Abstract
In April 2010, the European Medicines Agency Committee for Medicinal Products for Human Use recommended approval of roflumilast, a selective phosphodiesterase 4 inhibitor, for the “maintenance treatment of severe chronic obstructive pulmonary disease (COPD, FEV1 postbronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment”. This decision was based, in part, on the results of several large, international, multicenter, randomized, placebo-controlled trials of either six or 12 months’ duration that had been undertaken in COPD patients. Roflumilast 500 μg daily improved lung function and reduced exacerbations in patients with more severe COPD, especially those with chronic bronchitis, frequent exacerbations, or who required frequent rescue inhaler therapy in the placebo-controlled trials. It also improved lung function and reduced exacerbations in patients with moderately severe COPD treated with salmeterol or tiotropium. Advantages of roflumilast over inhaler therapy are that it is an oral tablet and only needs to be taken once daily. While taking roflumilast, the most common adverse effects patients experienced were gastrointestinal upset and headache. Weight loss, averaging 2.2 kg, occurred in patients treated with roflumilast. Patients taking roflumilast were more likely to drop out of the trials than patients in the control groups. Patients who discontinued therapy usually did so during the first few weeks and were more likely to have experienced gastrointestinal side effects. Roflumilast is the first selective phosphodiesterase 4 inhibitor and will offer physicians another treatment option for patients with more severe COPD.
Keywords: roflumilast, phosphodiesterase 4 inhibitor, chronic obstructive pulmonary disease, exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth most common cause of death in the US and is also a major cause of morbidity.1,2 Lung parenchymal destruction, ie, emphysema, and obstructive bronchiolitis are the typical pathologic changes in COPD and are characterized functionally by progressive airway obstruction.1 Inflammatory changes and mucus gland hyperplasia in the larger airways may also occur, and are accompanied by chronic cough and mucus hypersecretion. The clinical course of COPD is punctuated by exacerbations, periods of deterioration characterized by worsening dyspnea, and increases in cough, sputum volume, and sputum purulence usually associated with respiratory tract infection. Acute exacerbations of COPD are accompanied by acute deterioration in lung function and worsening disability.3 More frequent exacerbations are associated with a more rapid decline in lung function.3 Exacerbations that are severe enough to require hospitalization are particularly ominous because they are associated with significant inhospital mortality, and discharged patients have a 9% mortality rate within 30 days, and 28% are dead within one year.4,5
Inflammation in COPD
CD68+ macrophages and CD8+ T lymphocytes are the predominant inflammatory cells in COPD, with polymorphs increasing during acute exacerbations.6 The severity of inflammation in the small airways and lung parenchyma increases with worsening COPD.6 These alterations contribute to airways thickening, resulting in luminal narrowing, and parenchymal destruction diminishes elastic recoil. Along with mucus hypersecretion, these abnormalities contribute to airways obstruction and to the reduction in airflow.6 In COPD, blood levels of the proinflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNFα) are increased.7 The level of IL-32, a recently described cytokine expressed in bronchial epithelium, macrophages, and CD8+ cells, which promotes TNFα, IL-8, and CXCL2 expression, is also elevated and correlates with the reduction in forced expiratory volume in one second (FEV1) in COPD patients.7
COPD is associated with a variety of comorbidities and extrapulmonary symptoms.8,9 It has been suggested that the association between COPD and these other conditions is due to the inflammatory process extending systemically. Leukocyte counts and blood levels of C-reactive protein, fibrinogen, and TNFα are higher in COPD patients compared with matched controls.8 Systemic inflammation is associated with, and appears to be a risk factor for, a variety of symptoms and conditions including weight loss, muscle wasting, atherosclerosis, malignancy, osteoporosis, diabetes, and anemia.1,8,9
Treatment of inflammation in COPD
Although generally effective in asthma, inhaled corticosteroids (ICSs) provide relatively modest benefit in COPD.1,8 The predominance of CD68+ macrophages, CD8+ T lymphocytes, and neutrophils, rather than the more corticosteroid-responsive eosinophils and CD4+ T lymphocytes present in asthma, contribute to the relative resistance to corticosteroids seen in COPD.10 Although smoking cessation interventions and bronchodilators provide symptomatic relief, there are clear unmet clinical needs for patients with COPD. These include effective disease-modifying pharmacotherapies that target the inflammation and so arrest the relentless decline in lung function and reduce the frequency of exacerbations.
One novel class of compounds that may deliver therapeutic benefit in COPD are phosphodiesterase (PDE) 4 inhibitors. PDE is a generic term that describes a large superfamily of enzymes that catalyze the breakdown of cyclic adenosine-3’,5’-monophosphate (cAMP) and/or cyclic guanosine-3’,5’-monophosphate (cGMP) to their respective inactive nucleotide 5’-monophosphates.11 Eleven distinct PDE families have been identified,11 although most of the anti-inflammatory activity is believed to result from the inhibition of PDE4, for which there is clinical precedent.12 Indeed, theophylline is a weak, nonselective PDE inhibitor (see below) and has been used in clinical practice as a bronchodilator for more than 70 years. More recently, it has been reported that theophylline has immunomodulatory and anti-inflammatory activities in asthma and COPD at doses lower than those required to produce bronchodilation.13–19 Although mechanisms of action other than PDE4 inhibition have been hypothesized to account for the anti-inflammatory activity of theophylline,20 it seems more likely that its clinical activity reflects the concurrent (albeit modest) inhibition of multiple PDEs in target tissues, resulting in additive or even synergistic effects that combine to suppress inflammation.21
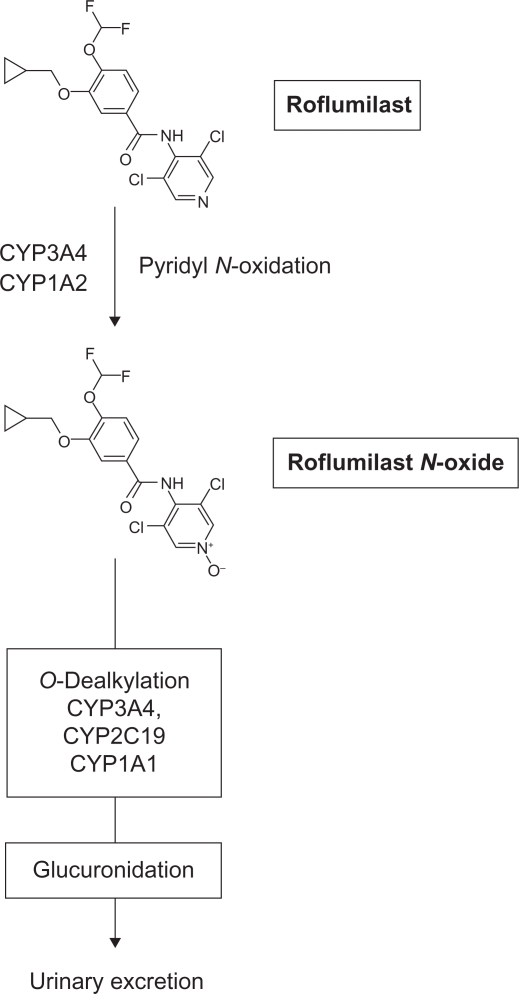
Unfortunately, theophylline has a narrow therapeutic margin, a poor adverse effect profile, and a proclivity to interact adversely with other drugs via competition with various cytochrome (CYP) 450 metabolizing enzymes, which severely limits its widespread clinical utility. In contrast, nonxanthine-based compounds that selectively inhibit PDE4 do not share these limitations of theophylline, and have undergone extensive preclinical and clinical evaluation.12,21,22 The most advanced compound within this class is the benzamide, roflumilast (see Figure 1), which is being developed jointly by Nycomed (Zurich, Switzerland, formerly Altana) in Europe and the Forest Research Institute in the US (ownership transferred from Nycomed in December 2009) for the treatment of COPD.
