest levels of activity, positive inspiratory pressure significantly increases blood flow to the locomotor muscles in patients with HF.
Acknowledgments
The authors would like to thank the participants who volunteered for this study. We also thank the dedicated support staff who provided critical technical support including: Minelle Hulsebus, Kathy O’Malley, Andrew Miller, Christopher Johnson, and Shelly Roberts. Supported by NIH grants HL71478 (B.D.J.), HL46493 (M.J.J.), 1KL2RR024151 (T.P.O.), the Frank R. and Shari Caywood Professorship (M.J.J.), AHA grant 0725715Z (T.P.O.), and the National Center for Research Resources (NIH) grant 1UL1RR024150. The authors have no conflicts of interest to disclose.
Glossary
Abbreviations
- CTL
control
- HF
heart failure
- HR
heart rate
- LBF
leg blood flow
- LVR
leg vascular resistance
- MAP
mean arterial pressure
- NB
normal breathing
- Ppl
index of pleural pressure
- RR
respiratory rate
- SV
stroke volume
Author contributions
T.P.O. contributed to study design, data collection, data analysis, and data interpretation. M.J.J. was involved with the conception of the study and study design, data collection, and data interpretation. N.M.D., J.H.E., and T.B.C. were instrumental in the collection of data and provided important intellectual contributions to the study design and data interpretation. This study was conducted in the laboratory of B.D.J. in cooperation with the Center for Translational Science Activities (CTSA). B.D.J. conceived and designed the experiments, contributed to data collection, data analysis, and data interpretation. All authors helped to draft and/or revise the manuscript critically. All authors approved the final version of the submitted manuscript.
References
- Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol. 1992;72:1818–1825. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- Agostoni PG, Bussotti M, Palermo P, Guazzi M. Does lung diffusion impairment affect exercise capacity in patients with heart failure. Heart. 2002;88:453–459. doi: 10.1136/heart.88.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KC, Hyatt RE, Mpougas P, Scanlon PD. Evaluation of pulmonary resistance and maximal expiratory flow measurements during exercise in humans. J Appl Physiol. 1999;86:1388–1395. doi: 10.1152/jappl.1999.86.4.1388. [DOI] [PubMed] [Google Scholar]
- Borghi-Silva A, Carrascosa C, Oliveira CC, Barroco AC, Berton DC, Vilaca D, Lira-Filho EB, Ribeiro D, Nery LE, Neder JA. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H2465–H2472. doi: 10.1152/ajpheart.91520.2007. [DOI] [PubMed] [Google Scholar]
- Bradley ME, Leith DE. Ventilatory muscle training and the oxygen cost of sustained hyperpnea. J Appl Physiol. 1978;45:885–892. doi: 10.1152/jappl.1978.45.6.885. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Johnson BD. Demand vs. capacity in the healthy pulmonary system. Schweiz Z Sportmed. 1992;40:55–64. [PubMed] [Google Scholar]
- Dimopoulou I, Daganou M, Tsintzas OK, Tzelepis GE. Effects of severity of long-standing congestive heart failure on pulmonary function. Respir Med. 1998;92:1321–1325. doi: 10.1016/s0954-6111(98)90136-6. [DOI] [PubMed] [Google Scholar]
- Franciosa JA, Ziesche S, Wilen M. Functional capacity of patients with chronic left ventricular failure. Relationship of bicycle exercise performance to clinical and hemodynamic characterization. Am J Med. 1979;67:460–466. doi: 10.1016/0002-9343(79)90794-0. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000a;117:321–332. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000b;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Johnson RLJ. Gas exchange efficiency in congestive heart failure. Circulation. 2000;101:2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- Johnson RLJ. Gas exchange efficiency in congestive heart failure II. Circulation. 2001;103:916–918. doi: 10.1161/01.cir.103.7.916. [DOI] [PubMed] [Google Scholar]
- Light RW, George RB. Serial pulmonary function in patients with acute heart failure. Arch Intern Med. 1983;143:429–433. [PubMed] [Google Scholar]
- Mancini D, Donchez L, Levine S. Acute unloading of the work of breathing extends exercise duration in patients with heart failure. J Am Coll Cardiol. 1997;29:590–596. doi: 10.1016/s0735-1097(96)00556-6. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Henson D, LaManca J, Levine S. Evidence of reduced respiratory endurance in patients with heart failue. J Am Coll Cardiol. 1994;24:972–981. doi: 10.1016/0735-1097(94)90858-3. [DOI] [PubMed] [Google Scholar]
- Miller JD, Curtis A, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H580–H592. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 1993;265:H1721–H1726. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, D’Arsigny C, Raj S, Abdollah H, Webb K. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med. 1999;160:1804–1811. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- Olson TP, Beck KC, Johnson JB, Johnson BD. Competition for intrathoracic space reduces lung capacity in patients with chronic heart failure: A radiographic study. Chest. 2006a;130:64–71. doi: 10.1378/chest.130.1.164. [DOI] [PubMed] [Google Scholar]
- Olson TP, Snyder EM, Johnson BD. Exercise disordered breathing in chronic heart failure. Exerc Sport Sci Rev. 2006b;34:194–201. doi: 10.1249/01.jes.0000240022.30373.a2. [DOI] [PubMed] [Google Scholar]
- Pina IL, Madonna DW, Sinnamon EA. Exercise test interpretation. Cardiol Clin. 1993;11:215–227. [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff T, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistance. Am J Physiol Heart Circ Physiol. 2002;282:H1732–H1738. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985;55:1037–1042. doi: 10.1016/0002-9149(85)90742-8. [DOI] [PubMed] [Google Scholar]
- Terakado S, Takeuchi T, Miura T, Sato H, Nishioka N, Fujieda Y, Kobayashi R, Ibukiyama C. Early occurrence of respiratory muscle deoxygenation assessed by near-infared spectroscopy during leg exercise in patients with chronic heart failure. Jpn Circ J. 1999;63:97–103. doi: 10.1253/jcj.63.97. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Adjustments in the peripheral circulation in chronic heart failure. Eur Heart J. 1983;4(suppl. A):67–83. doi: 10.1093/eurheartj/4.suppl_a.67. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Athanasopoulos D, Habazettl H, Kuebler WM, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol. 2009;587:3665–3677. doi: 10.1113/jphysiol.2009.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Wilson JR, Janicki JS, Likoff MJ. Exercise testing in the evaluation of the patient with chronic cardiac failure. Am Rev Respir Dis. 1984;129:S60–S62. doi: 10.1164/arrd.1984.129.2P2.S60. [DOI] [PubMed] [Google Scholar]
-
Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on
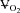 and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar] - Wright RS, Levine MS, Bellamy PE, Simmons MS, Batra P, Stevenson LW, Walden JA, Laks H, Tashkin DP. Ventilatory and diffusion abnormalities in potential heart transplant recipients. Chest. 1990;98:816–820. doi: 10.1378/chest.98.4.816. [DOI] [PubMed] [Google Scholar]
- Yeh MP, Gardner RM, Adams TD, Yanowitz FB. Computerized determination of pneumotachometer characteristics using a calibrated syringe. J Appl Physiol. 1982;53:280–285. doi: 10.1152/jappl.1982.53.1.280. [DOI] [PubMed] [Google Scholar]
- Zelis R, Flaim SF, Liedtke AJ, Nellis SH. Cardiocirculatory dynamics in the normal and failing heart. Ann Rev Physiol. 1981;43:455–476. doi: 10.1146/annurev.ph.43.030181.002323. [DOI] [PubMed] [Google Scholar]