Figure 1.
Structure of roflumilast and its metabolic inactivation.
Chemistry
The IUPAC name for roflumilast is 3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benz-amide; CAS 162401-32-3). The trade name is Daxas® and research codes are APTA 2217, B9302–107, BY 217, and BYK 20869.23 Roflumilast is synthesized in five steps from 3-(cyclopropylmethoxy)-4-hydroxybenzaldehyde.24 The compound is achiral, and appears as a white crystalline solid with a melting point, parent molecular weight, and empirical formula of 158°C, 403.22, and C17H14Cl2F2N2O3, respectively. The compound is sparingly soluble in aqueous media but is soluble in organic solvents, including dimethylsulfoxide. The structure of roflumilast docked in the catalytic sites of PDE4 isoforms has been solved by x-ray co-crystallography.25
Biochemistry and enzymology
Roflumilast and its primary metabolite, roflumilast N-oxide, are potent and competitive inhibitors of PDE4.26 However, PDE4 is not a single enzyme and, in humans, in excess of 50 different variants have been identified that are encoded by four genes, PDE4A, PDE4B, PDE4C, and PDE4D.27 These enzymes have absolute specificity for cAMP and are expressed across almost all immune and proinflammatory cells that are believed to contribute to disease pathogenesis.12 The finding that elevation of cAMP within the lung exerts anti-inflammatory activity in a variety of preclinical models fuelled the idea that PDE4 could be exploited to therapeutic advantage in COPD with small molecule inhibitors, such as roflumilast.26
Using PDE4 isolated from human neutrophils, which contains a mixture of different PDE4s, roflumilast and roflumilast N-oxide have IC50 values of 800 pM and 2 nM, respectively.26 However, neither of these compounds discriminate between PDE4 gene variants, and it is possible21 that this lack of subtype selectivity contributes to its improved therapeutic ratio compared with several of its predecessors. Indeed, cilomilast and a PDE4 inhibitor from Purdue-Frederick, V-11294A, preferentially inhibit (by 10- and 30-fold, respectively) PDE4D,28,29 which has been linked with gastrointestinal (GI) events of concern that are often associated with this class of drugs.21,30,31 Both of these compounds were discontinued from development because of unfavorable adverse effect profiles and/or lack of efficacy.32 Both roflumilast and its N-oxide are highly selective PDE4 inhibitors, and are essentially inactive against PDEs 1, 2, 3, 5, and 7 at concentrations up to 10 μM.26
Pharmacodynamics of roflumilast: Preclinical and clinical data
The rationale for developing selective PDE4 inhibitors is based on three critical findings: PDE4 regulates cAMP degradation in most immune and proinflammatory cells; in cell-based systems, PDE4 inhibitors of varied structural classes suppress a plethora of responses that are considered to be proinflammatory; and PDE4 inhibitors are efficacious in preclinical animal models that attempt to reproduce specific facets of COPD pathobiology.12,33,34 If these findings are confirmed in humans, PDE4 inhibitors could provide a potential disease-modifying therapy in COPD.35
With the exception of the platelet, all immune and proinflammatory cells express PDE4.12 PDE4 variants are also abundant in structural cells including airway smooth muscle, epithelial cells, and fibroblasts.38 Without exception, each of these cell types coexpress multiple PDE4 variants derived from PDE4A, PDE4B, and PDE4D12 and, currently, the isoform(s) that must be inhibited for the anti-inflammatory actions of PDE4 inhibitors to be realized is largely unknown. Nevertheless, there are considerable in vitro data describing the inhibitory effect of the nonselective PDE4 inhibitor, roflumilast, on a variety of proinflammatory responses.26,37,38 Similarly, in preclinical animal models that reproduce specific components of COPD, roflumilast is efficacious, suggesting that it might be disease-modifying.39–48 For example, in a chronically cigarette-exposed murine model, roflumilast significantly reduced the characteristic increase in pulmonary neutrophil and macrophage burden and also increased IL-10, although goblet cell metaplasia was unaffected.41 Roflumilast also prevented the development of experimental emphysema in the same cigarette smoke-exposed murine model.42 In another study, using cigarette smoke-exposed guinea pigs, roflumilast reduced the numbers of neutrophils, eosinophils, and lymphocytes, as well as protein concentration, in bronchoalveolar lavage fluid, whereas methylprednisolone only attenuated the eosinophilia.43 There are also in vitro data supporting the idea that PDE4 inhibitors, including roflumilast and roflumilast N-oxide, may alleviate airway remodeling.44–46 Collectively, therefore, this class of drugs may exert multiple beneficial effects that combine to arrest the progressive decline in lung function that is a defining characteristic of COPD.
Despite PDE4 inhibitors being in development for more than 20 years, their mechanism(s) of action has not, unequivocally, been established. In animals, roflumilast does not protect against bronchoconstriction induced by leukotriene D4 and 5-hydroxytryptamine.47,48 Similarly, there is no evidence that PDE4 inhibitors cause bronchodilation in human COPD.49 Thus, an anti-inflammatory effect rather than a reduction in airway smooth muscle tone may account for the clinical efficacy of this and other PDE4 inhibitors. Unfortunately, there are few studies that have examined the potential anti-inflammatory effects of roflumilast in humans and, therefore, the available data are limited and inconclusive. In a double-blind, crossover, placebo-controlled study of four weeks’ duration involving 38 patients with COPD (mean post-bronchodilator FEV1 61% predicted), oral roflumilast 500 μg daily reduced the absolute number of neutrophils, eosinophils, and lymphocytes in induced sputum by 36%, 50%, and 35%, respectively, relative to placebo by the end of the study.50 Significant reductions in eosinophil cationic protein, IL-8, neutrophil elastase, and α2-macroglobulin, a marker of microvascular leak, were also reported.50 The ex vivo generation of TNFα induced by lipopolysaccharide (LPS) in whole blood, a biomarker of systemic inflammation, was reduced by 10.4%. These effects on inflammatory indices were accompanied by significant improvements in pre- and postbronchodilator FEV1 (mean change 80 mL and 69 mL, respectively) compared with placebo. A concern with these data is that the statistical significance for most inflammatory endpoint measures was driven by placebo and this may have overestimated the magnitude of the anti-inflammatory effect produced. Thus, at the end of the study, the absolute number of neutrophils and eosinophils were increased by approximately 20%–40% in the placebo arm relative to baseline.50 Similar effects were also seen for neutrophil elastase and α2-macroglobulin. The mechanism responsible for this rapid apparent “deterioration” in inflammatory status after placebo is unclear.
Despite difficulties interpreting these results, other studies with roflumilast and with the PDE4 inhibitors, cilomilast and Bay 19-8004, are consistent with these drugs having anti-inflammatory activity in airway diseases. Thus, roflumilast significantly decreased neutrophils in the BAL fluid of healthy subjects following segmental challenge with LPS51 and, in a separate investigation, reduced LPS-induced TNFα generation ex vivo.52 Cilomilast, given 15 mg twice daily for 12 weeks, significantly reduced the numbers of subepithelial CD8+ T lymphocytes and CD68+ macrophages in bronchial biopsies in COPD patients.53 Finally, Bay 19-8004 reduced levels of albumin and eosinophil cationic protein in sputum samples obtained from patients with COPD.54
Pharmacokinetics of roflumilast and roflumilast N-oxide
The absorption, distribution, metabolism, and excretion of roflumilast delivered by the oral route have been examined in several populations including healthy adults, adolescents, and children,55–57 as well as in patients with COPD (see www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf). In an open, randomized, two-period crossover study involving 12 healthy, fasted, white adult subjects, the absorption of roflumilast, administered orally in two 250 μg immediate-release tablets, is rapid and complete, with the time to peak plasma concentration (Tmax) being reached after approximately one hour.55 Roflumilast given orally is highly bioavailable (F = 0.79), binds extensively (98.9%) to plasma proteins, achieves steady-state levels within four days of once-daily dosing, has an elimination half-life (t1/2) of between seven and 25 hours (mean about 17 hours) and is subject to negligible first-pass hepatic metabolism.55–58 The clearance (Cl) and volume of distribution (Vd) were 13 L/hour and 2.92 L/kg, respectively, after a single intravenous dose (120 μg) of roflumilast in healthy adult subjects, indicating pronounced distribution in tissues.59 In patients with COPD, exposure to roflumilast estimated from the area under the concentration-time curve (AUC) up to nine hours and the maximum observed plasma concentration (Cmax) was 60% and 6% higher, respectively, when compared with normal healthy individuals (www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf).
An open, randomized, two-period, two-sequence, crossover study established that oral ingestion (both single and repeat doses) of roflumilast (250 μg and 500 μg) provides dose-proportional systemic exposure with no difference between the single and repeat dose regimens. Similar dose proportionality data also were observed for roflumilast N-oxide indicating that both compounds display linear pharmacokinetics.59
In humans, the metabolism of roflumilast is extensive, involving both Phase I (CYP P450) and Phase II (conjugation) reactions, with unchanged drug in urine accounting for less than 1% of the administered oral dose. The major metabolic pathway for roflumilast elimination after oral administration is pyridine N-oxidation with the formation of roflumilast N-oxide (see Figure). This process is catalyzed primarily by the mixed function oxidases, CYP3A4 and CYP1A2. The pharmacokinetics of roflumilast N-oxide are distinct from the parent compound. The Tmax is between four hours and 12 hours, and the Cmax is typically 1- to 2-fold higher.55,58,60,61 Steady-state plasma levels of roflumilast N-oxide are usually achieved within six days of once-daily oral administration and the elimination t1/2 is approximately 27 hours, which is significantly prolonged relative to the parent compound. Roflumilast N-oxide is also highly bound (97%) to plasma proteins. Finally, total systemic exposure, estimated from the AUC, exceeds that of roflumilast by about 10-fold.59 Taken together, these data indicate that the N-oxide metabolite accounts for about 90% of the biologic action of roflumilast and produces long-lasting, competitive PDE4 inhibition over 24 hours, making once-daily roflumilast administration a realistic treatment regimen. Roflumilast N-oxide is O-dealkylated primarily by CYP3A4, with a small contribution by CYP2C19 and extrahepatic CYP1A, glucuronidated, and eliminated via the kidney (see Figure).61
Clinical trials of roflumilast in COPD
Outcome measures of efficacy in the roflumilast clinical development program have been evaluated in several international, prospective, randomized, double-blind, placebo-controlled trials involving over 9000 patients with COPD.
RECORD
The first large clinical trial involved 1411 patients with moderately severe disease (mean post-bronchodilator FEV1 1.5 L, 54% predicted) with a lack of reversibility to 400 μg albuterol and compared the effect of daily treatment with roflumilast 250 μg or 500 μg for 24 weeks with placebo62 (Table 1). The only other respiratory medications allowed during the study were short-acting β2-agonists (SABAs) and short-acting anticholinergics (SAACs). Approximately a quarter of the patients were treated with xanthines, 20% with ICSs, and 15% with long-acting β2 agonists (LABAs) prior to study entry. RECORD (M2-107) was initiated prior to the marketing of tiotropium.62 There were two primary outcome measures, ie, the change from baseline in post-bronchodilator FEV1 and the St George’s respiratory questionnaire score (SGRQ). At the end of the study, roflumilast-treated patients experienced greater improvements in postbronchodilator FEV1 (74 mL and 97 mL for the 250 μg and 500 μg dose, respectively) and health-related quality of life, although the difference from baseline did not reach the clinically significant threshold of −4 units (Table 2). In addition, exacerbations, primarily of mild intensity, were decreased but adverse events were similar in the two groups.62 More patients discontinued treatment in the roflumilast arms than in the placebo arm. COPD exacerbations were the most common adverse effects, followed by nasopharyngitis, diarrhea, upper respiratory tract infections, and nausea, in descending order of frequency.62
Table 1.
Patient demographics in the large, randomized roflumilast treatment trials
| Rabe 200562 | Calverley 200763 | Calverley 200965 |
Fabbri 200968 |
||
|---|---|---|---|---|---|
| Salmeterol | Tiotropium | ||||
| Number randomized | 1411 | 1513 | 3096 | 933 | 743 |
| Mean age (years) | 64 | 65 | 64 | 65 | 64 |
| % male | 74 | 76 | 75 | 66 | 72 |
| Smoking history (pack years) | 43 | 44 | 48 | 43 | 43 |
| Current smokers (%) | 46 | 37 | 41 | 39 | 40 |
| FEV1 prebronchodilator | 1.4 | 1.0 | 1.0 | 1.4 | 1.5 |
| FEV1 (% predicted) | 54 | 37 | 33 | 52 | 53 |
Notes: Data include number of patients randomized in each trial, mean age in years of patients in each trial, percentage of male patients, average smoking history in pack years (one pack year = one pack/day for one year), percentage of current smokers, mean prebronchodilator FEV1, and mean FEV1 as a percentage of the predicted value.
Abbreviation: FEV1, forced expiratory volume in one second.
Table 2.
Patient outcomes in the roflumilast treatment trials
| Rabe 200562500 μg | Calverley 200763 | Calverley 200965 |
Fabbri 200968 |
||
|---|---|---|---|---|---|
| Salmeterol | Tiotropium | ||||
| Pre-FEV1 (mL) | 88 | 36 | 48 | 49 | 80 |
| Post-FEV1 (mL) | 97 | 39 | 55 | 60 | 81 |
| SGRQ | –1.7 | +0.3 | |||
| Dropouts (%) | 22/11 | 29/22 | 33/31 | 23/18 | 17/10 |
| Exacerbation rate | 0.28/0.30 | 0.86/0.92 | 1.14/1.37 | 18/11 | 11/16 |
| Weight loss (kg) | N/A | N/A | 2.2 | 2.2 | 2.1 |
| Diarrhea (%) | 9/2 | 9/3 | 8/3 | 8/3 | 9/1 |
| Nausea (%) | 5/1 | 5/1 | 4/2 | 5/1 | 3/1 |
| Headache (%) | N/A | 6/2 | 3/1 | 3/1 | 2/0 |
Notes: Pre-FEV1 refers to mean change in prebronchodilator FEV1 at the end of the trial. Post-FEV1 refers to the mean change in postbronchodilator FEV1 at the end of the trial. SGRQ refers to change in mean St George Respiratory Question Score at the end of the treatment period. A reduction in score represents an improvement. Dropouts represent the percentage of patients that did not complete the treatment period. For each study, the first percentage represents the percentage of subjects in the roflumilast treatment arm that did not complete the study and the second percentage represents dropouts in the placebo arm. In each study, a greater percentage dropped out of the roflumilast treatment arms. The exacerbation rate refers to the number of moderate and severe exacerbations, exacerbation rate per patient, or the percentage of patients experiencing exacerbations during the study. In each case, the first number represents the roflumilast arm and the second the placebo arm of the studies. The next row contains the average difference in weight loss between the placebo arm and the treatment arms. In the three studies reporting weight loss, patients receiving roflumilast lost an average of slightly more than 2 kg more than the placebo-treated patients (N/A, data are not available). The last three rows represent the percentages of patients reporting diarrhea, nausea, and headache. In each case, the first percentage represents the percentage of patients reporting the side effect in the roflumilast arm and the second represents the percentage reporting the side effect in the placebo arm.
RATIO and OPUS
In two subsequent identical studies, (RATIO, M2-112; NCT00430729) and (OPUS, M2-111; NCT00076089), the effects of daily roflumilast 500 μg for 12 months were compared with placebo in 1513 patients and 1173 patients with more severe COPD.64 In RATIO (mean post-bronchodilator FEV1 1.0 L, 41% predicted) 65% of subjects were taking ICSs prior to the study and were allowed to continue this medication at a steady dose (Table 1). Forty-five percent of the patients were taking LABAs and 5% were taking tiotropium which were discontinued prior to the study.63 All participants were allowed SABAs for rescue. The primary outcome measures were the change from baseline in postbronchodilator FEV1 (as in RECORD) and the number of moderate or severe exacerbations per patient per year. At 12 months, mean postbronchodilator FEV1 had increased by 39 mL compared with the placebo group but the exacerbation rate was unchanged with roflumilast treatment,63 possibly because it was too low for a statistically significant difference to be detected (Table 2). However, in a post hoc analysis of a subgroup of patients with GOLD stage IV disease, roflumilast significantly reduced exacerbation frequency.63 Roflumilast did not significantly improve the SGRQ, which was used as a secondary outcome, in either patient population.63
The results of the OPUS study have not yet been published. However, because roflumilast failed to reduce the frequency of exacerbations in RATIO, a pooled analysis of the RATIO and OPUS datasets has been performed to increase the statistical power.64 In this combined group of 2686 patients (mean postbronchodilator FEV1 39% predicted), roflumilast significantly reduced exacerbation frequency by 13% relative to placebo. This effect was most pronounced in those patients with a diagnosis of chronic bronchitis (24% reduction) indicating that this phenotype of COPD may benefit most from the anti-inflammatory actions of PDE4 inhibition.
AURA and HERMES
Based on the post hoc analysis in RATIO, in which the exacerbation rate was less in roflumilast-treated patients with GOLD Stage IV disease,63 two identically designed studies (AURA [M2-124; NCT00297102] and HERMES [M2-125; NCT00297115]) involving 3096 patients with chronic bronchitis (mean postbronchodilator FEV1 1.1 L, 36% of predicted) compared the effect of daily roflumilast 500 μg for 12 months with that of placebo (Table 1). Inclusion in the trial required all enrollees to have had experienced at least one exacerbation serious enough to require systemic corticosteroids and/or hospitalization in the previous year.65 All patients were allowed to continue their SABAs, SAACs, and/or LABAs, but had to discontinue LAACs and ICSs. On entry, 42% of the patients were treated with an ICS and 50% with a LABA. The primary outcome measures were change from baseline in prebronchodilator FEV1 (in contrast with postbronchodilator FEV1 in RATIO) and rate of moderate or severe acute exacerbations. The use of prebronchodilator FEV1 as an outcome measure has been developed to assess the efficacy of nonbronchodilators and is recommended by the FDA in clinical trials of COPD (see: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071575.pdf). In this highly selected group, patients experienced an improvement in pre- and postbronchodilator FEV1 (48 mL and 55 mL, respectively, for the pooled data); a reduction in the exacerbation rate was also noted (Table 2). Compared with the placebo arm, the reduction in moderate or severe exacerbations was 17% in the roflumilast-treated patients.65 Of the secondary outcome measures, there were small statistically, but not clinically, significant improvements in transition dyspnea index scores for the roflumilast-treated patients, although there was no difference in the health utility assessment tool, ie, the Euroquol-5 dimension total score, between the roflumilast-treated and placebo groups. Mortality was similar in the two study arms. C-reactive protein concentration was used as a marker of systemic inflammation but was not different in the roflumilast and placebo groups. Roflumilast-treated patients experienced more adverse events including weight loss, which averaged 2.2 kg, compared with the placebo arm. To summarize, COPD patients with more severe airway obstruction, GOLD stage IV disease, those with chronic bronchitis, and those who had at least one exacerbation in the previous year, experienced improvement in pre- and postbronchodilator FEV1 and a reduction in moderate and severe exacerbations, and these improvements were independent of smoking status or LABA use.65
EOS and HELIOS
A combination of an ICS and a LABA has been shown to be more effective than either class of drug individually at improving flow rates and health status, and reducing COPD exacerbations.66 Despite the benefits from combination therapy in COPD, treatment has not been shown to statistically decrease mortality.66,67 Moreover, there are concerns about adverse effects, including pneumonia, cataracts, glaucoma, and reductions in bone density in ICS-treated patients. Two trials comparing the effects of roflumilast with placebo added to long-acting bronchodilators for 24 weeks, one with salmeterol (EOS M2-127; NCT00313209) and one with tiotropium (HELIOS M2-128; NCT00424268), were recently reported.68 The COPD patients in this report had milder obstruction (mean postbronchodilator FEV1 1.5–1.6 L, 55%–56% of predicted) than those in the AURA and HERMES trials (mean postbronchodilator FEV1 1.1 L, 36% predicted, Table 1).65 In the salmeterol study, although chronic bronchitis was not a prerequisite, 79% of patients had chronic cough and sputum production.68 Chronic bronchitis and the use of a minimum of 28 puffs of rescue inhaler per week were prerequisites for the tiotropium study.68 Similar to the earlier report,65 more patients dropped out of the roflumilast study arms whether they were treated with salmeterol or tiotropium. The primary outcome measure was change from baseline in prebronchodilator FEV1. In both trials with long-acting bronchodilators, pre- and postbronchodilator FEV1 improved in patients treated with roflumilast.68 In the EOS trial, pre-and postbronchodilator FEV1 were greater in those patients given roflumilast plus salmeterol when compared with patients taking salmeterol as monotherapy, and this effect was similar in magnitude to the increase in postbronchodilator FEV1 reported with the addition of ICS to salmeterol in other studies.66,69 Similarly, in the tiotropium study (HELIOS) roflumilast improved prebronchodilator FEV1 by 80 mL when compared with patients using the LAAC alone (Table 2).68 In the salmeterol trial, the time to first moderate or severe exacerbation, and the proportion of patients experiencing an exacerbation, were better in the roflumilast arm. In the tiotropium study, median time to any exacerbation and the proportion of patients experiencing any exacerbation were decreased in the roflumilast study arm.68
Pending trials
The OPUS trial has been completed and a full report is expected in 2010.64 As stated above, this study is a replica of RATIO and was designed to evaluate the efficacy of roflumilast on exacerbation rate and health-related quality of life, and on the economic impact of managing patients with COPD (see: http://clinicaltrials.gov/ct2/show/NCT00076089?term=roflumilast&rank=7).
There is good evidence that FEV1 alone may have limitations as a clinical outcome measure of efficacy.70 As an alternative, it has been advocated that static or dynamic lung volume measurements may provide more instructive information pertaining to the impairment of lung function, especially in subjects who are poorly reversible.71 The HERO study (M2-121) was designed to evaluate the effect of roflumilast on air trapping and measures of hyperinflation in subjects with COPD. The trial has been completed but not published (http://clinicaltrials.gov/ct2/show/NCT00108823?term=roflumilast&rank=20).
Safety and tolerability
In the roflumilast clinical development program, patient withdrawals were similar in those patients who received roflumilast when compared with placebo, although more patients taking roflumilast withdrew within the first 12 weeks of treatment.62–66,68 The most common reasons for withdrawal in the roflumilast group were GI adverse events or headache.62–66,68 whereas the most prevalent adverse events were exacerbations of COPD and respiratory infections, followed by GI symptoms including diarrhea, weight loss, and nausea. Weight loss is a concern in COPD patients, especially those with advanced disease who are often underweight. The weight loss averaged slightly more than 2 kg per patient and was greater in patients with GI symptoms and who had particularly severe COPD. Interestingly, there was an inverse relationship between the magnitude of the weight loss and body mass index; thin patients lost less weight than heavier ones. Although adverse events were more frequent in roflumilast-treated patients, serious adverse events generally were not more common (see below).62–66,68 ICS therapy has been a risk factor for developing pneumonia in some COPD clinical trials, but no evidence has emerged that this is more common in roflumilast-treated patients.72 The rates of atrial fibrillation were not increased with roflumilast.63
Neuropsychiatric adverse events were more common in patients who received 500 μg of roflumilast when compared with the lower 250 μg dose or placebo. Indeed, 403 (7%) adverse events were documented in the 5677 patients who received roflumilast (500 μg once daily) whereas only 190 (3.5%) adverse events were reported in the 5491 patients who were given placebo (see www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf). In particular, the incidence of insomnia, anxiety, and depression was two to three times higher in the 500 μg roflumilast-treated group when compared with placebo. A potentially significant cause for concern was that of the 12054 patients in the roflumilast COPD database, three completed suicides (all in males) were reported in those patients given roflumilast compared with none in patients taking placebo. It is noteworthy that none of these individuals had a prior history of depression. There were also two suicide attempts (both in females). However, in these cases both individuals had prior psychiatric histories (see www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf).
Another potential serious adverse event was cancer. Analysis of the overall roflumilast clinical development program revealed a total of 218 cancers/tumors in 208 patients. Disproportionately more (n = 131, 60%) of these lesions were in the roflumilast-treatment group when compared with placebo. Specifically, there was a greater incidence of lung and prostate cancer reported in patients given roflumilast than in those individuals given placebo (see www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf). The significance of this observation is unclear. If roflumilast is carcinogenic, one might expect a greater than one-year latency period before tumors develop. Indeed, people smoke cigarettes for decades before they get lung cancer. Thus, the possibility that these findings are a chance observation, similar to the initial reports with omalizumab,73 rather than a response to treatment, should not be dismissed.
Contraindications, effects of food, and drug–drug interactions
No potential contraindications have, thus far, been identified in the roflumilast clinical development program. Although the metabolism of roflumilast is significantly arrested in patients with mild and moderate hepatic insufficiency, leading to increased systemic exposure (AUC0–24 = 51% and 92% higher in patients meeting Child-Pugh A and Child-Pugh B criteria, respectively, when compared with healthy subjects), changes to the pharmacokinetics of roflumilast N-oxide are relatively modest.74 Since the primary metabolite is believed to account for approximately 90% of the pharmacodynamic impact of roflumilast, the small pharmacokinetic changes reported are not believed to be clinically relevant. Thus, no dose adjustments are predicted to be required in patients with mild and moderate liver cirrhosis.74
Similarly, although a high-fat meal decreases Cmax and delays Tmax of roflumilast versus the fasted state, the same pharmacokinetic parameters are not changed for roflumilast N-oxide.75 Thus, because the primary metabolite mediates most of the pharmacologic effects of roflumilast, these data strongly suggest that the parent drug can be taken with or without food.
Many patients with COPD have multiple comorbidities which require other medications. The possibility that roflumilast and/or its N-oxide could interact unfavorably with drugs commonly used in COPD has therefore been evaluated. Initial in vitro studies using human liver microsomes established that neither roflumilast nor roflu-milast N-oxide inhibit CYP3A4, CYP1A2,76 CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A5, or 4A9/11. Conversely, roflumilast does not induce CYP1A2, 2A6, 2C9, 2C19, and 3A4/5, and is only a weak inducer of CYP2B6. Thus, there is a low potential for roflumilast to interact adversely with other drugs, including midazolam,76 montelukast,77 budesonide,79 salbutamol,80 formoterol, warfarin, sildenafil, digoxin (see www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf), and antacids containing magnesium hydroxide/aluminum hydroxide.60 This is important to determine because inducers of these enzymes have the potential to increase the clearance of roflumilast, thereby lowering its efficacy. Conversely, xenobiotics that are metabolized by the same enzyme(s) could compete with roflumilast, delay its inactivation, and so increase systemic exposure, with the potential for adverse events. However, rifampicin has been shown to limit the efficacy of roflumilast significantly due to its ability to induce enzymes that include CYP3A4, CYP2C19, and extrahepatic CYP1A2.58 Similarly, coadministration of erythromycin,78 ketoconazole,61 fluvoxamine, theophylline, cimetidine, enoxacin, and minulet significantly influence systemic exposure to roflumilast and the N-oxide (see www.fda.gov/downloads/AdvisoryCommittees/Com-mitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrug-sAdvisoryCommittee/UCM207377.pdf).
Polyaromatic hydrocarbons, which are constituents of cigarette smoke, are known to induce CYP1A1 and CYP1A2.81–83 Although CYP1A2 contributes to roflumilast metabolism and may enhance the rate at which the N-oxide is produced in cigarette smokers (see above), the N-oxide is not a substrate for CYP1A2.74 Accordingly, no dose adjustments are likely to be required in smoking patients with COPD.
Positioning roflumilast in clinical management of COPD
There are insufficient data to recommend the use of roflumilast in patients with mild COPD. The clinical trials with roflumilast have been conducted in patients with moderate or severe COPD.62–66,68 Roflumilast improved lung function in patients with more severe COPD, especially in those with chronic bronchitis, those with recent exacerbations, and those requiring frequent rescue inhaler use, whether given alone or in combination with the long-acting bronchodilators, salmeterol or tiotropium.62–66,68 In these patients, roflumilast also reduced exacerbations when given alone or in combination with long-acting bronchodilators. No studies have addressed whether roflumilast might supplant ICSs in combinations with long-acting bronchodilators or whether there is a benefit to adding it to combinations of ICSs and long-acting bronchodilators.
Although the studies reviewed herein were done in different COPD populations adhering to different protocols, the reported results with roflumilast are similar to the outcome in patients with COPD of similar severity treated with ICSs.66 In the TORCH (Towards a Revolution in COPD Health) study, postbronchodilator FEV1 declined 47 mL less per year in patients treated with fluticasone propionate compared with placebo-treated patients.66 In the roflumilast studies, post-bronchodilator FEV1 declined 39 mL, 49 mL, and 61 mL less in roflumilast-treated compared with placebo-treated patients over the one year of the study.63,65 In the TORCH study, postbronchodilator FEV1 was 50 mL/year in patients treated with salmeterol-fluticasone compared with those treated with salmeterol alone.66 Postbronchodilator FEV1 was 60 mL greater in patients treated with the combination of roflumilast and salmeterol for one year compared with patients treated with salmeterol alone.68 The combined moderate and severe exacerbation rate was reduced 18% in ICS-treated patients compared with placebo in the TORCH study.66 In comparison, the exacerbation rate was reduced 17% in the roflumilast-treated group compared with placebo.65
The optimal placement of roflumilast in the treatment algorithm remains uncertain. Possible indications include patients with more severe COPD who remain inadequately controlled despite the use of combination therapy. In the absence of sufficient clinical trial data, potential positioning in the treatment algorithm include use in patients inadequately controlled on a combination of LAAC and LABA and use in patients inadequately controlled with a LAAC, LABA, and ICS or where theophylline is generally used. Important advantages are that it avoids many drug interactions, narrow therapeutic index of theophylline, and the need for regular blood level monitoring.38 Potential disadvantages are that it is not a bronchodilator, lacks the clinical track record of a drug used for over 70 years, and the cost of a new drug versus an older generic medication.
Compared with ICSs, a potential advantage of combining roflumilast with long-acting bronchodilators is the ease of taking a pill once daily rather than having to be able to use an inhaler properly, and which needs to be taken twice daily. Roflumilast is not related to an increased risk of pneumonia or other adverse affects associated with ICSs use, such as osteoporosis, glaucoma, cataracts, and skin thinning. However, roflumilast is associated with other adverse effects, including weight loss and a greater risk of discontinuation of therapy whether given alone, or with short- and/or long-acting bronchodilators.62–66,68 Moreover, serious adverse events include an apparently higher incidence of neuropsychiatric abnormalities and of certain cancers. In this respect, it is salient that in January 2010 Forest Laboratories submitted to the FDA a new indication for roflumilast together with associated labeling changes and a warning regarding neuropsychiatric events. The revised indication for roflumilast is for the “maintenance treatment of chronic obstructive pulmonary disease (COPD) associated with chronic bronchitis in patients at risk of exacerbations” and is more restrictive than broad maintenance treatment of COPD (www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM207377.pdf). In April 2010, the Pulmonary-Allergy Drugs Advisory Committee (PADAC) convened by the FDA voted by 10 votes to five against approving roflumilast for the treatment of COPD (www.medscape.com/viewarticle/720010). Although the PADAC believe roflumilast to be safe and modestly effective, it seems likely that this negative outcome reflects a concern that the potential adverse events outweigh the modest improvements in lung function. However, on 22 April 2010, the European Medicines Agency Committee for Medicinal Products for Human Use adopted an opposite stance to PADAC and recommended the granting of a marketing authorization for roflumilast. The approved indication is for the “… maintenance treatment of severe chronic obstructive pulmonary disease (COPD, FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment” (www.ema.europa.eu/pdfs/human/opinion/Daxas_15986110en.pdf). A final decision by the FDA is expected in May 2010 after negotiations with Forest. Regardless of the outcome of those talks, in the European Union at least, after more than 20 years of development, roflumilast will become the first class PDE4 inhibitor for the treatment of COPD and will provide physicians with another treatment option for patients with more severe disease.
Footnotes
Disclosure
The authors were solely responsible for the content and writing of the review. MAG has received honoraria, consultancy fees, and/or research grants from AstraZeneca, Forest, Gilead Sciences, GlaxoSmithKline, Nycomed, Otsuka, Proctor and Gamble, Sanofi-Aventis, Schering-Plough, and the GSK (Canada)/Collaborative Innovation Research Fund. SKF has received honoraria, consultancy fees, and/or research grants from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Ception, GlaxoSmithKline, MedImmune, Novartis, Nycomed, and Pfizer.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance: United States, 1971–2000. MMWR CDC Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 3.Donaldson GC, Seemungal TA, Bhomik, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nie JX, Wang L, Upshur REG. Mortality of elderly patients in Ontario after hospital admission for chronic obstructive pulmonary disease. Can Respir J. 2009;14(8):485–489. doi: 10.1155/2007/425248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med. 1996;154(4 Pt1):959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. New Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. The cytokine network in COPD. Am J Respir Cell Mol Biol. 2009;41(6):631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 8.Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systemic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ, Celli B. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Immunol Rev. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 11.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58(3):488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 12.Press NJ, Banner KH. PDE4 inhibitors – a review of the current field. Prog Med Chem. 2009;47(1):37–74. doi: 10.1016/S0079-6468(08)00202-6. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343(8904):1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 14.Kidney J, Dominguez M, Taylor PM, Rose M, Chung KF, Barnes PJ. Immunomodulation by theophylline in asthma. Demonstration by withdrawal of therapy. Am J Respir Crit Care Med. 1995;151(6):1907–1914. doi: 10.1164/ajrccm.151.6.7767539. [DOI] [PubMed] [Google Scholar]
- 15.Djukanovic R, Finnerty JP, Lee C, Wilson S, Madden J, Holgate ST. The effects of theophylline on mucosal inflammation in asthmatic airways: Biopsy results. Eur Respir J. 1995;8(5):831–833. [PubMed] [Google Scholar]
- 16.Finnerty JP, Lee C, Wilson S, Madden J, Djukanovic R, Holgate ST. Effects of theophylline on inflammatory cells and cytokines in asthmatic subjects: A placebo-controlled parallel group study. Eur Respir J. 1996;9(8):1672–1677. doi: 10.1183/09031936.96.09081672. [DOI] [PubMed] [Google Scholar]
- 17.Culpitt SV, de Matos C, Russell RE, Donnelly LE, Rogers DF, Barnes PJ. Effect of theophylline on induced sputum inflammatory indices and neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165(10):1371–1376. doi: 10.1164/rccm.2105106. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Nasuhara Y, Betsuyaku T, et al. Effect of low-dose theophylline on airway inflammation in COPD. Respirology. 2004;9(2):249–254. doi: 10.1111/j.1440-1843.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 19.Iiboshi H, Ashitani J, Katoh S, et al. Long-term treatment with theophylline reduces neutrophils, interleukin-8 and tumor necrosis factor-α in the sputum of patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2007;20(1):46–51. doi: 10.1016/j.pupt.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Barnes PJ. Theophylline in chronic obstructive pulmonary disease. New horizons. Proc Am Thorac Soc. 2005;2(4):334–339. doi: 10.1513/pats.200504-024SR. [DOI] [PubMed] [Google Scholar]
- 21.Giembycz MA. Can the anti-inflammatory potential of PDE4 inhibitors be realized: Guarded optimism or wishful thinking? Br J Pharmacol. 2008;155(3):288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pages L, Gavalda A, Lehner MD. PDE4 inhibitors: A review of current developments (2005–2009) Expert Opin Ther Pat. 2009;19(11):1501–1519. doi: 10.1517/13543770903313753. [DOI] [PubMed] [Google Scholar]
- 23.Altana Roflumilast – APTA 2217, B9302–107, BY 217, BYK 20869. Drugs R D. 2004;5(3):176–181. doi: 10.2165/00126839-200405030-00009. [DOI] [PubMed] [Google Scholar]
- 24.Sorbera LA, Leeson PA, Casterer J. Roflumilast. Drugs Future. 2000;25(12):1261–1264. [Google Scholar]
- 25.Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12(12):2233–2247. doi: 10.1016/j.str.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297(1):267–279. [PubMed] [Google Scholar]
- 27.Houslay MD, Schafer P, Zhang KY. Phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10(22):1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 28.Torphy TJ, Barnette MS, Underwood DC, et al. Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: From concept to clinic. Pulm Pharmacol Ther. 1999;12(2):131–135. doi: 10.1006/pupt.1999.0181. [DOI] [PubMed] [Google Scholar]
- 29.Gale DD, Hofer P, Spina D, et al. Pharmacology of a new cyclic nucleotide phosphodiesterase type 4 inhibitor, V11294. Pulm Pharmacol Ther. 2003;16(2):97–104. doi: 10.1016/S1094-5539(02)00175-X. [DOI] [PubMed] [Google Scholar]
- 30.Robichaud A, Stamatiou PB, Jin SL, et al. Deletion of phosphodiesterase 4D in mice shortens α2-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110(7):1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giembycz MA. 4D or not 4D – the emetogenic basis of PDE4 inhibitors uncovered? Trends Pharmacol Sci. 2002;23(12):548. doi: 10.1016/s0165-6147(02)02089-8. [DOI] [PubMed] [Google Scholar]
- 32.Giembycz MA. An update and appraisal of the cilomilast phase III clinical development programme for chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2006;62(2):138–152. doi: 10.1111/j.1365-2125.2006.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torphy TJ. Phosphodiesterase isozymes: Molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998;157(2):351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- 34.Giembycz MA. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of bronchial asthma? Biochem Pharmacol. 1992;43(10):2041–2051. doi: 10.1016/0006-2952(92)90160-k. [DOI] [PubMed] [Google Scholar]
- 35.Currie GP, Butler CA, Anderson WJ, Skinner C. Phosphodiesterase 4 inhibitors in chronic obstructive pulmonary disease: A new approach to oral treatment. Br J Clin Pharmacol. 2008;65(6):803–810. doi: 10.1111/j.1365-2125.2008.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spina D. Phosphodiesterase-4 inhibitors in the treatment of inflammatory lung disease. Drugs. 2003;63(23):2575–2594. doi: 10.2165/00003495-200363230-00002. [DOI] [PubMed] [Google Scholar]
- 37.Hatzelmann A, Morcillo EJ, Lungarella G, et al. The preclinical pharmacology of roflumilast – A selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease Pulm Pharmac Ther 2010April7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Field SK. Roflumilast: An oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin Investig Drugs. 2008;17(5):811–818. doi: 10.1517/13543784.17.5.811. [DOI] [PubMed] [Google Scholar]
- 39.Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297(1):280–290. [PubMed] [Google Scholar]
- 40.Sanz MJ, Cortijo J, Taha MA, et al. Roflumilast inhibits leukocyte-endothelial cell interactions, expression of adhesion molecules and microvascular permeability. Br J Pharmacol. 2007;152(4):481–492. doi: 10.1038/sj.bjp.0707428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martorana PA, Lunghi B, Lucatelli M, De Cunto G, Beume R, Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke-exposed mice. BMC Pulm Med. 2008;8:17. doi: 10.1186/1471-2466-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martorana PA, Beume R, Lucatelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172(7):848–853. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald MF, Spicer D, McAulay AE, et al. Roflumilast but not methylprednisolone inhibited cigarette-smoke-induced pulmonary inflammation in guinea pigs. Eur Respir J. 2006;28(Suppl 50):663S. [Google Scholar]
- 44.Togo S, Liu X, Wang X, et al. PDE4 inhibitors roflumilast and rolipram augment PGE2 inhibition of TGF-β1-stimulated fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L959–L969. doi: 10.1152/ajplung.00508.2007. [DOI] [PubMed] [Google Scholar]
- 45.Sabatini F, Petechia L, Boero S, et al. A phosphodiesterase 4 inhibitor, roflumilast N-oxide, inhibits human lung fibroblast functions in vitro Pulm Pharmacol Ther 2010March11 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Burgess JK, Oliver BG, Poniris MH, et al. A phosphodiesterase 4 inhibitor inhibits matrix protein deposition in airways in vitro. J Allergy Clin Immunol. 2006;118(3):649–657. doi: 10.1016/j.jaci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Wollin L, Bundshuh DS, Wohlsen A, Marx D, Beume R. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm Pharmacol Ther. 2006;19(5):343–352. doi: 10.1016/j.pupt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Wollin L, Marx D, Wohlsen A, Beume R. Roflumilast inhibition of pulmonary leukotriene production and bronchoconstriction in ovalbumin-sensitized and -challenged guinea pigs. J Asthma. 2005;42(10):873–878. doi: 10.1080/02770900500370858. [DOI] [PubMed] [Google Scholar]
- 49.Grootendorst DC, Gauw SA, Baan R, et al. Does a single dose of the phosphodiesterase 4 inhibitor, cilomilast (15 mg), induce bronchodilation in patients with chronic obstructive pulmonary disease? Pulm Pharmacol Ther. 2003;16(2):115–120. doi: 10.1016/S1094-5539(02)00172-4. [DOI] [PubMed] [Google Scholar]
- 50.Grootendorst DC, Gauw SA, Verhoosel RM, et al. The PDE4 inhibitor roflumilast reduces sputum neutrophil and eosinophil numbers in patients with COPD. Thorax. 2007;62(12):1081–1087. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hohlfeld JM, Schoenfeld K, Lavae-Mokhtari M, et al. Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: A randomized placebo-controlled trial. Pulm Pharmacol Ther. 2008;21(4):616–623. doi: 10.1016/j.pupt.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Timmer W, Leclerc V, Birraux J, et al. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-alpha ex vivo. J Clin Pharmacol. 2002;42(3):297–303. doi: 10.1177/00912700222011328. [DOI] [PubMed] [Google Scholar]
- 53.Gamble E, Grootendorst DC, Brightling CE, et al. Anti-inflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(8):976–982. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- 54.Grootendorst DC, Gauw SA, Benschop PN, Sterk PJ, Hiemstra PS, Rabe KF. Efficacy of the novel phosphodiesterase-4 inhibitor BAY 19-8004 on lung function and airway inflammation in asthma and chronic obstructive pulmonary disease (COPD) Pulm Pharmacol Ther. 2003;16(6):341–347. doi: 10.1016/S1094-5539(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 55.Bethke TD, Bohmer GM, Hermann R, et al. Dose-proportional intra-individual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47(1):26–36. doi: 10.1177/0091270006294529. [DOI] [PubMed] [Google Scholar]
- 56.Hauns B, Hermaan R, Hunnemeyer A, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(10):1146–1153. doi: 10.1177/0091270006291621. [DOI] [PubMed] [Google Scholar]
- 57.Neville KA, Szefler SJ, Abdel-Rahman SM, et al. Single-dose pharmacokinetics of roflumilast in children and adolescents. J Clin Pharmacol. 2008;48(8):978–985. doi: 10.1177/0091270008319466. [DOI] [PubMed] [Google Scholar]
- 58.Nassr N, Huennemeyer A, Herzog R, et al. Effects of rifampicin on the pharmacokinetics of roflumilast and roflumilast N-oxide in healthy subjects. Br J Clin Pharmacol. 2009;68(4):580–587. doi: 10.1111/j.1365-2125.2009.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David M, Zech K, Seiberling M, Weimar C, Bethke TD. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high absolute bioavailability. J Allergy Clin Immunol. 2004;113(1):S220–S221. [Google Scholar]
- 60.Nassr N, Lahu G, Hunnemeyer A, et al. Magnesium hydroxide/aluminium hydroxide-containing antacid does not affect the pharmacokinetics of the targeted phosphodiesterase 4 inhibitor roflumilast. J Clin Pharmacol. 2007;47(5):660–666. doi: 10.1177/0091270006297920. [DOI] [PubMed] [Google Scholar]
- 61.Lahu G, Huennemeyer A, von Richter O, et al. Effect of single and repeated doses of ketoconazole on the pharmacokinetics of roflumilast and roflumilast N-oxide. J Clin Pharmacol. 2008;48(11):1339–1339. doi: 10.1177/0091270008321941. [DOI] [PubMed] [Google Scholar]
- 62.Rabe KF, Bateman ED, O’Donnell DE, et al. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: A randomized controlled trial. Lancet. 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 63.Calverley PMA, Sanchez-Toril F, McIvor A, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 64.Rennard SI, Calverley PMA, Rempel A, et al. The effect of roflumilast treatment on exacerbations in patients with COPD – results of a pooled analysis of two 1-year studies. Am J Respir Crit Care Med. 2008;177:A963. [Google Scholar]
- 65.Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomized clinical trials. Lancet. 2009;374(9691):684–695. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 66.Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 67.Tashkin DP, Celli B, Senn S, et al. A four-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 68.Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: Two randomized clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 69.Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007;146(8):545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 70.Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(3):967–975. doi: 10.1164/ajrccm.153.3.8630581. [DOI] [PubMed] [Google Scholar]
- 71.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 72.Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162–166. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 73.Corren J, Casale TB, Lanier B, Buhl R, Holgate S, Jimenez P. Safety and tolerability of omalizumab. Clin Exp Allergy. 39(6):788–797. doi: 10.1111/j.1365-2222.2009.03214.x. [DOI] [PubMed] [Google Scholar]
- 74.Hermann R, Nassr N, Lahu G, et al. Steady-state pharmacokinetics of roflumilast and roflumilast N-oxide in patients with mild and moderate liver cirrhosis. Clin Pharmacokinet. 2007;46(5):403–416. doi: 10.2165/00003088-200746050-00003. [DOI] [PubMed] [Google Scholar]
- 75.Hauns B, Hermann R, Hunnemeyer A, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(10):1146–1153. doi: 10.1177/0091270006291621. [DOI] [PubMed] [Google Scholar]
- 76.Nassr N, Lahu G, von Richter O, et al. Lack of a pharmacokinetic interaction between steady-state roflumilast and single-dose midazolam in healthy subjects. Br J Clin Pharmacol. 2007;63(3):365–370. doi: 10.1111/j.1365-2125.2006.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bohmer GM, Nassr N, Wenger M, et al. The targeted oral, once-daily phosphodiesterase 4 inhibitor roflumilast and the leukotriene receptor antagonist montelukast do not exhibit significant pharmacokinetic interactions. J Clin Pharmacol. 2009;49(4):389–397. doi: 10.1177/0091270008330980. [DOI] [PubMed] [Google Scholar]
- 78.Lahu G, Huennemeyer A, Herzog R, et al. Effect of repeated dose of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide. Int J Clin Pharmacol Ther. 2009;47(4):236–245. doi: 10.5414/cpp47236. [DOI] [PubMed] [Google Scholar]
- 79.Hermann R, Siegmund W, Giessmann T, et al. The oral, once-daily phosphodiesterase 4 inhibitor roflumilast lacks relevant pharmacokinetic interactions with inhaled budesonide. J Clin Pharmacol. 2007;47(8):1005–1013. doi: 10.1177/0091270007300950. [DOI] [PubMed] [Google Scholar]
- 80.Bethke TD, Giessmann T, Westphal K, et al. Roflumilast, a once-daily oral phosphodiesterase 4 inhibitor, lacks relevant pharmacokinetic interactions with inhaled salbutamol when co-administered in healthy subjects. Int J Clin Pharmacol Ther. 2006;44(11):572–579. doi: 10.5414/cpp44572. [DOI] [PubMed] [Google Scholar]
- 81.Jusko WJ. Influence of cigarette smoking on drug metabolism in man. Drug Metab Rev. 1979;9(2):221–236. doi: 10.3109/03602537908993892. [DOI] [PubMed] [Google Scholar]
- 82.Hunt SN, Jusko WJ, Yurchak AM. Effect of smoking on theophylline disposition. Clin Pharmacol Ther. 1976;19(5 Pt 1):546–551. doi: 10.1002/cpt1976195part1546. [DOI] [PubMed] [Google Scholar]
- 83.Powell JR, Vozeh S, Hopewell P, Costello J, Sheiner LB, Riegelman S. Theophylline disposition in acutely ill hospitalized patients. The effect of smoking, heart failure, severe airway obstruction, and pneumonia. Am Rev Respir Dis. 1978;118(2):229–238. doi: 10.1164/arrd.1978.118.2.229. [DOI] [PubMed] [Google Scholar]